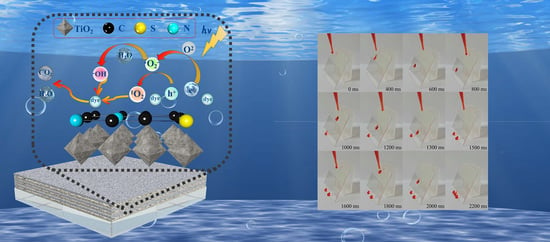
Photocatalytic Properties of a Novel Keratin char-TiO2 Composite Films Made through the Calcination of Wool Keratin Coatings Containing TiO2 Precursors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Weight of Wool Keratins
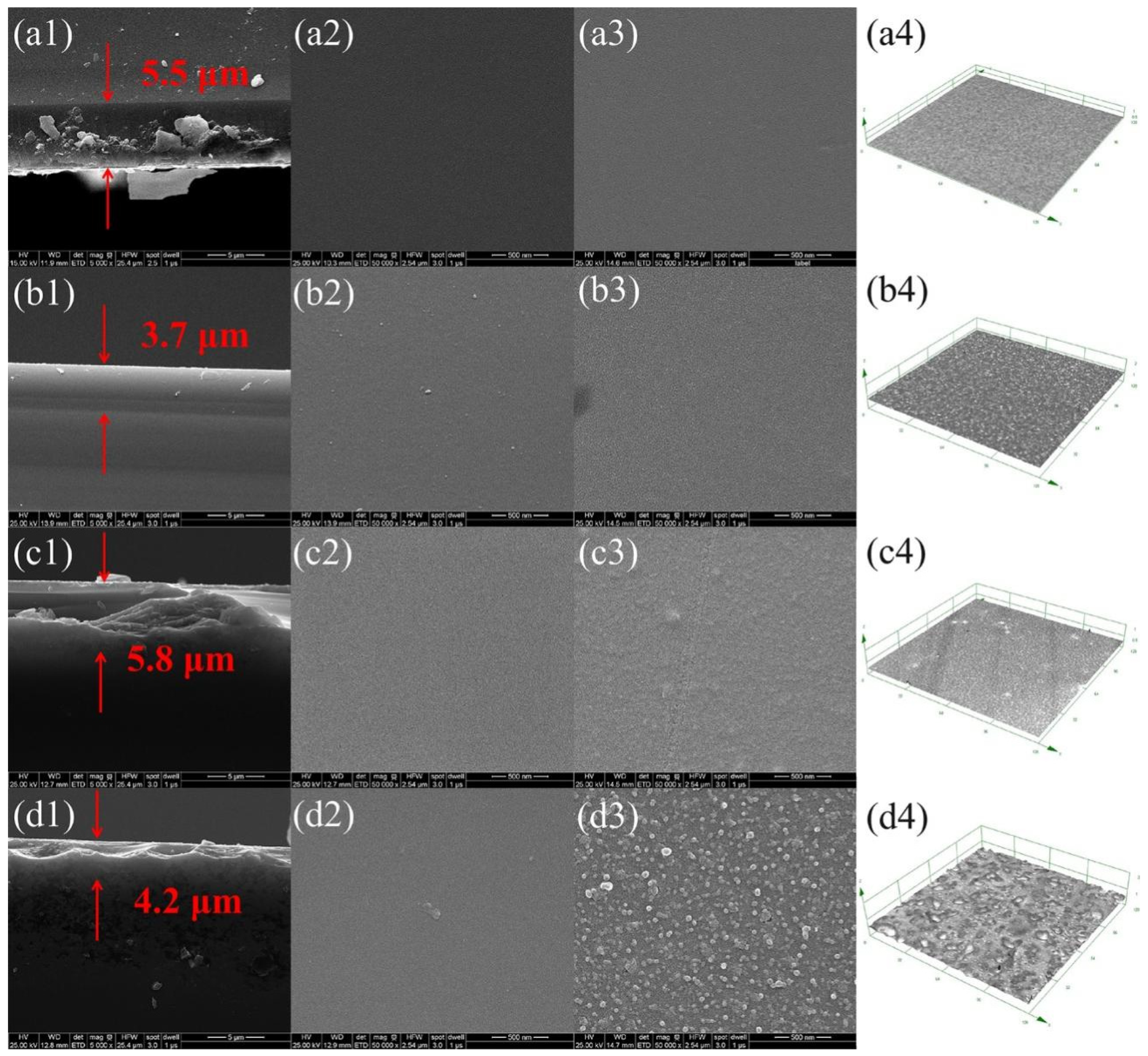
2.2. Morphology and Surface Roughness of As-Prepared Keratin char-TiO2 Films
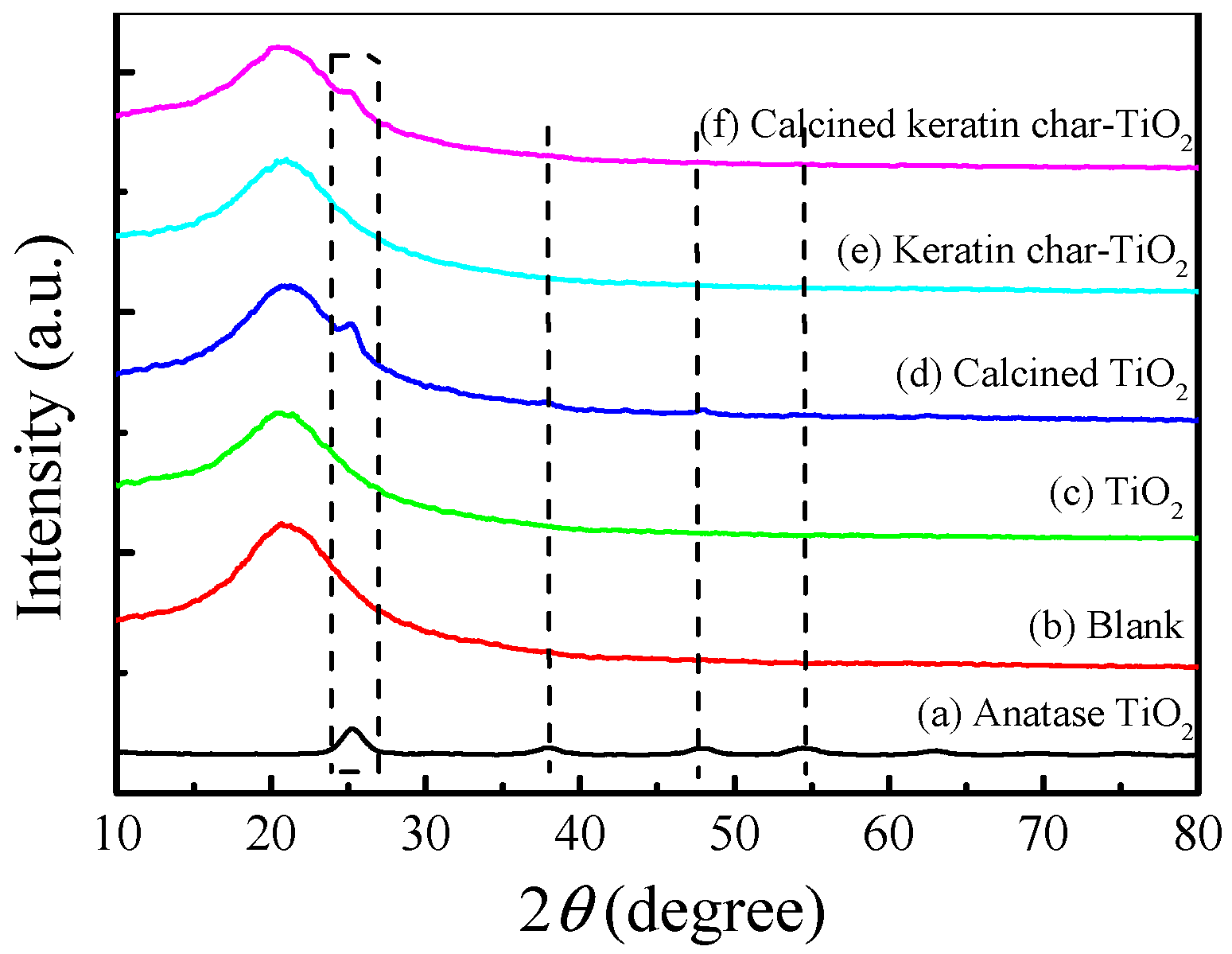
2.3. Crystal Structures
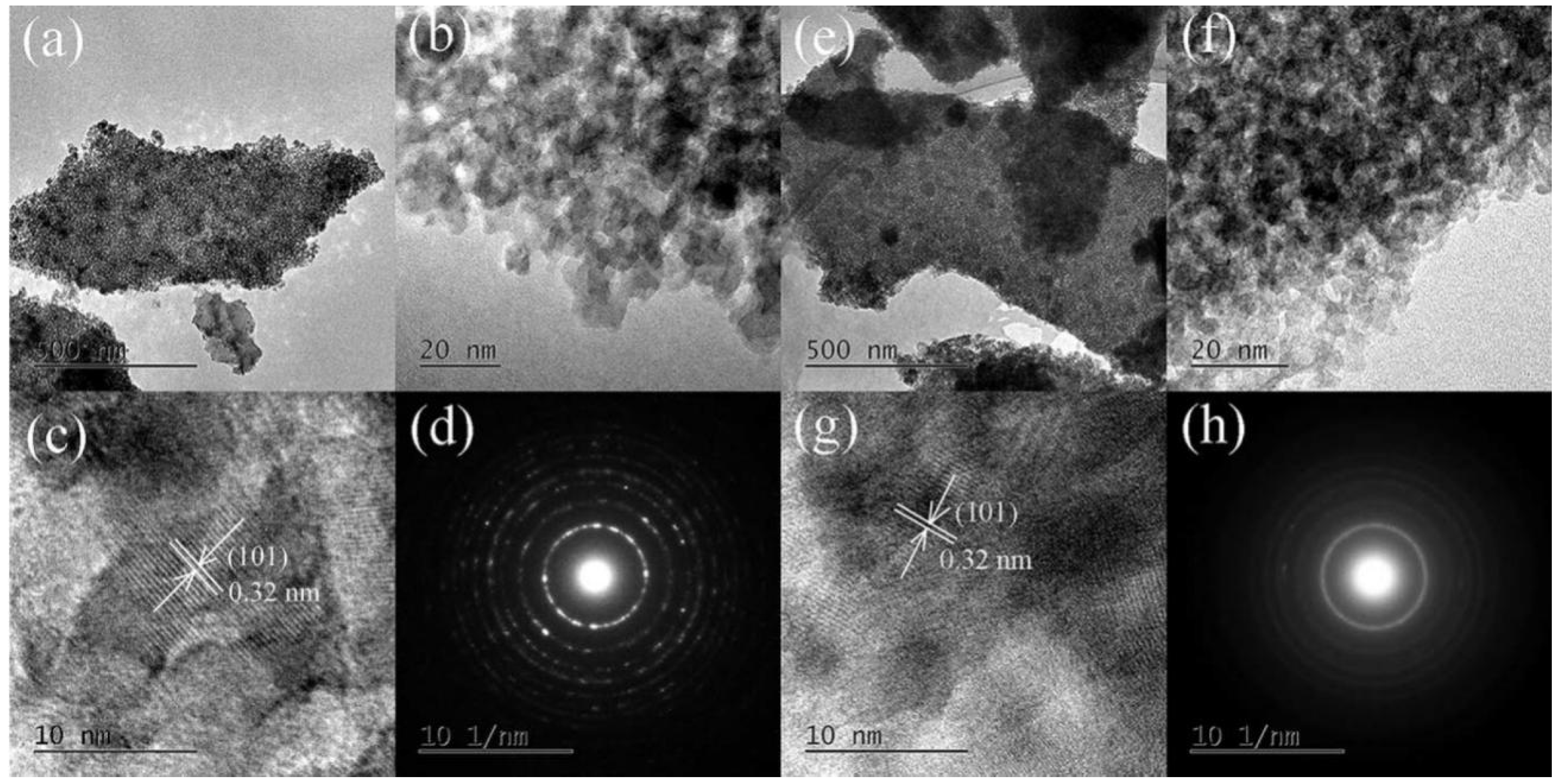
2.4. Microstructures of the Calcined Films
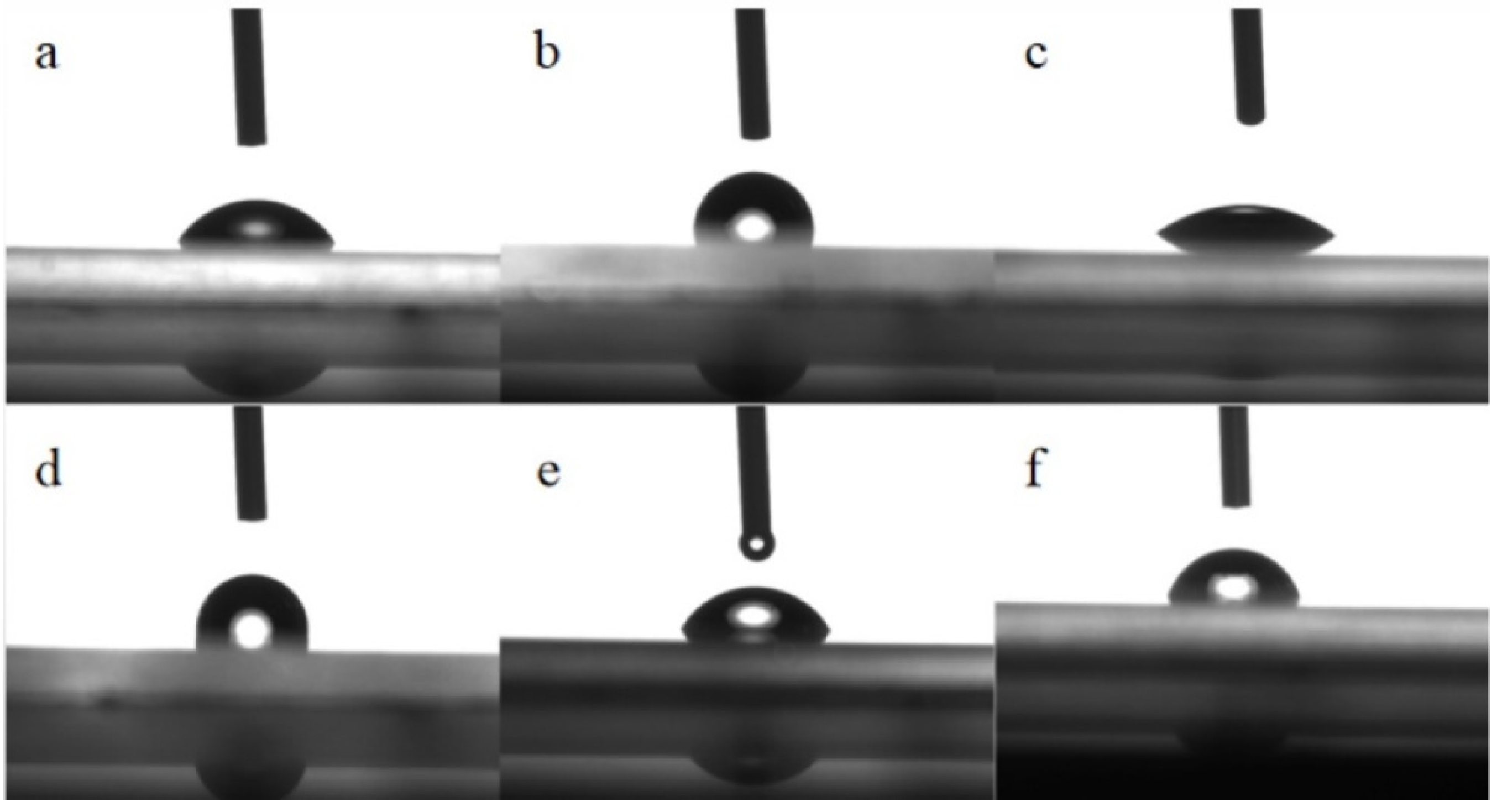
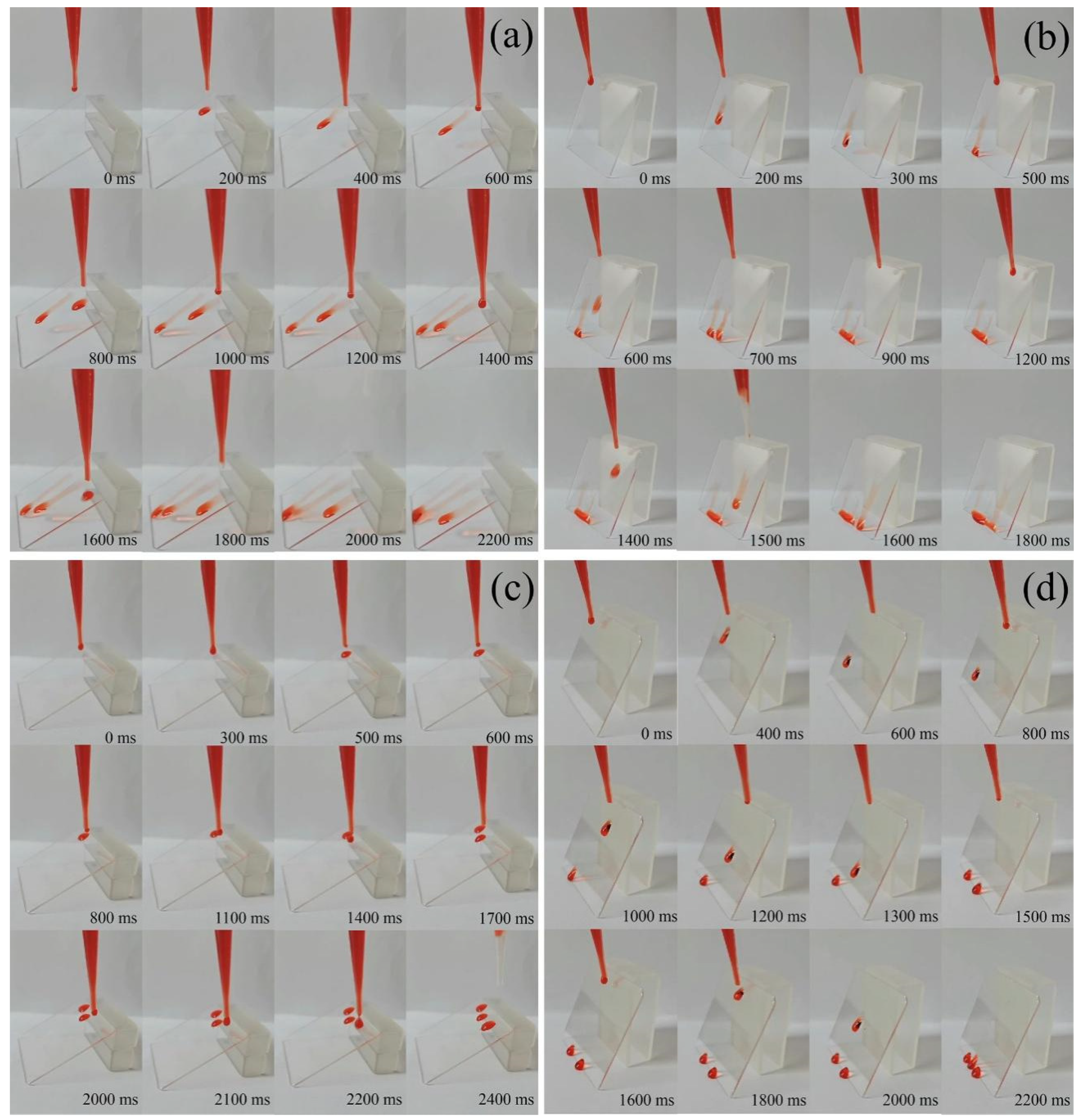
2.5. Surface Contact Angles
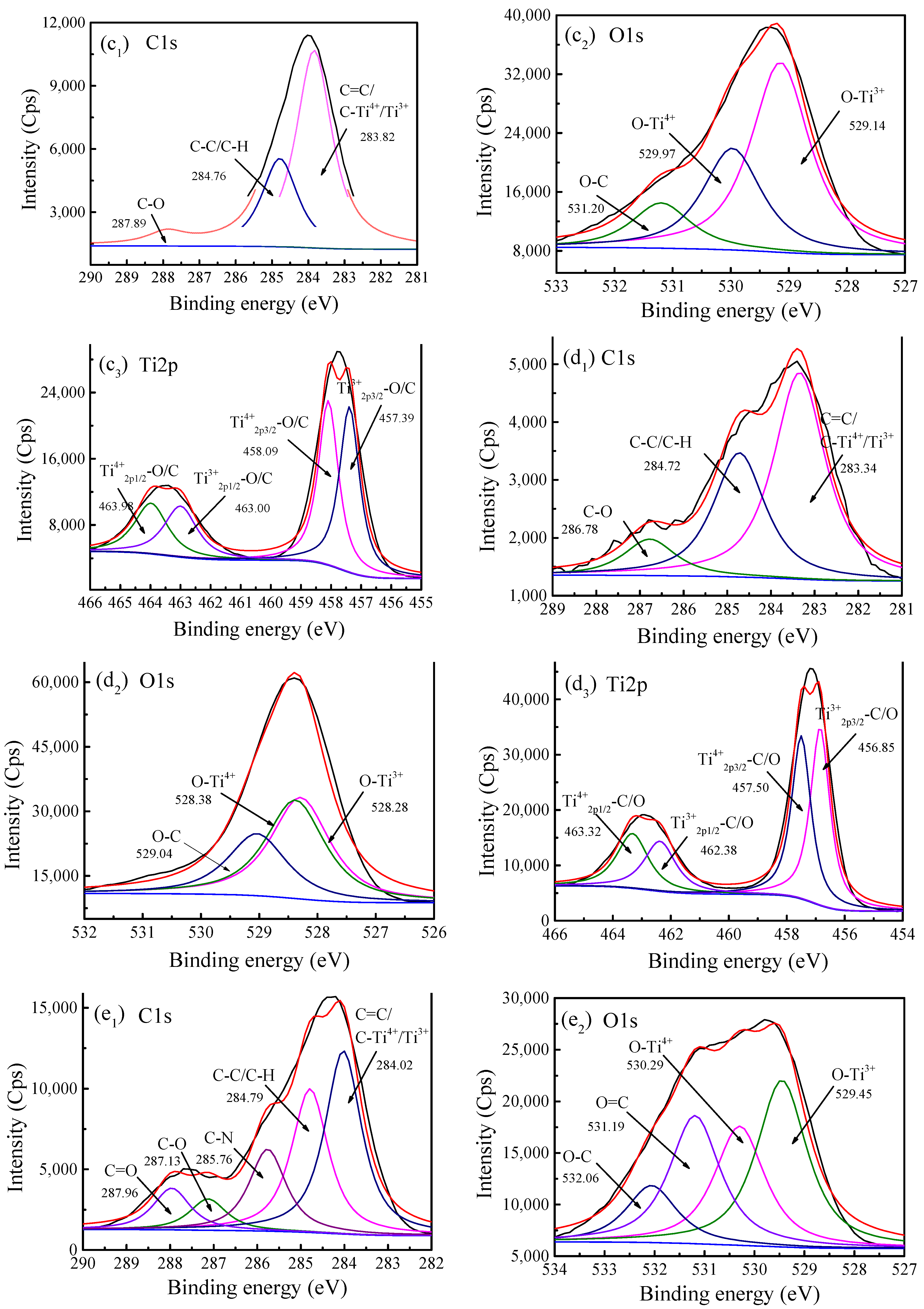
2.6. Chemical Bonds and Element Analyses
2.7. Energy Band Gap Structures
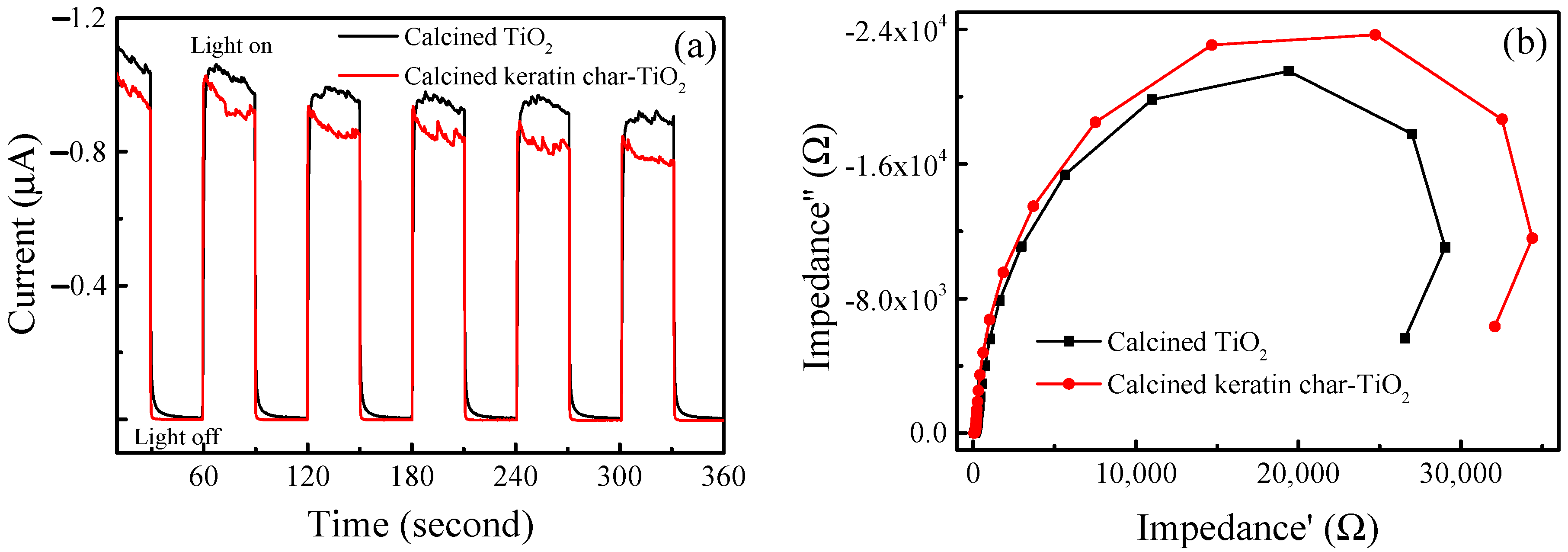
2.8. Separation Efficiency of Photogenerated Electron–Hole Pairs
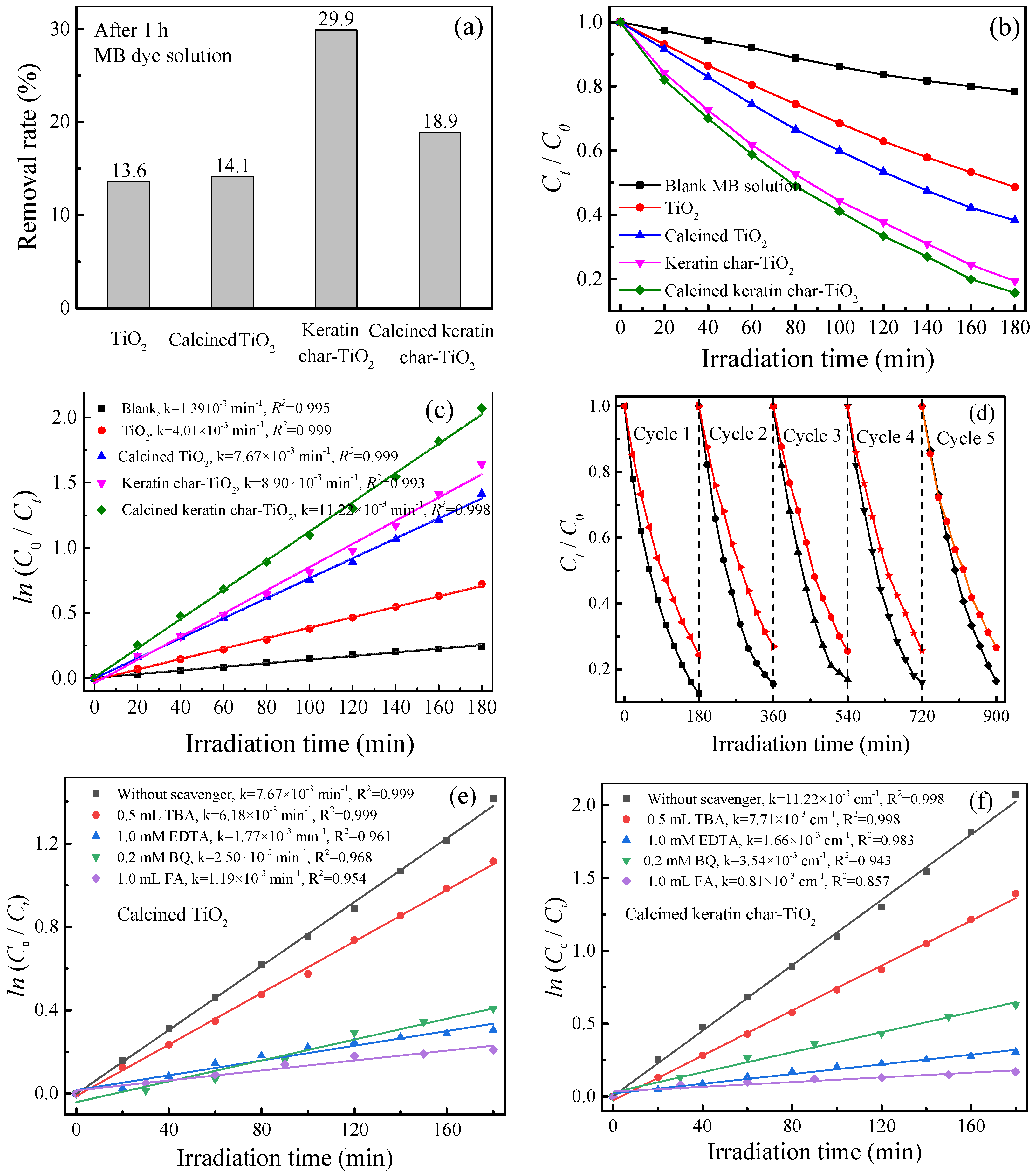
2.9. Photocatalysis Performance
2.10. DFT Theoretical Calculations
2.11. Hydrophobic Behaviors
2.12. Photocatalytic Reaction Mechanism
3. Experimental Section
3.1. Materials and Reagents
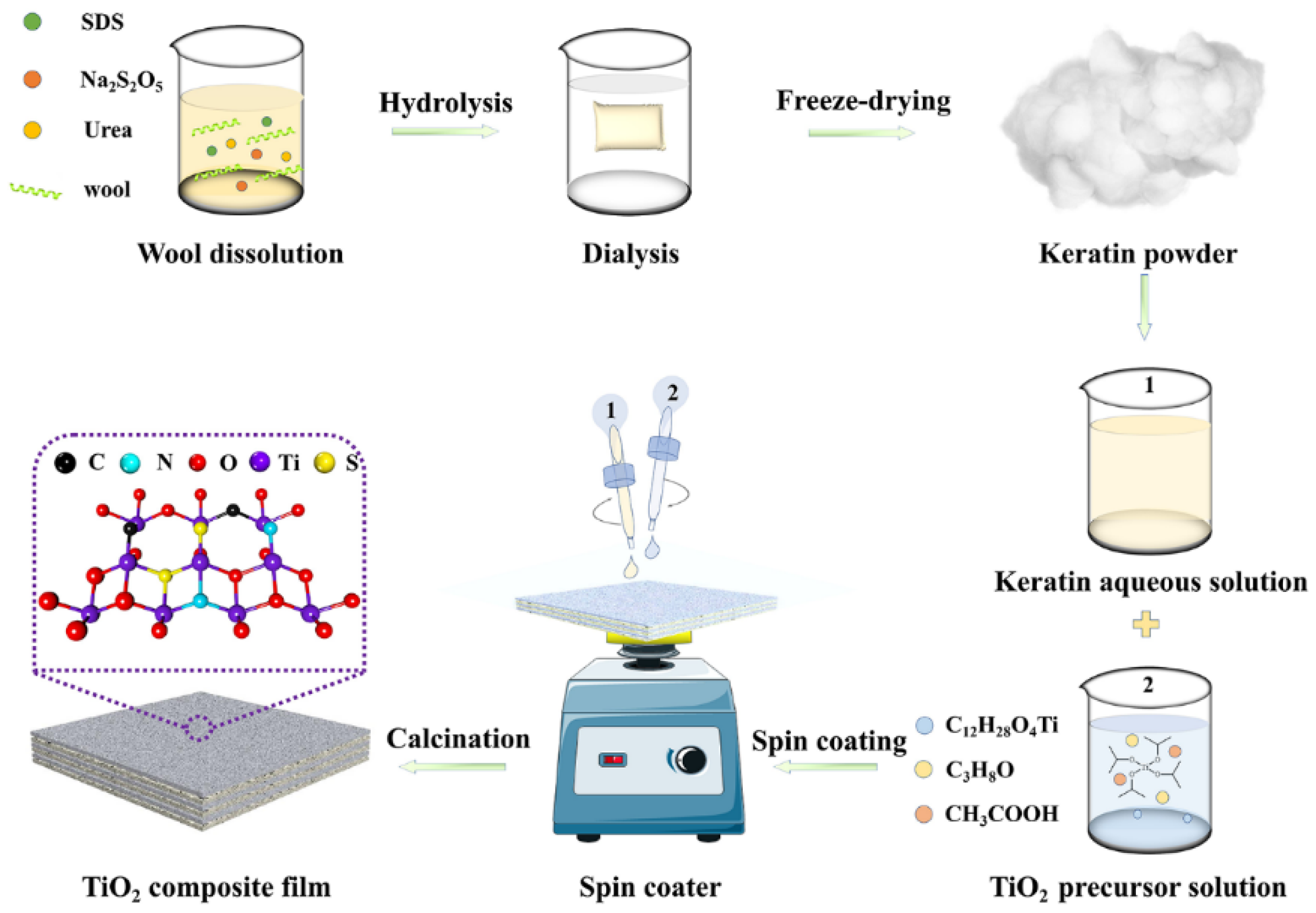
3.2. Preparation of Keratin char-TiO2 Composite Films
3.2.1. Extraction of Wool Keratins
3.2.2. Spin-Coating and Calcinations of Wool Keratin-TiO2 Composite Films
3.3. Characterization Methods
3.4. Measurements of Photocatalytic Properties
3.5. Theoretical Calculation Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demirci, S.; Dikici, T.; Yurddaskal, M.; Gultekind, S.; Toparli, M.; Celik, E. Synthesis and characterization of Ag doped TiO2 heterojunction films and their photocatalytic performances. Appl. Surf. Sci. 2016, 390, 591–601. [Google Scholar] [CrossRef]
- Fang, W.F.; Xin, M.Y.; Zhang, J.L. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Photocatalytic formulations for protein fibers: Experimental analysis of the effect of preparation on compatibility and photocatalytic activities. J. Colloid Interface Sci. 2008, 326, 283–288. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Structural, optical properties and photocatalytic activity of Fe3+ doped TiO2 thin films deposited by sol-gel spin coating. Surf. Interfaces 2019, 17, 100368. [Google Scholar] [CrossRef]
- Perez, J.A.B.; Courel, M.; Pal, M.; Delgado, F.P.; Mathews, N.R. Effect of ytterbium doping concentration on structural, optical and photocatalytic properties of TiO2 thin films. Ceram. Int. 2017, 43, 15777–15784. [Google Scholar] [CrossRef]
- Anas, M.; Han, D.S.; Mahmoud, K.; Park, H.; Abdel, -W.A. Photocatalytic degradation of organic dye using titanium dioxide modified with metal and non-metal deposition. Mat. Sci. Semicon. Proc. 2016, 41, 209–218. [Google Scholar] [CrossRef]
- Datcu, A.; Mendoza, M.L.; del Pino, A.P.; Logofatu, C.; Luculescu, C.; Gyorgy, E. UV-visible light induced photocatalytic activity of TiO2/graphene oxide nanocomposite coatings. Catal. Today 2019, 321, 81–86. [Google Scholar] [CrossRef]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.J.; Kim, H.Y. General one-pot strategy to prepare Ag-TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloys Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.; Barakat, N.A.M.; Park, M.; Han, T.H.; Lim, B.H.; Kim, H.Y. Incorporation of cadmium sulfide nanoparticles on the cadmium titanate nanofibers for enhanced organic dye degradation and hydrogen release. Ceram. Int. 2014, 40, 1553–1559. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. Chemsuschem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- El, N.A.; Helmy, E.T.; Gomaa, E.A.; Eldafrawy, S.; Mousac, M. Photocatalytic and biological activities of undoped and doped TiO2 prepared by green method for water treatment. J. Environ. Chem. Eng. 2019, 7, 103385. [Google Scholar]
- Zhang, G.Z.; Teng, F.; Wang, Y.Q.; Zhang, P.; Gong, C.S.; Chen, L.L.; Zhao, C.H.; Xie, E.Q. Preparation of carbon-TiO2 nanocomposites by a hydrothermal method and their enhanced photocatalytic activity. RSC Adv. 2013, 3, 24644–24649. [Google Scholar] [CrossRef]
- Kisch, H.; Sakthivel, S.; Janczarek, M.; Mitoraj, D. A low-band gap, nitrogen-modified titania visible-light photocatalyst. J. Phys. Chem. C 2007, 111, 11445–11449. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P.; Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, R.; Delegan, N.; Dirany, A.; Drogui, P.; El, K.M.A. Probing the electronic surface properties and bandgap narrowing of in-situ N, W and (W, N) doped magnetron-sputtered TiO2 films intended for electro-photocatalytic applications. J. Phys. Chem. 2016, 120, 631–638. [Google Scholar] [CrossRef]
- Fan, M.N.; Lin, Z.H.; Zhang, P.; Ma, X.D.; Wu, K.P.; Liu, M.L.; Xiong, X.H. Synergistic effect of nitrogen and sulfur dual-doping endows TiO2 with exceptional sodium storage performance. Adv. Energy Mater. 2021, 11, 2003037. [Google Scholar] [CrossRef]
- Khan, M.I.; KBhatti, A.; Qindeel, R.; Althobaiti, H.S.; Alonizan, N. Structural, electrical and optical properties of multilayer TiO2 thin films deposited by sol-gel spin coating. Results Phys. 2017, 7, 1437–1439. [Google Scholar] [CrossRef]
- Dahnoun, M.; Attaf, A.; Saidi, H.; Benatia, R.; Yahia, A.; Khelifi, C.; Saadi, A.; Attaf, N.; Ezzaouia, H.; Guerbous, L. High transparent titanium dioxide-anatase thin films deposited by spin coating technique: Effect of annealing temperature. J. Nanoelectron. Optoelectron. 2018, 13, 1359–1365. [Google Scholar] [CrossRef]
- Rajendra, K.; Senthil, K.V.; Anitha, R.K. Synthesis and characterization of immobilized activated carbon doped TiO2 thin films. Optik 2014, 125, 1993–1996. [Google Scholar] [CrossRef]
- Li, J.X.; Chen, Z.; Fang, J.F.; Yang, Q.; Yang, X.R.; Zhao, W.; Zhou, D.T.; Qian, X.X.; Liu, C.X.; Shao, J.Z. Facile synthesis of TiO2 film on glass for the photocatalytic removal of rhodamine B and tetracycline hydrochloride. Mater. Express 2019, 9, 437–443. [Google Scholar] [CrossRef]
- Varshney, G.; Kanel, S.R.; Kempisty, D.M.; Varshney, V.; Agrawal, A.; Sahle-Demessie, E.; Varma, R.S.; Nadagouda, M.N. Nanoscale TiO2 films and their application in remediation of organic pollutants. Coordin. Chem. Rev. 2016, 306, 43–64. [Google Scholar] [CrossRef]
- Breeson, A.C.; Sankar, G.; Goh, G.K.L.; Palgrave, R.G. Rutile to anatase phase transition induced by N doping in highly oriented TiO2 films. Phys. Chem. Chem. Phys. 2016, 18, 24722–24728. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Bekhit, A.E.A. An improved method for solubilisation of wool keratin using peracetic acid. J. Environ. Chem. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.H.; Xu, D.; Li, J.Y.; Li, L.F.; Li, W.B.; Cui, W.G.; Liu, K.S. Highly efficient extraction of large molecular-weight keratin from wool in a water/ethanol co-solvent. Text Res. J. 2019, 90, 1084–1093. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Ma, J.H.; Jian, Y.K.; Che, J.N. Extracting keratin from wool by using L-cysteine. Green Chem. 2016, 18, 476–481. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Physicochemical properties of keratin extracted from wool by various methods. Text. Res. J. 2018, 8, 2415–2424. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Han, Y.; Tang, Q.; Wu, H.L.; Mao, N.T. High photoactivity rutile-type TiO2 particles co-doped with multiple elements under visible light irradiation. Mater. Res. Express 2018, 5, 105015. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.S.; Zhang, H.; Zhang, X.Y.; Guo, Y.N.; Yang, L.M.; Wu, H.L.; Mao, N.T. Photocatalytic properties of core–shell structured wool-TiO2 hybrid composite powders. Catalysts 2021, 11, 12. [Google Scholar] [CrossRef]
- Ruzgar, D.G.; Kurtoglu, S.A.; Bhullar, S.K. A study on extraction and characterization of keratin films and nanofibers from waste wool fiber. J. Nat. Fibers 2020, 17, 427–436. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Puca, M.; Bravo, J.P.; Bafico, J.; Orto, V.C.D.; Copello, G.J. Dual adsorbent-photocatalytic keratin-TiO2 nanocomposite for trimethoprim removal from wastewater. J. Chem. 2020, 44, 10964–10972. [Google Scholar] [CrossRef]
- Siriorn, I.N.A.; Jatuphorn, W. Investigation of morphology and photocatalytic activities of electrospun chicken feather keratin/PLA/TiO2/clay nanofibers. E3S Web Conf. 2020, 141, 01003. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoumi, A.; Albetran, H.M.; Alamri, H.R.; Alamri, S.N.; Low, I.M. Impact of annealing temperature on structural, morphological and optical properties of GO-TiO2 thin films prepared by spin coating technique. Superlattices Microst. 2020, 139, 106423. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Liu, Q.R.; Hubert, J.; Huang, W.D.; Baert, K.; Wallaert, G.; Terryn, H.; Delplancke, -O.M.P.; Reniers, F. Deposition of photocatalytic anatase titanium dioxide films by atmospheric dielectric barrier discharge. Surf. Coat. Technol. 2017, 310, 173–179. [Google Scholar] [CrossRef]
- Guayaquil, -S.F.; Serrano, -R.B.; Valades, -P.P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B-Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Bsiri, N.; Ghrairi, N.; Rabha, M.B.; Bouaicha, M. Comparative study of TiO2 films prepared by EPD and sol-gel techniques and their use in DSSCs. Int. J. Nanotechnol. 2014, 11, 946–954. [Google Scholar] [CrossRef]
- Zhen, D.S.; Guo, M.; Li, X.F.; Bouaicha, M. Eu–TiO2 nanocomposite with high photoelectrochemical activity for enhanced photocatalysis of rhodamine B. J. Nanosci. Nanotechnol. 2019, 19, 7758–7763. [Google Scholar] [CrossRef] [PubMed]
- Vorokh, A.S. Scherrer formula: Estimation of error in determining small nanoparticle size. Nanosyst. Phys. Chem. Math. 2018, 9, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Bao, T.; Ning, J.; Bolag, A.; Narengerile, N. Microstructure and optical and electrical properties of TiO2 nanotube thin films prepared by spin-coating method. Micro Nano Lett. 2019, 14, 1208–1212. [Google Scholar] [CrossRef]
- Lu, B.; Ma, X.G.; Pan, C.S.; Zhu, Y.F. Photocatalytic and photoelectrochemical properties of in situ carbon hybridized BiPO4 films. Appl. Catal. A-Gen. 2012, 435, 93–98. [Google Scholar] [CrossRef]
- Tang, B.W.; Niu, S.; Sun, C.; Li, Z.Q.; Su, X.H.; Wang, S. The superhydrophilicity and photocatalytic property of Zn-doped TiO2 thin films. Ferroelectrics 2019, 549, 96–103. [Google Scholar]
- Ferraris, S.; Prato, M.; Vineis, C.; Varesano, A.A.; di Confiengo, G.G.; Spriano, S. Coupling of keratin with titanium: A physico-chemical characterization of functionalized or coated surfaces. Sur. Coat. Technol. 2020, 397, 126057. [Google Scholar] [CrossRef]
- Spiridonova, J.; Katerski, A.; Danilson, M.; Krichevskaya, M.; Krunks, M.; Acik, I.O. Effect of the titanium isopropoxide: Acetylacetone molar ratio on the photocatalytic activity of TiO2 thin films. Molecules 2019, 24, 4326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.I.J.; Thiripuranthagan, S.H. Non-metal doped titania photocatalysts for the degradation of neonicotinoid insecticides under visible light irradiation. J. Nanosci. Nanotechnol. 2018, 18, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Khedr, T.M.; El, -S.S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly active non-metals doped mixed-phase TiO2 for photocatalytic oxidation of ibuprofen under visible light. J. Photochem. Photobiol. A 2017, 346, 530–540. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.W.; Zhang, X.T.; Mao, N.T. Photocatalytic effects of wool fibers modified with solely TiO2 nanoparticles and N-doped TiO2 nanoparticles by using hydrothermal method. Chem. Eng. J. 2014, 254, 106–114. [Google Scholar] [CrossRef]
- Wang, M.C.; Lin, H.J.; Yang, T.S. Characteristics and optical properties of iron ion Fe3+-doped titanium oxide thin films prepared by a sol–gel spin coating. J. Alloys Compd. 2009, 473, 394–400. [Google Scholar] [CrossRef]
- Mozaffari, N.; Elahi, S.H.; Parhizgar, S.S.; Mozaffari, N.; Elahi, S.M. The effect of annealing and layer numbers on the optical and electrical properties of cobalt-doped TiO2 thin films. Mater. Res. Express 2019, 6, 116428. [Google Scholar] [CrossRef]
- Peng, C.; Xie, X.; Xu, W.K.; Zhou, T.; Wei, P.; Jia, J.B.; Zhang, K.; Cao, Y.H.; Wang, H.J.; Peng, F.; et al. Engineering highly active Ag/Nb2O5@Nb2CT x (MXene) photocatalysts via steering charge kinetics strategy. Chem. Eng. J. 2021, 421, 128766. [Google Scholar] [CrossRef]
- Yi, S.S.; Yan, J.M.; Wulan, B.R.; Li, S.J.; Liu, K.H.; Jiang, Q. Noble-metal-free cobalt phosphide modified carbon nitride: An efficient photocatalyst for hydrogen generation. Appl. Catal. B-Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Shi, H.Y.; Li, N.; Sun, Z.X.; Wang, T.Q.; Xu, L. Interface modification of titanium dioxide nanoparticles by titanium-substituted polyoxometalate doping for improvement of photoconductivity and gas sensing applications. J. Phys. Chem. Solids 2018, 120, 57–63. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z.; Han, C.; Li, Q.; Xue, X.X. Photocatalytic activity of Fe-doped diopside. Trans. Nonferr. Metal. Soc. 2012, 22, 3053–3058. [Google Scholar] [CrossRef]
- Leichner, C.; Steinbring, C.; Baus, R.A.; Baecker, D.; Gust, R.A. Bernkop-Schnürch, reactive keratin derivatives: A promising strategy for covalent binding to hair. J. Colloid Interf. Sci. 2019, 534, 533–541. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Esfandiar, A.; Mohatashamifar, M. Photodegradation of graphene oxide sheets by TiO2 nanoparticles after a photocatalytic reduction. J. Phys. Chem. C. 2010, 114, 12955–12959. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Zhou, L.; Sun, H.Q.; Wang, G.X.; Wang, S.B. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B-Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Xue, G.; Liu, H.H.; Chen, Q.Y.; Hills, C.; Tyrer, M.; Innocent, F. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard Mater. 2011, 186, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Lv, J.L.; Zhang, J.F.; Dai, K.; Liang, C.H. Facile synthesis of Z-scheme BiVO4/porous graphite carbon nitride heterojunction for enhanced visible-light-driven photocatalyst. Appl. Surf. Sci. 2018, 430, 595–602. [Google Scholar] [CrossRef]
- Ran, M.H.; Yang, C.X.; Fang, Y.; Zhao, K.K.; Ruan, Y.Q.; Wu, J.; Yang, H.; Liu, Y.F. Model for rolling angle. J. Phys. Chem. C 2012, 116, 8449–8455. [Google Scholar]
- Hua, Y.W.; Huang, S.Y.; Liu, S.; Pan, W. A corrosion-resistance superhydrophobic TiO2 film. Appl. Sur. Sci. 2012, 258, 7460–7464. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Hussein, M.A.; Adesina, A.Y.; Al, A.N. In vitro corrosion and bioactivity performance of surface-treated Ti-20Nb-13Zr Alloys for orthopedic applications. Coatings 2019, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Annalisa, A.; Giovanna, S.; Armida, T.; Guerrini, A.; Orlandi, V.T.; Corticelli, F.; Varchi, G. Methylene blue doped films of wool keratin with antimicrobial photodynamic activity. ACS Appl. Mater. Inter. 2015, 7, 17416–17424. [Google Scholar]
- Shavandi, A.; Bekhit, A.E.A.; Carne, A.; Bekhit, A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J.Bioact. Compat. Pol. 2017, 32, 163–177. [Google Scholar] [CrossRef]
- Bezir, N.C.; Evcin, A.; Kayali, R.; Ozen, M.K.; Balyaci, G. Comparison of pure and doped TiO2 thin films prepared by sol-gel spin-coating method. Acta Phys. Pol. A 2017, 132, 620–624. [Google Scholar] [CrossRef]
- Kumar, A.; Mondal, S.K.S. DLTS analysis of amphoteric interface defects in high-TiO2 MOS structures prepared by sol-gel spin-coating. AIP Adv. 2015, 5, 117122. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Lin, C.P.; Bi, H.J.; Liu, Y.G.; Yan, Q.S. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2016, 392, 701–707. [Google Scholar] [CrossRef]
- Ma, L.N.; Wang, G.H.; Jiang, C.J.; Bao, H.L.; Xu, Q.C. Synthesis of core-shell TiO2@g-C3N4, hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Sur. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Xu, S.B.; Jiang, L.; Yang, H.G.; Song, Y.Q.; Dan, Y. Structure and photocatalytic activity of polythiophene/TiO2 composite particles prepared by photoinduced polymerization. J. Catal. 2011, 32, 536–545. [Google Scholar] [CrossRef]
- Shu, J.X.; Wang, Z.H.; Xia, G.Q.; Zheng, Y.Y.; Yang, L.H.; Zhang, W. One-pot synthesis of AgCl@Ag hybrid photocatalyst with high photocatalytic activity and photostability under visible light and sunlight irradiation. Chem. Eng. J. 2014, 252, 374–381. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for abinitio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, H.; Li, W.; Yang, L.; Wu, H.; Mao, N. Photocatalytic Properties of a Novel Keratin char-TiO2 Composite Films Made through the Calcination of Wool Keratin Coatings Containing TiO2 Precursors. Catalysts 2021, 11, 1366. https://doi.org/10.3390/catal11111366
Zhang J, Zhang H, Li W, Yang L, Wu H, Mao N. Photocatalytic Properties of a Novel Keratin char-TiO2 Composite Films Made through the Calcination of Wool Keratin Coatings Containing TiO2 Precursors. Catalysts. 2021; 11(11):1366. https://doi.org/10.3390/catal11111366
Chicago/Turabian StyleZhang, Jinyuan, Hui Zhang, Wenjun Li, Limeng Yang, Hailiang Wu, and Ningtao Mao. 2021. "Photocatalytic Properties of a Novel Keratin char-TiO2 Composite Films Made through the Calcination of Wool Keratin Coatings Containing TiO2 Precursors" Catalysts 11, no. 11: 1366. https://doi.org/10.3390/catal11111366