Raw Biogas as Feedstock for the OCM Process
Abstract
:1. Introduction
2. Results and Discussion
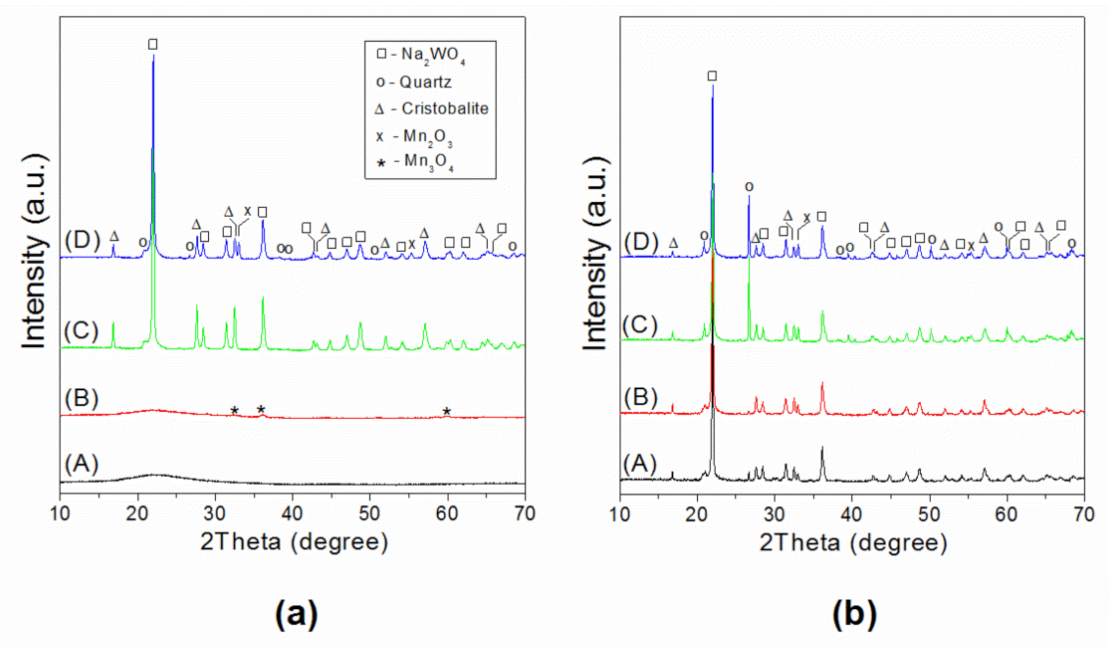
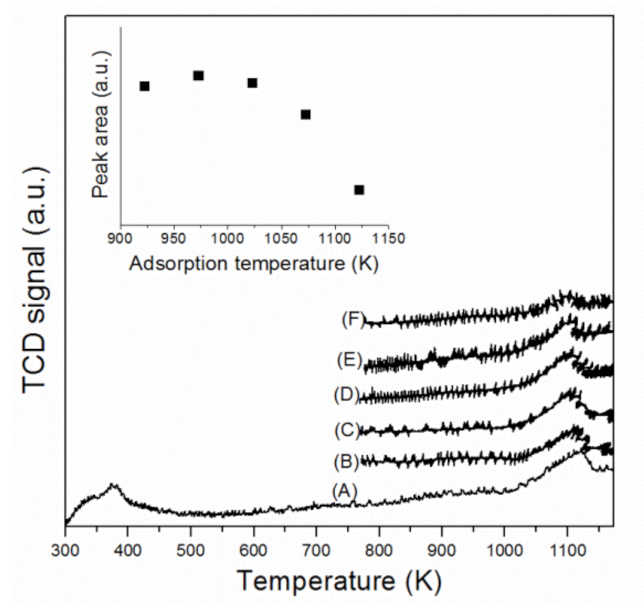
2.1. Physicochemical Characterization
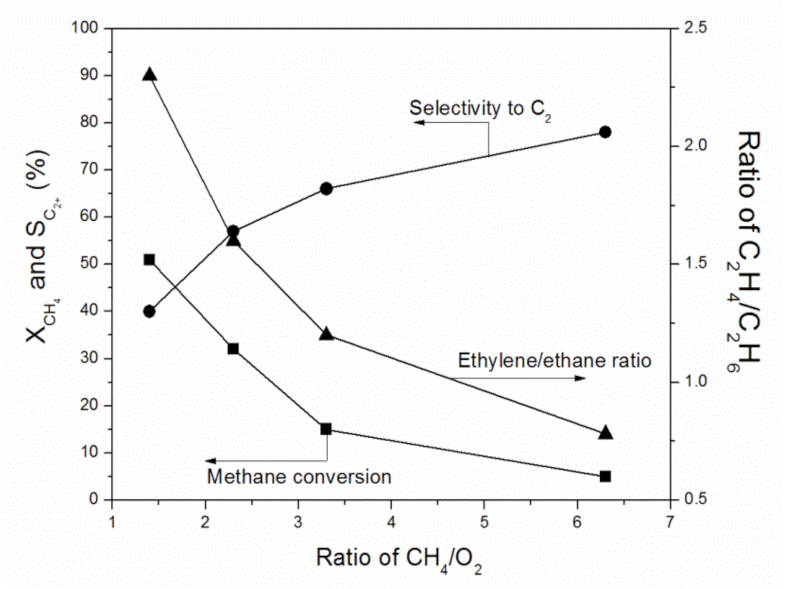
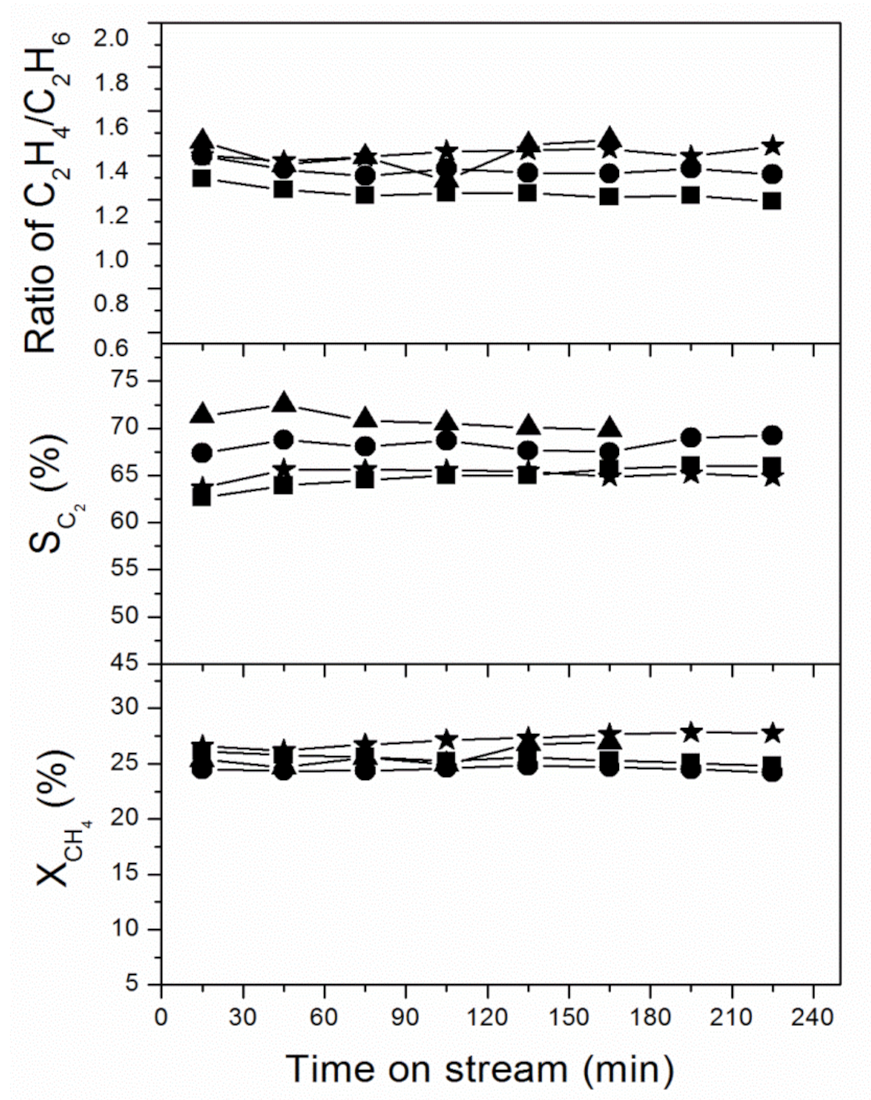
2.2. Catalytic Performance
3. Materials and Methods
3.1. Feedstock
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ali, M.M.; Ndongo, M.; Bilal, B.; Yetilmezsoy, K.; Youm, I.; Bahramian, M. Mapping of biogas production potential from livestock manures and slaughterhouse waste: A case study for African countries. J. Clean. Prod. 2020, 256, 120499. [Google Scholar] [CrossRef]
- Kazimierowicz, A.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Otero, A.; Mendoza, M.; Carreras, R.; Fernandez, B. Biogas production from slaughterhouse waste: Effect of blood content and fat saponification. Waste Manag. 2021, 133, 119–126. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2021, 14, 150. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L. Giant Miscanthus as a Substrate for Biogas Production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, B.; Yao, F.; He, L.; Chen, F.; Ma, Y.; Shu, X.; Hou, K.; Wang, D.; Li, X. Biogas production from anaerobic co-digestion of waste activated sludge: Co-substrates and influencing parameters. Rev. Environ. Sci. Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Bücker, F.; Marder, M.; Peiter, M.R.; Neutzling Lehn, D.; Mendonça Esquerdo, V.; de Almeida Pinto, L.A.; Konrad, O. Fish waste: An efficient alternative to biogas and methane production in an anaerobic mono-digestion system. Renew. Energy 2020, 147, 798–805. [Google Scholar] [CrossRef]
- Rasi, S.; Läntelä, J.; Rintala, J. Trace compounds affecting biogas energy utilisation—A review. Energy Convers. Manag. 2011, 52, 3369–3375. [Google Scholar] [CrossRef]
- Li, Y.; Alaimo, C.P.; Kim, M.; Kado, N.Y.; Peppers, J.; Xue, J.; Wan, C.; Green, P.G.; Zhang, R.; Jenkins, B.M.; et al. Composition and Toxicity of Biogas Produced from Different Feedstocks in California. Environ. Sci. Technol. 2019, 53, 11569–11579. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sust. Energ. Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Schildhauer, T.J. Direct Methanation of Biogas—Technical Challenges and Recent Progress. Front. Energy Res. 2020, 8, 570887. [Google Scholar] [CrossRef]
- Dannesboe, C.; Bøgild Hansen, J.; Johannsen, I. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 Methanation of Biogas over 20 wt% Ni-Mg-Al Catalyst: On the Effect of N2, CH4, and O2 on CO2 Conversion Rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Sebastia-Saez, D.; Wang, Q.; Reina, T.R. Is the production of biofuels and bio-chemicals always profitable? Co-production of biomethane and urea from biogas as case study. Energy Convers. Manag. 2020, 220, 113058. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sust. Energ. Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Minutillo, M.; Perna, A.; Sorce, A. Green hydrogen production plants via biogas steam and autothermal reforming processes: Energy and exergy analyses. Appl. Energy 2020, 277, 115452. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming. Chem. Proc. 2020, 2, 10. [Google Scholar]
- Zhumabek, M.; Xanthopoulou, G.; Tungatarova, S.A.; Baizhumanova, T.S.; Vekinis, G.; Murzin, D.Y. Biogas Reforming over Al-Co Catalyst Prepared by Solution Combustion Synthesis Method. Catalysts 2021, 11, 274. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrog. Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Alfaro, C.; Bimbela, F.; Gandía, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]
- Habibi, N.; Wang, Y.; Arandiyan, H.; Rezaei, M. Effect of substitution by Ni in MgAl2O4 spinel for biogas dry reforming. Int. J. Hydrog. Energy 2017, 42, 24159–24168. [Google Scholar] [CrossRef]
- Madeira, J.G.F.; Boloy, R.A.M.; Delgado, A.R.S.; Lima, F.R.; Coutinho, E.R.; de Castro Pereira Filho, R. Ecological analysis of hydrogen production via biogas steam reforming from cassava flour processing wastewater. J. Clean. Prod. 2017, 162, 709–716. [Google Scholar] [CrossRef]
- Yao, J.; Kraussler, M.; Benedikt, F.; Hofbauer, H. Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes. Energy Convers. Manag. 2017, 145, 278–292. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Wang, L.; Wuc, C.F.; Wang, J.Q.; Shen, B.X.; Tu, X. Low temperature reforming of biogas over K-, Mg- and Ce-promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B 2018, 224, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Kan, X.; Zhou, D.; Yang, W.; Zhai, X.; Wang, C.H. An investigation on utilization of biogas and syngas produced from biomass waste in premixed spark ignition engine. Appl. Energy 2018, 212, 210–222. [Google Scholar] [CrossRef]
- Chiodo, V.; Maisano, S.; Zafarana, G.; Urbani, F. Effect of pollutants on biogas steam reforming. Int. J. Hydrog. Energy 2017, 42, 1622–1628. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Elwell, A.; Joseph, B.; Kuhn, J.N. Effect of silicon poisoning on catalytic dry reforming of simulated biogas. Appl. Catal. A Gen. 2017, 538, 157–164. [Google Scholar] [CrossRef]
- Chein, R.; Yang, Z.W. H2S effect on dry reforming of biogas for syngas production. Int. J. Energy Res. 2019, 43, 3330–3345. [Google Scholar] [CrossRef]
- Gunnerman, R.W.; Gunnerman, P.W. Process for Conversion of Biogas to Liquid Fuels. U.S. Patent 20110000128A1, 6 January 2011. [Google Scholar]
- Ge, X.; Yang, L.; Sheets, J.P.; Yu, Z.; Li, Y. Biological conversion of methane to liquid fuels: Status and opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Mardina, P.; Kim, D.; Kim, S.Y.; Kalia, V.C.; Kim, I.W.; Lee, J.K. Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Bioresour. Technol. 2016, 218, 202–208. [Google Scholar] [CrossRef]
- Sheets, J.P.; Ge, X.; Li, Y.F.; Yu, Z.; Li, Y. Biological conversion of biogas to methanol using methanotrophs isolated from solid-state anaerobic digestate. Bioresour. Technol. 2016, 201, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Abatzoglou, N.; Boivin, S. A review of biogas purification Processes. Biofuels Bioprod. Bioref. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Miltner, M.; Makaruk, A.; Harasek, M. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.; Ghosh, P.; Tyagi, B.; Vijay, V.K.; Vijay, V.; Thakur, I.S.; Kamyab, H.; Nguyen, D.D.; Kumar, A. Advances in biogas valorization and utilization and utilization system: A comprehensive review. J. Clean. Prod. 2020, 273, 123052. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Budzianowski, W.M. A review of potential innovations for production, conditioning and utilization of biogas with multiple-criteria assessment. Renew. Sust. Energ. Rev. 2016, 54, 1148–1171. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-Dasht Arzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology—A review of biogas cleaning, upgrading and utilisation. Renew. Sust. Energ. Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valor. 2017, 8, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas Management: Advanced Utilization for Production of Renewable Energy and Added-value Chemicals. Front. Environ. Sci. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; Assad, M.E.H.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2021, 1–24. [Google Scholar] [CrossRef]
- Penteado, A.T.; Kim, M.; Godini, H.R.; Esche, E.; Repke, J.-U. Biogas as a Renewable Feedstock for Green Ethylene Production via Oxidative Coupling of Methane: Preliminary Feasibility Study. Chem. Eng. Trans. 2017, 61, 589–594. [Google Scholar]
- Penteado, A.T.; Kim, M.; Godini, H.R.; Esche, E.; Repke, J.-U. Techno-economic evaluation of a biogas-based oxidative coupling of methane process for ethylene production. Front. Chem. Sci. Eng. 2018, 12, 598–618. [Google Scholar]
- Gu, S.; Choi, J.-W.; Suh, D.J.; Park, Y.-K.; Choi, J.; Ha, J.-M. Upgrading of sulfur-containing biogas into high quality fuel via oxidative coupling of methane. Int. J. Energy Res. 2021, 45, 19363–19377. [Google Scholar] [CrossRef]
- Sokolov, S.; Seeburg, D.; Wohlrab, S.; Friedel, M.; Nitzsche, J.; Kondratenko, E.V. An Approach Using Oxidative Coupling of Methane for Converting Biogas and Acid Natural Gas into High-Calorific Fuels. Ind. Eng. Chem. Res. 2019, 58, 2454–2459. [Google Scholar] [CrossRef]
- Pak, S.; Lunsford, J. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar] [CrossRef]
- Michorczyk, B.; Ogonowski, J.; Michorczyk, P. Oxidative coupling of methane in the presence of various gaseous additives. Przemysł. Chem. 2015, 94, 572–576. [Google Scholar]
- Takanabe, K.; Iglesia, E. Rate and Selectivity Enhancements Mediated by OH Radicals in the Oxidative Coupling of Methane Catalyzed by Mn/Na2WO4/SiO2. Angew. Chem. Int. Ed. 2008, 47, 7689–7693. [Google Scholar] [CrossRef]
- Takanabe, K.; Iglesia, E. Mechanistic Aspects and Reaction Pathways for Oxidative Coupling of Methane on Mn/Na2WO4/SiO2 Catalysts. J. Phys. Chem. C 2009, 113, 10131–10145. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Yao, L.; Hu, C. Effect of CO2 on the structural variation of Na2WO4/Mn/SiO2 catalyst for oxidative coupling of methane to ethylene. J. Energy Chem. 2015, 24, 394–400. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Yu, C.J.; Fang, X.P.; Li, S.B.; Wang, H.L. Oxide/Support Interaction and Surface Reconstruction in the Na2WO4/SiO2 System. J. Phys. Chem. 1993, 97, 12870–12875. [Google Scholar] [CrossRef]
- Palermo, A.; Vazquezy, J.P.H.; Lee, A.F.; Tikhov, M.S.; Lambert, R.M. Critical influence of the amorphous silica-to-cristobalite phase transition on the performance of Mn/Na2WO4/SiO2 catalysts for the oxidative coupling of methane. J. Catal. 1998, 177, 259–266. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Niu, J.; Fang, X. Mechanistic Study of Oxidative Coupling of Methane over Mn2O3-Na2WO4/SiO2 Catalyst. Appl. Catal. A Gen. 1995, 124, 9–18. [Google Scholar] [CrossRef]
- Chua, Y.; Mohamed, A.; Bhatia, S. Oxidative coupling of methane for the production of ethylene over sodium-tungsten-manganese-supported-silica catalyst (Na-W-Mn/SiO2). Appl. Catal. A Gen. 2008, 343, 142–148. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Qin, S.; Hu, C. Study of the effect of gas space time on the combination of methane gas-phase oxidation and catalytic oxidative coupling over Mn/Na2WO4/SiO2 catalyst. Ind. Eng. Chem. Res. 2006, 45, 7090–7095. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, Y.; Jin, Y.; Chen, Y. Methane oxidative coupling over Na2WO4/SiO2. Catal. Lett. 1992, 13, 221–228. [Google Scholar] [CrossRef]
- Fleischer, V.; Simon, U.; Parishan, S.; Colmenares, M.G.; Görke, O.; Gurlo, A.; Riedel, W.; Thum, L.; Schmidt, J.; Risse, T.; et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments. J. Catal. 2018, 360, 102–117. [Google Scholar] [CrossRef]
- Wang, D.; Xu, M.; Shi, C.; Lunsford, J.H. Effect of carbon dioxide on the selectivities obtained during the partial oxidation of methane and ethane over Li+/MgO catalysts. Catal. Lett. 1993, 18, 323–328. [Google Scholar] [CrossRef]
- Aika, K.; Nishiyama, T. Utilisation of CO2 in the oxidative coupling of methane over PbO-MgO and PbO-CaO. Chem. Commun. 1988, 1, 70–71. [Google Scholar] [CrossRef]
- Thien, C.Y.; Mohamed, A.R.; Bhatia, S. Process optimization of oxidative coupling of methane for ethylene production using response surface methodology. J. Chem. Technol. Biotechnol. 2007, 82, 81–91. [Google Scholar] [CrossRef]
- Ehsani, M.R.; Bateni, H.; Parchikolaei, G.R. Modeling the oxidative coupling of methane using artificial neural network and optimizing of its operational conditions using genetic algorithm. Korean J. Chem. Eng. 2012, 29, 855–861. [Google Scholar] [CrossRef]
- Murthy, P.R.; Liu, Y.; Wu, G.; Diao, Y.; Shi, C. Oxidative Coupling of Methane: Perspective for High-Value C2 Chemicals. Crystals 2021, 11, 1011–1030. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, S.; Choi, J.-W.; Suh, D.J.; Song, K.H.; Ha, J.-M. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O2, surface lattice oxygen, and CO2 on the C2+ selectivity. RSC Adv. 2020, 10, 35889–35897. [Google Scholar] [CrossRef]
- Papa, F.; Gingasu, D.; Patron, L.; Miyazaki, A.; Balint, I. On the nature of active sites and catalytic activity for OCM reaction of alkaline-earth oxides-neodymia catalytic systems. Appl. Catal. A Gen. 2010, 375, 172–178. [Google Scholar] [CrossRef]
- Suzuki, T.; Wada, K.; Watanabe, Y. Effects of carbon dioxide and catalyst preparation on the oxidative dimerization of methane. Appl. Catal. A Gen. 1990, 59, 213–225. [Google Scholar] [CrossRef]
- Dissanayake, D.; Lunsford, J.H.; Rosynek, M.P. Oxidative coupling of methane over oxide-supported barium catalysts. J. Catal. 1993, 143, 286–298. [Google Scholar] [CrossRef]
- Korf, S.J.; Roos, J.A.; de Bruijn, N.A.; van Ommen, J.G.; Ross, J.R.H. Influence of CO2 on the oxidative coupling of methane over a lithium promoted magnesium oxide catalyst. Chem. Commun. 1987, 19, 1433–1434. [Google Scholar] [CrossRef]
- Wang, D.; Rosynek, M.P.; Lunsford, J.H. Oxidative coupling of methane over oxide-supported sodium-manganese catalysts. J. Catal. 1995, 155, 390–402. [Google Scholar] [CrossRef]
- Pak, S.; Qiu, P.; Lunsford, J.H. Elementary Reactions in the Oxidative Coupling of Methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO Catalysts. J. Catal. 1998, 179, 222–230. [Google Scholar] [CrossRef]
- Daneshpayeh, M.; Khodadadi, A.; Mostoufi, N.; Mortazavi, Y.; Sotudeh-Gharebagh, R.; Talebizadeh, A. Kinetic modeling of oxidative coupling of methane over Mn/Na2WO4/SiO2 catalyst. Fuel Process. Technol. 2009, 90, 403–410. [Google Scholar] [CrossRef]
- Bogdan, B.; Michorczyk, B.; Rokicińska, A.; Basta, M.; Myradova, M.; Kuśtrowski, P.; Michorczyk, P. Monolithic composites with geometry controlled by polymeric 3D printed templates: Characterization and catalytic performance in OCM. Appl. Surf. Sci. 2021, 553, 149554. [Google Scholar] [CrossRef]
- Michorczyk, P.; Hędrzak, E.; Węgrzyniak, A. Preparation of monolithic catalysts using 3D printed templates for oxidative coupling of methane. J. Mater. Chem. A 2016, 4, 18753–18756. [Google Scholar] [CrossRef]
- Sikora, J.; Mruk, B. Quantitative and qualitative analysis of biogas emitted from batches composed on the basis of available factions on the farm. Infrastruct. Ecol. Rural. Areas 2016, 3, 907–917. [Google Scholar]
- Sikora, J.; Niemiec, M.; Szeląg-Sikora, A.; Kuboń, M.; Olech, E.; Marczuk, A. Biogasification of wastes from industrial processing of carps. Przemysł. Chem. 2017, 96, 2275–2278. [Google Scholar]



| Sample | SBET (m2g−1) | VTotal (cm3g−1) |
|---|---|---|
| SiO2 | 474 | 0.83 |
| Mn/SiO2 | 427 | 0.78 |
| Na2WO4/SiO2 | 2.1 | <0.01 |
| Mn-Na2WO4/SiO2 | 1.9 | <0.01 |
| Mn-Na2WO4/SiO2 a | 1.9 | <0.01 |
| Mn-Na2WO4/SiO2 b | 2.0 | <0.01 |
| Mn-Na2WO4/SiO2 c | 1.9 | <0.01 |
| Mn-Na2WO4/SiO2 d | 1.9 | <0.01 |
| Feedstock | Na (1s) | W (4f) | Mn (2p) | O (1s) SiO2 | O (1s) MOx | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | In (at %) | BE (eV) | In (at.%) | BE (eV) | In (at.%) | BE (eV) | In (at.%) | BE (eV) | In (at.%) | |
| Fresh catalyst | 1071.7 | 4.48 | 36.0 | 0.71 | 642.7 | 0.62 | 533.1 | 30.96 | 530.7 | 9.25 |
| CH4 + O2 +He | 1071.7 | 4.89 | 36.0 | 0.82 | 642.5 | 1.57 | 533.0 | 45.71 | 530.6 | 12.9 |
| Biogas 1 + O2 + He | 1071.9 | 2.24 | 35.9 | 0.61 | 641.6 | 0.96 | 533.0 | 55.22 | 530.7 | 6.37 |
| Biogas 2 + O2 + He | 1072.1 | 3.27 | 35.9 | 0.58 | 642.1 | 1.16 | 532.8 | 54.37 | 530.5 | 7.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michorczyk, B.; Sikora, J.; Kordon-Łapczyńska, B.; Gaweł, D.; Czekaj, I. Raw Biogas as Feedstock for the OCM Process. Catalysts 2022, 12, 54. https://doi.org/10.3390/catal12010054
Michorczyk B, Sikora J, Kordon-Łapczyńska B, Gaweł D, Czekaj I. Raw Biogas as Feedstock for the OCM Process. Catalysts. 2022; 12(1):54. https://doi.org/10.3390/catal12010054
Chicago/Turabian StyleMichorczyk, Barbara, Jakub Sikora, Bogusława Kordon-Łapczyńska, Dorota Gaweł, and Izabela Czekaj. 2022. "Raw Biogas as Feedstock for the OCM Process" Catalysts 12, no. 1: 54. https://doi.org/10.3390/catal12010054







