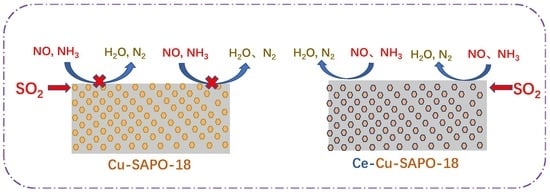
Improved SO2 Tolerance of Cu-SAPO-18 by Ce-Doping in the Selective Catalytic Reduction of NO with NH3
Abstract
:1. Introduction
2. Results and Discussion
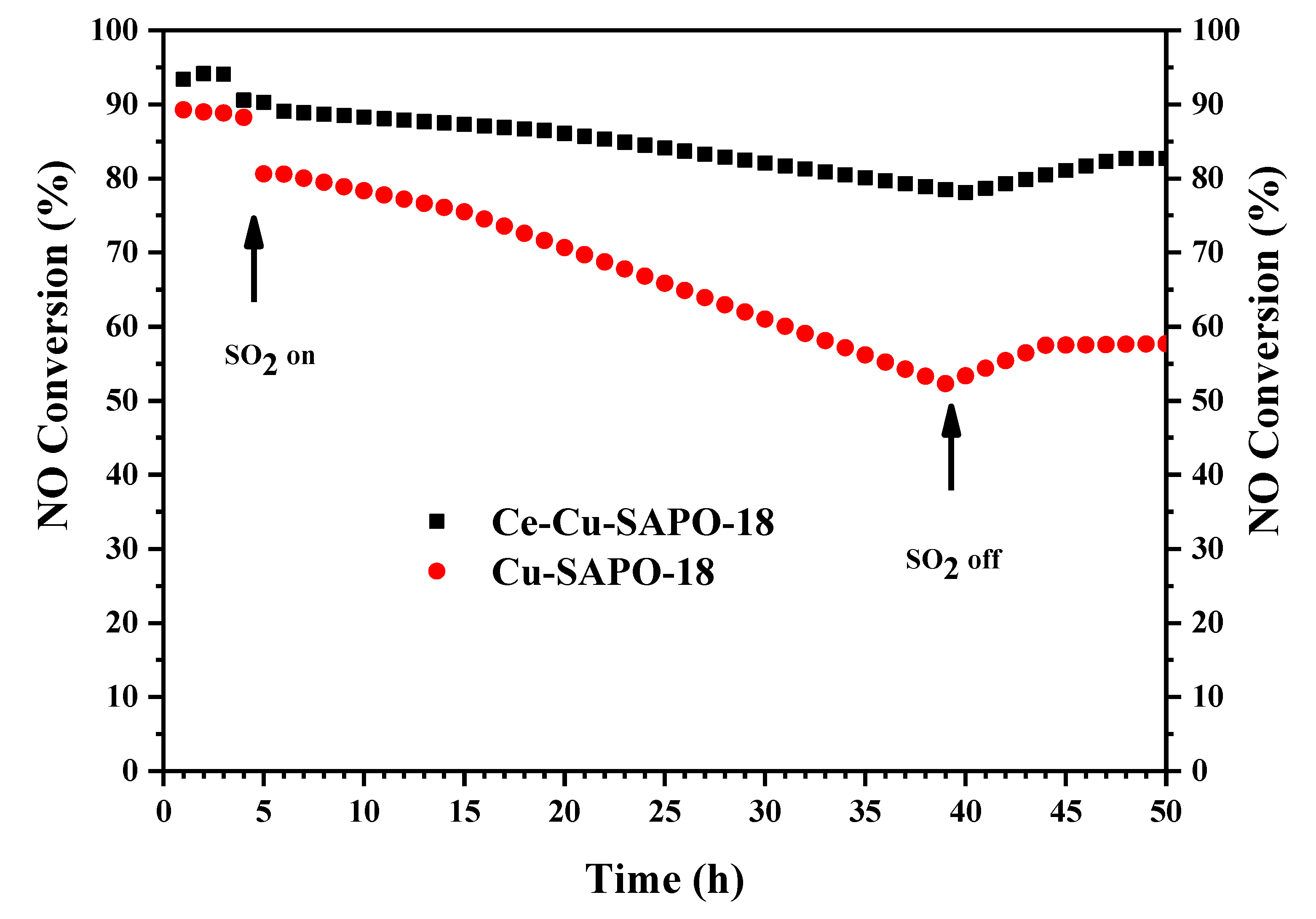
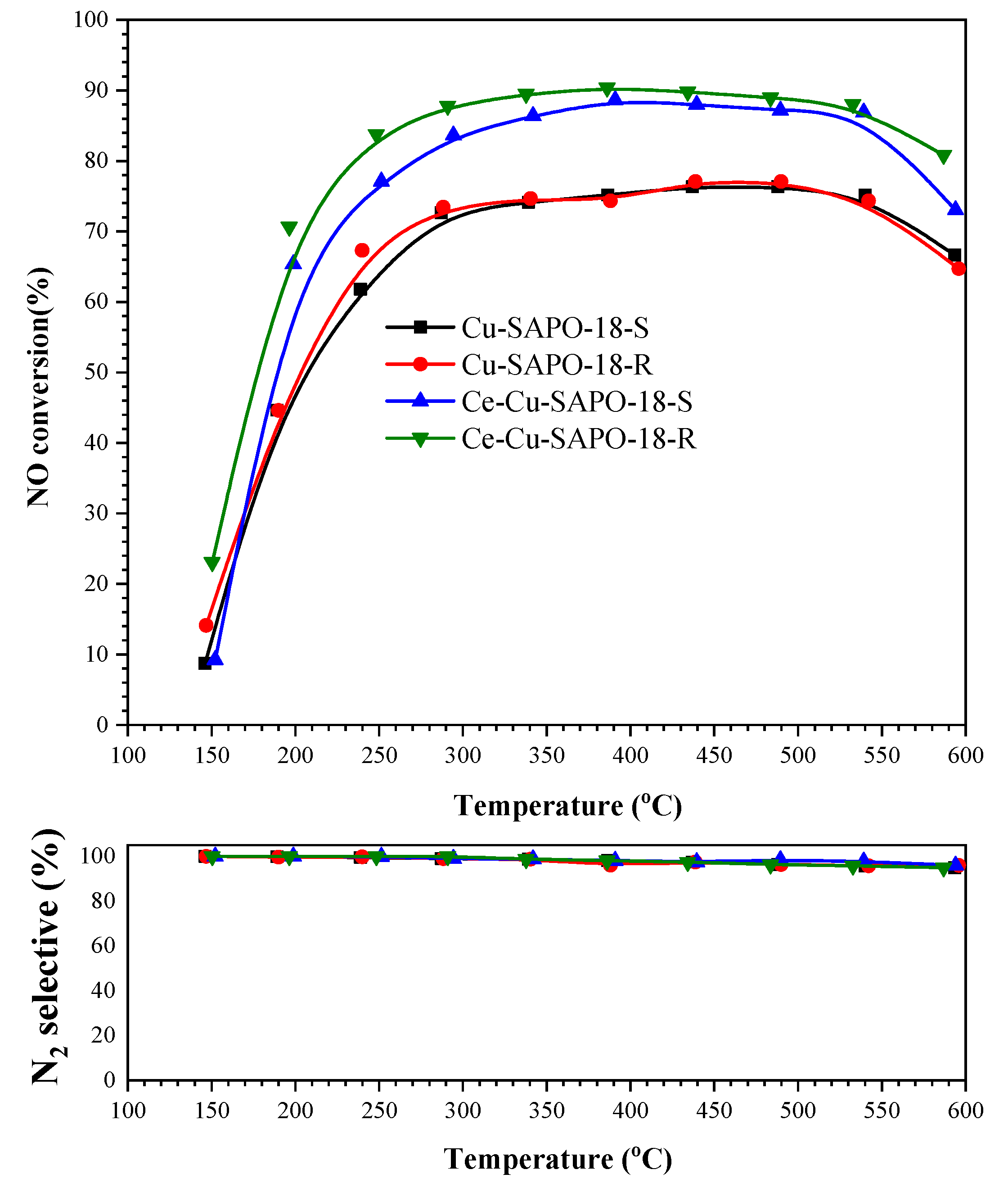
2.1. Impact of SO2 Exposure on NH3-SCR Performance
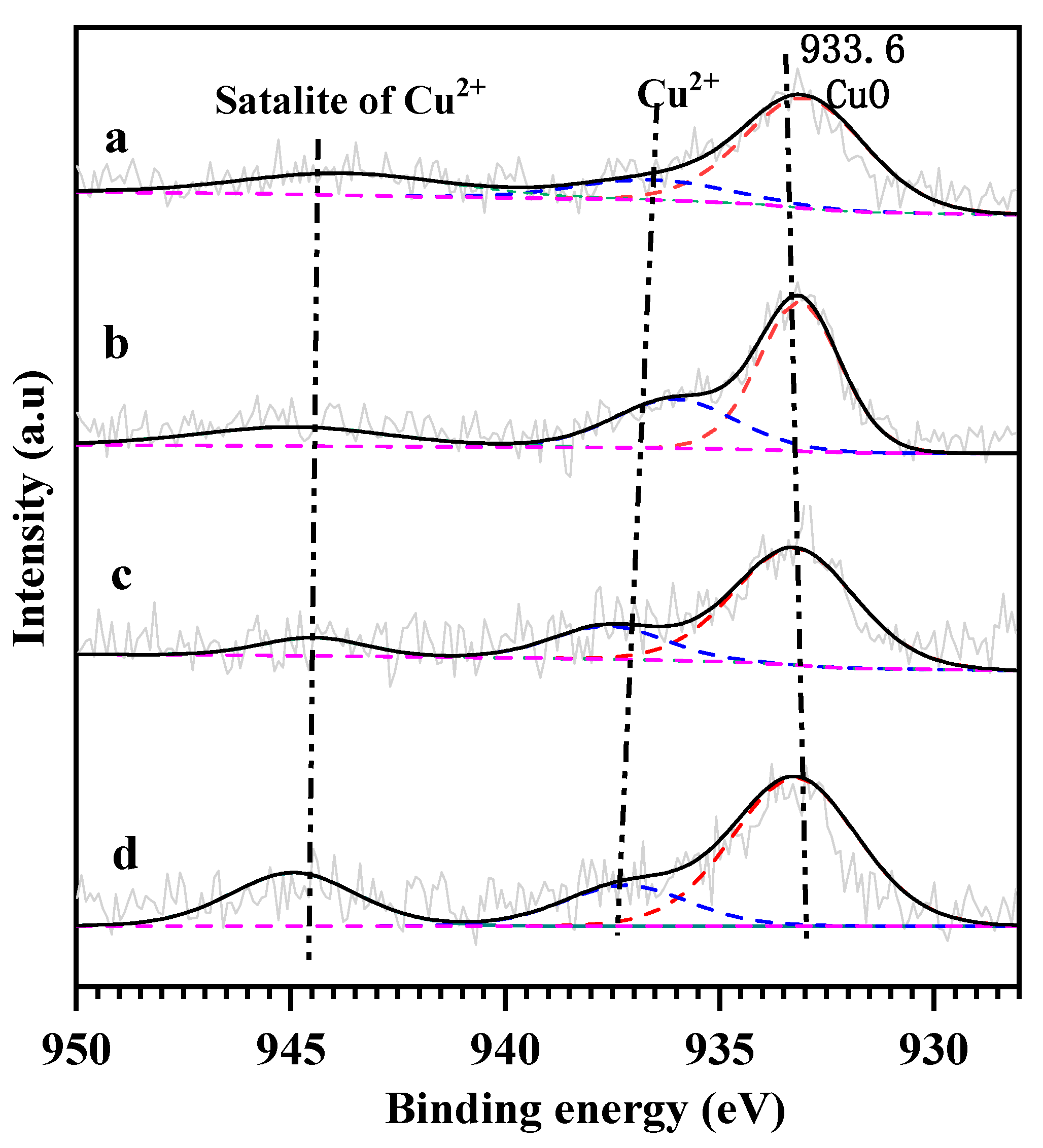
2.2. Surface Property
2.3. Morphology
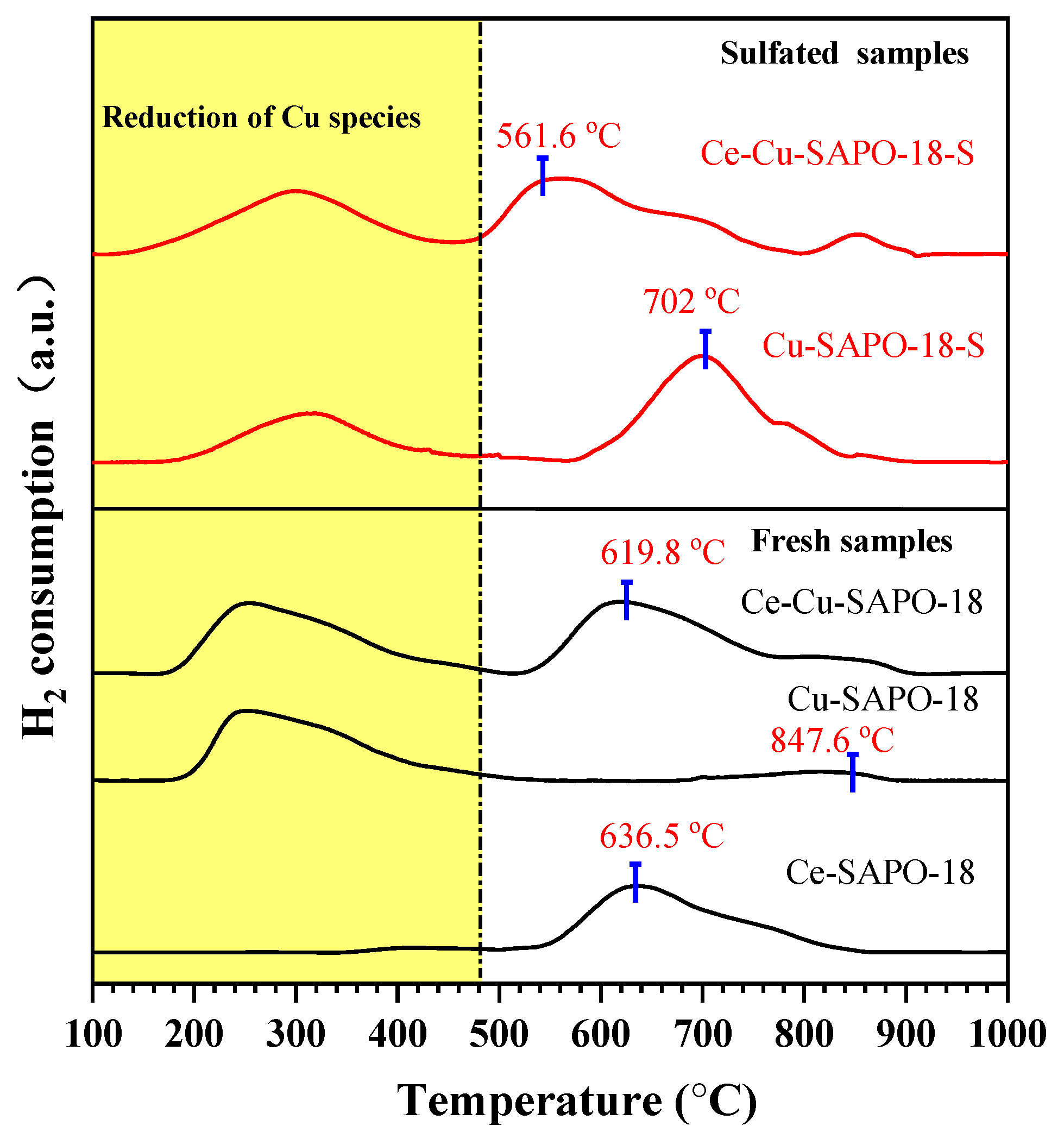
2.4. Reducibility
2.5. Adsorption of NH3 by the Sulfated and Fresh Samples
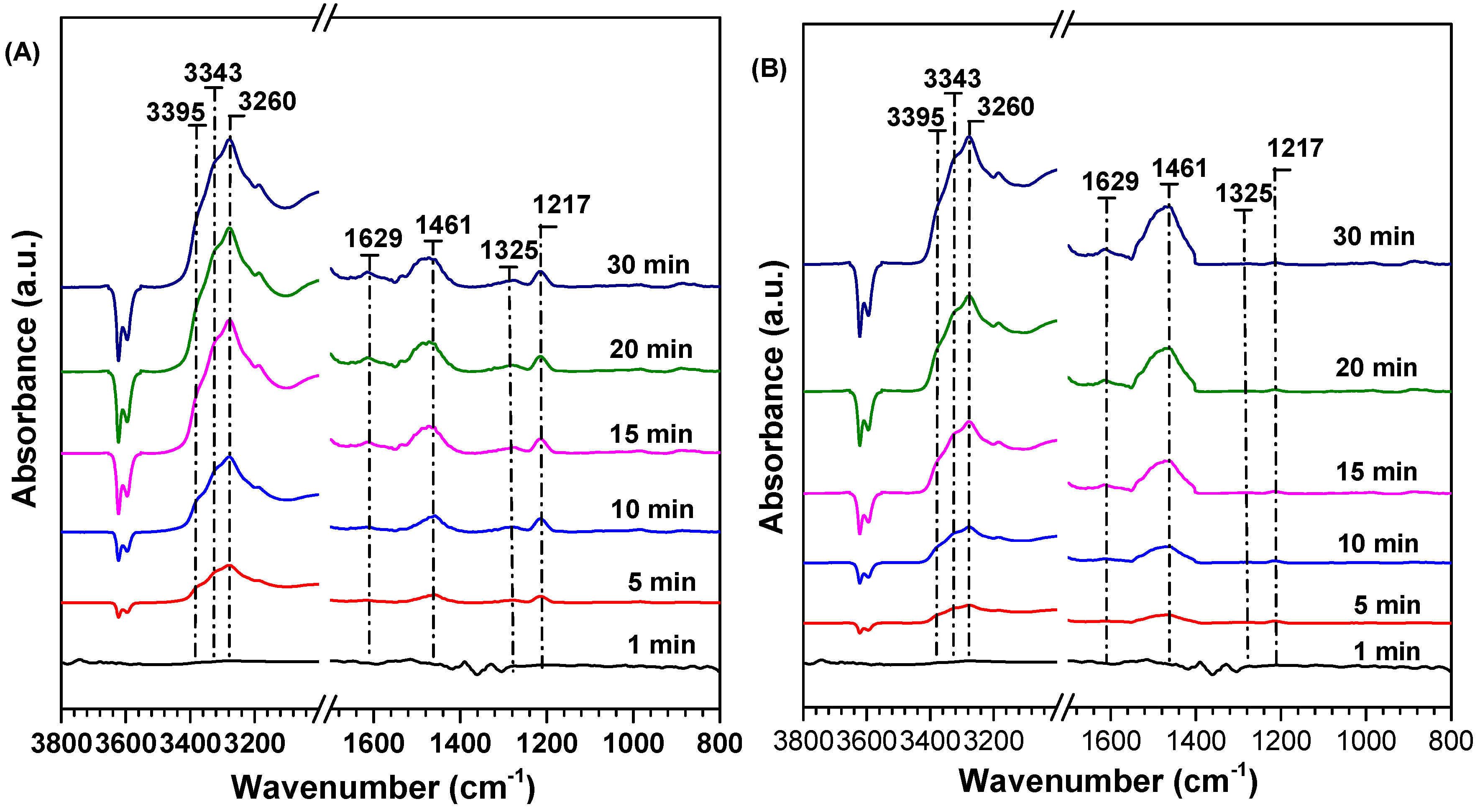
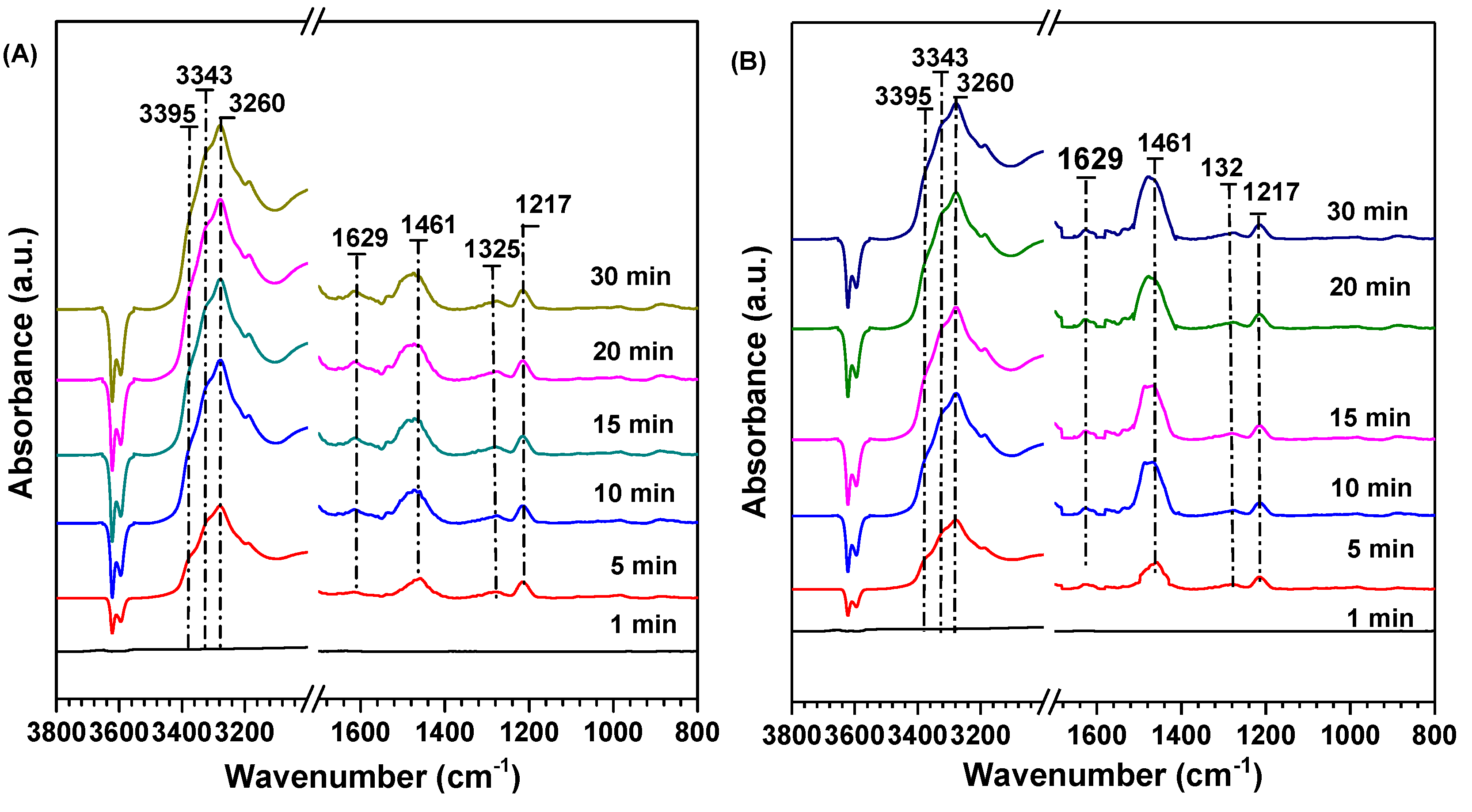
2.6. Competitive Adsorption between SO2 and NO on the Sample Surface
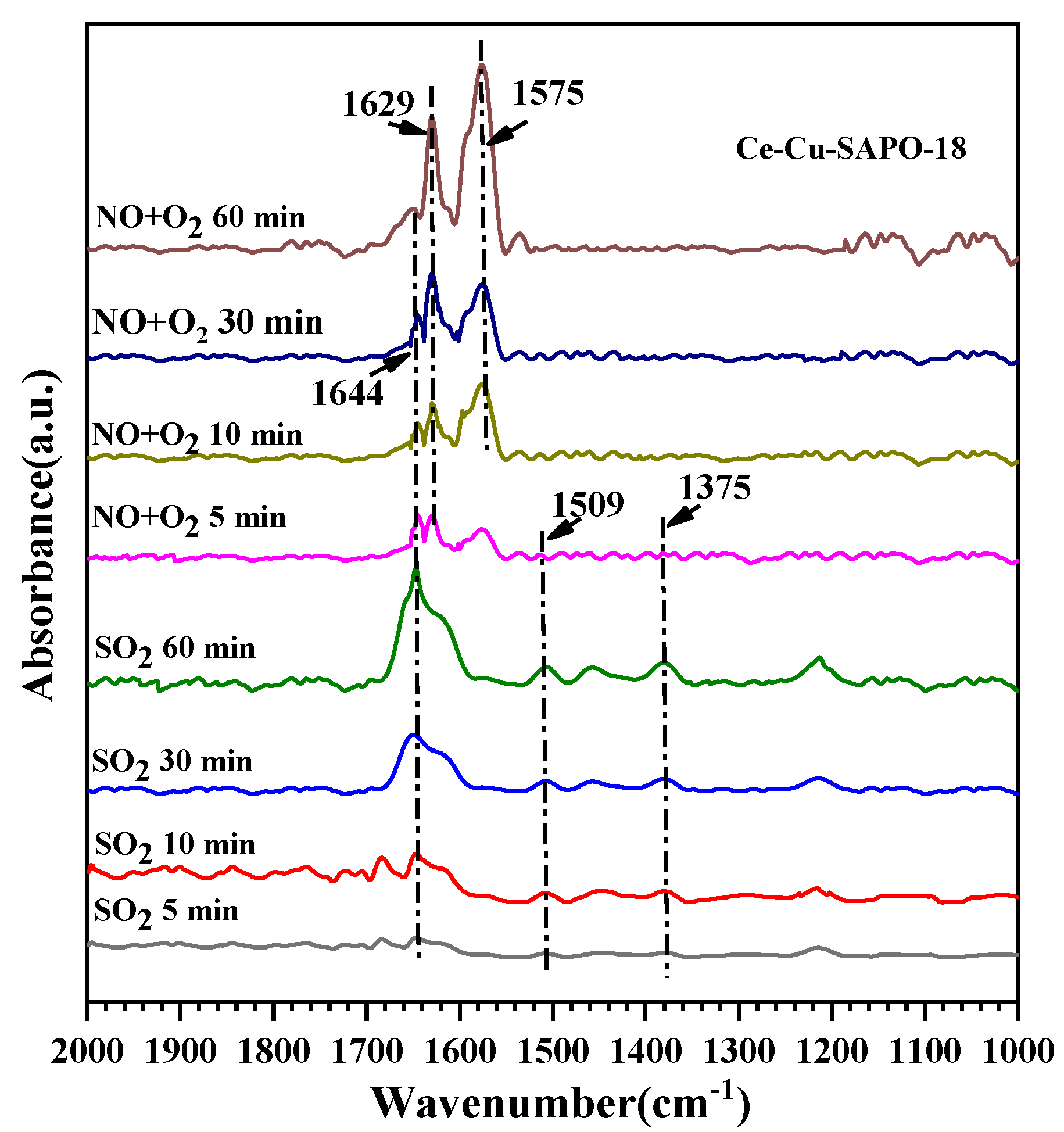
2.7. Thermal Stability of Surface Species during the NH3-SCR Reaction Process
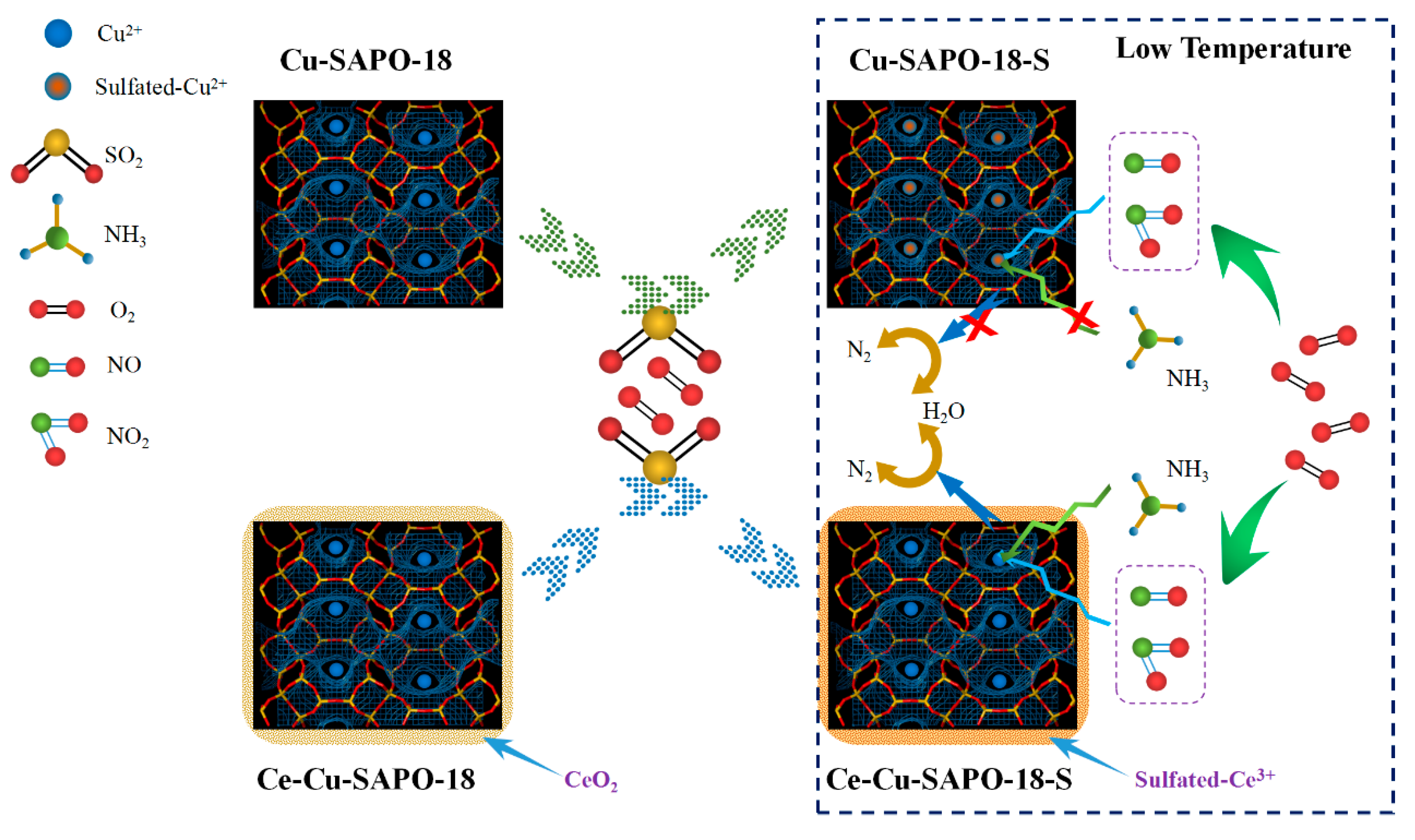
2.8. Possible Mechanism for Improved SO2 Resistance of Cu-SAPO-18 by Ce Doping
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V.W. Reversible and irreversible deactivation of Cu-CHA NH3-SCR catalysts by SO2 and SO3. Appl. Catal. B-Environ. 2018, 226, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Deka, U.; Juhin, A.; Eilertsen, E.A.; Emerich, H.; Green, M.A.; Korhonen, S.T.; Weckhuysen, B.M.; Beale, A.M. Confirmation of isolated cu2+ ions in SSZ-13 zeolite as active sites in NH3 selective catalytic reduction. J. Phys. Chem. C 2012, 116, 4809–4818. [Google Scholar] [CrossRef]
- Giordanino, F.; Vennestrøm, P.N.R.; Lundegaard, L.F.; Stappen, F.N.; Mossin, S.; Beato, P.; Bordiga, S.; Lamberti, C. Characterization of Cu-exchanged SSZ-13: A comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans. 2013, 42, 12741. [Google Scholar] [CrossRef] [PubMed]
- Lomachenko, K.A.; Borfecchia, E.; Negri, C.; Berlier, G.; Lamberti, C.; Beato, P.; Falsig, H.; Bordiga, S. The Cu-CHA deNOx Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Brylewska, K.; Tarach, K.A.; Rutkowska, M.; Jabłońska, M.; Choi, M.; Chmielarz, L. IR studies of Fe modified ZSM-5 zeolites of diverse mesopore topologies in the terms of their catalytic performance in NH3 -SCR and NH3 -SCO processes. Appl. Catal. B-Environ. 2015, 179, 589–598. [Google Scholar] [CrossRef]
- Chen, B.; Xu, R.; Zhang, R.; Liu, N. Economical Way to Synthesize SSZ-13 with Abundant Ion-Exchanged Cu+ for an Extraordinary Performance in Selective Catalytic Reduction (SCR) of NOx by Ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W. Recent NH3 -SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Zhao, L. Nature of cu species in Cu-SAPO-18 catalyst for NH3-SCR: Combination of experiments and DFT calculations. J. Phys. Chem. C 2016, 120, 14669–14680. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.; Li, J.; Kamasamudram, K.; Epling, W.S. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B-Environ. 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Zhang, N.; He, H.; Wang, D.; Li, Y. Challenges and Opportunities for Manganese Oxides in Low-temperature Selective Catalytic Reduction of NOx with NH3: H2O Resistance Ability. J. Solid State Chem. 2020, 289, 121464. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, S.; Li, S.; Zhao, J.; Xia, Y.; Lv, B.; Li, W. Inhibitory effect of SO2 on side reactions of NH3-SCR over olivine. Catal. Sci. Technol. 2015, 5, 3613–3623. [Google Scholar] [CrossRef]
- Shen, M.; Wen, H.; Hao, T.; Yu, T.; Fan, D.; Wang, J.; Li, W.; Wang, J. Deactivation mechanism of SO2 on Cu/SAPO-34 NH3-SCR catalysts: Structure and active Cu2+. Catal. Sci. Technol. 2015, 5, 1741–1749. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, T.; Wang, X.; Qi, G.; Xue, J.; Shen, M.; Li, W. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B-Environ. 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, W.; Miao, P.; Li, F.; Guo, Z.; Sun, Q. Intergrowth Silicoaluminophosphate Molecular Sieves Synthesized and Their Catalytic Performances for Methanol to Olefins Reaction. Ind. Eng. Chem. Res. 2018, 57, 10398–10402. [Google Scholar] [CrossRef]
- Ono, K.; Miyake, K.; Nakai, M.; Al Jabri, H.; Hirota, Y.; Uchida, Y.; Tanaka, S.; Miyamoto, M.; Nishiyama, N. Development of AEI type germanoaluminophosphate (GeSAPO-18) with ultra-weak acid sites and its catalytic properties for the methanol to olefin (MTO) reaction. Catal. Sci. Technol. 2017, 7, 4622–4628. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Ce doping to Cu-SAPO-18: Enhanced catalytic performance for the NH3-SCR of NO in simulated diesel exhaust. Microporous Mesoporous Mater. 2019, 276, 133–146. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Wang, Q.; Deng, J. The protection of CeO2 thin film on Cu-SAPO-18 catalyst for highly stable catalytic NH3-SCR performance. Chem. Eng. J. 2017, 330, 926–935. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Qiao, H.; Yu, H.; Hu, Y.; Chang, L.; Bao, W. Cerium-Stabilized Cu-SSZ-13 Catalyst for the Catalytic Removal of NOx by NH3. Ind. Eng. Chem. Res. 2016, 55, 1174–1182. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, X.; Xu, H.; Lan, L.; Gong, M.; Chen, Y. Novel promotional effect of yttrium on Cu-SAPO-34 monolith catalyst for selective catalytic reduction of NOx by NH3 (NH3-SCR). Catal. Commun. 2016, 76, 33–36. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, S.; Lan, L.; Yang, Z.; Xu, H.; Lin, T.; Gong, M.; Chen, Y. Promotional effect of Ce on Cu-SAPO-34 monolith catalyst for selective catalytic reduction of NOx with ammonia. J. Mol.Catal. A-Chem. 2015, 398, 304–311. [Google Scholar] [CrossRef]
- Wu, R.; Li, L.; Zhang, N.; He, J.; Song, L.; Zhang, G.; Zhang, Z.; He, H. Enhancement of low-temperature NH3-SCR catalytic activity and H2O & SO2 resistance over commercial V2O5-MoO3/TiO2 catalyst by high shear-induced doping of expanded graphite. Catal. Today 2020. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Guo, Y.; He, J.; Wu, R.; Song, L.; Zhang, G.; Zhao, J.; Wang, D.; He, H. A MnO2-based catalyst with H2O Resistance for NH3-SCR: Study of Catalytic Activity and Reactants-H2O Competitive Adsorption. Appl. Catal. B-Environ. 2020, 270, 118860. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B-Environ. 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Zhang, B.; Yong, Y.; Song, L.; Wu, R.; He, H. Polytetrafluoroethylene Doping: A Low Cost and Easy Way to Improve the H2O Resistance Ability over MnO2 Catalyst for Low-temperature NH3-SCR. J. Environ. Chem. Eng. 2019, 7, 103044. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Zheng, C.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of Si/Al ratio on catalytic performance of hydrothermally aged Cu-SSZ-13 for the NH3 -SCR of NO in simulated diesel exhaust. Appl. Surf. Sci. 2017, 419, 382–392. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Feng, G.; Chang, L.; Bao, W. In situ synthesis of CuSAPO-34/cordierite and its selective catalytic reduction of nitrogen oxides in vehicle exhaust: The effect of HF. Fuel 2013, 109, 101–109. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Rawski, M.; Vakros, G.J. Avgouropoulos A Novel Post-Synthesis Modification of CuO-CeO2 Catalysts: Effect on Their Activity for Selective CO Oxidation. ChemCatChem 2018, 10, 2096–2106. [Google Scholar] [CrossRef]
- Karthe, S.; Szargan, R.; Suoninen, E. Oxidation of pyrite surfaces—A photoelectron spectroscopic study. Appl. Surf. Sci. 1993, 72, 157–170. [Google Scholar] [CrossRef]
- Lavrenyuk, H.; Kochubei, V.; Mykhalichko, O.; Mykhalichko, B. A new flame retardant on the basis of diethylenetriamine copper(II) sulfate complex for combustibility suppressing of epoxy-amine composites. Fire Saf. J. 2016, 80, 30–37. [Google Scholar] [CrossRef]
- Ma, L.; Seo, Y.; Nahata, M.C.; Chen, X.; Li, J.; Chwank, J.W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. B-Environ. 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Richter, M.; Fait, M.J.G.; Eckelt, R.; Schneider, M.; Radnik, J.; Heidemann, D.; Fricke, R. Gas-phase carbonylation of methanol to dimethyl carbonate on chloride-free Cu-precipitated zeolite Y at normal pressure. J. Catal. 2007, 245, 11–24. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B-Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Lietti, L.; Ramis, G.; Berti, F.; Toledo, G.; Robba, D.; Busca, G.; Forzatti, P. Chemical, structural and mechanistic aspects on NOx SCR over commercial and model oxide catalysts. Catal. Today 1998, 42, 101–116. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Liu, K.; Shi, X.; He, H. Inhibitory effect of NO2 on the selective catalytic reduction of NOx with NH3 over one-pot-synthesized Cu-SSZ-13 catalyst. Catal. Sci. Technol. 2014, 4, 1104–1110. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Li, J. Design and synthesis of core-shell structured meso-Cu-SSZ-13@mesoporous aluminosilicate catalyst for SCR of NOx with NH3: Enhancement of activity, hydrothermal stability and propene poisoning resistance. Appl. Catal. B-Environ. 2016, 195, 48–58. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Reaction mechanism of selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Xie, S.; Wang, J.; He, H. Poisoning effect of sulphate on the selective catalytic reduction of NOx by C3H6 over Ag-Pd/Al2O3. J. Mol.Catal. A-Chem. 2007, 266, 166–172. [Google Scholar] [CrossRef]
- Waqif, M.; Bazin, P.; Saur, O.; Lavalley, J.C.; Blanchard, G.; Touret, O. Study of ceria sulfation. Appl. Catal. B-Environ. 1997, 11, 193–205. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B-Environ. 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT Study of the SO2 Effect on Low-Temperature SCR Reaction over Fe−Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Ye, Q.; Gao, Q.; Dai, H. Improved SO2 Tolerance of Cu-SAPO-18 by Ce-Doping in the Selective Catalytic Reduction of NO with NH3. Catalysts 2020, 10, 783. https://doi.org/10.3390/catal10070783
Han S, Ye Q, Gao Q, Dai H. Improved SO2 Tolerance of Cu-SAPO-18 by Ce-Doping in the Selective Catalytic Reduction of NO with NH3. Catalysts. 2020; 10(7):783. https://doi.org/10.3390/catal10070783
Chicago/Turabian StyleHan, Shuai, Qing Ye, Qi Gao, and Hongxing Dai. 2020. "Improved SO2 Tolerance of Cu-SAPO-18 by Ce-Doping in the Selective Catalytic Reduction of NO with NH3" Catalysts 10, no. 7: 783. https://doi.org/10.3390/catal10070783