A Study of Support Effects for the Water-Gas-Shift Reaction over Cu
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Supports
2.2. Water–Gas-Shift Measurements
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bartholomew, C.H.; Farrauto, R.J. Hydrogen Production and Synthesis Gas Reactions. In Fundamentals of Industrial Catalytic Processes; John Wiley and Sons: Hoboken, NJ, USA, 2005; pp. 339–486. [Google Scholar]
- Opalka, S.M.; Vanderspurt, T.H.; Radhakrishnan, R.; She, Y.; Willigan, R.R. Design of Water Gas Shift Catalysts for Hydrogen Production in Fuel Processors. J. Phys. Condens. Matter 2008, 20, 64237. [Google Scholar] [CrossRef]
- Yaccato, K.; Carhart, R.; Hagemeyer, A.; Herrmann, M.; Lesik, A.; Strasser, P.; Volpe, A.; Turner, H.; Weinberg, H.; Grasselli, R.K.; et al. High Throughput Discovery of Families of High Activity WGS Catalysts: Part I—History and Methodology. Comb. Chem. High Throughput Screen. 2010, 13, 318–330. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. Studies of the Water-Gas-Shift Reaction on Ceria-Supported Pt, Pd, and Rh: Implications for Oxygen-Storage Properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Madras, G. Support-Dependent Activity of Noble Metal Substituted Oxide Catalysts for the Water Gas Shift Reaction. AIChE J. 2010, 56, 2662–2676. [Google Scholar] [CrossRef]
- Han, W.-Q.; Wen, W.; Hanson, J.C.; Teng, X.; Marinkovic, N.; Rodriguez, J.A. One-Dimensional Ceria as Catalyst for the Low-Temperature Water−Gas Shift Reaction. J. Phys. Chem. C 2009, 113, 21949–21955. [Google Scholar] [CrossRef]
- Kinch, R.T.; Cabrera, C.R.; Ishikawa, Y. A Density-Functional Theory Study of the Water−Gas Shift Mechanism on Pt/Ceria(111). J. Phys. Chem. C 2009, 113, 9239–9250. [Google Scholar] [CrossRef]
- Meira, D.M.; Ribeiro, R.U.; Mathon, O.; Pascarelli, S.; Bueno, J.M.C.; Zanchet, D. Complex Interplay of Structural and Surface Properties of Ceria on Platinum Supported Catalyst under Water Gas Shift Reaction. Appl. Catal. B Environ. 2016, 197, 73–85. [Google Scholar] [CrossRef]
- González, I.D.; Navarro, R.M.; Wen, W.; Marinkovic, N.; Rodriguéz, J.A.; Rosa, F.; Fierro, J.L.G. A Comparative Study of the Water Gas Shift Reaction over Platinum Catalysts Supported on CeO2, TiO2 and Ce-Modified TiO2. Catal. Today 2010, 149, 372–379. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Panagiotopoulou, P.; Kondarides, D.I.; Efstathiou, A.M. Kinetic and Mechanistic Studies of the Water–Gas Shift Reaction on Pt/TiO2 Catalyst. J. Catal. 2009, 264, 117–129. [Google Scholar] [CrossRef]
- Azzam, K.G.; Babich, I.V.; Seshan, K.; Lefferts, L. Bifunctional Catalysts for Single-Stage Water–Gas Shift Reaction in Fuel Cell Applications.: Part 1. Effect of the Support on the Reaction Sequence. J. Catal. 2007, 251, 153–162. [Google Scholar] [CrossRef]
- Shah, P.R.; Kim, T.; Zhou, G.; Fornasiero, P.; Gorte, R.J. Evidence for Entropy Effects in the Reduction of Ceria−Zirconia Solutions. Chem. Mater. 2006, 18, 5363–5369. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the Mechanism of Low-Temperature Water Gas Shift Reaction on Copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Colussi, S.; Katta, L.; Amoroso, F.; Farrauto, R.J.; Trovarelli, A. Ceria-Based Palladium Zinc Catalysts as Promising Materials for Water Gas Shift Reaction. Catal. Commun. 2014, 47, 63–66. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of Water Gas Shift Reaction Catalysts: A Review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Yu, W.-Z.; Wang, W.-W.; Ma, C.; Li, S.-Q.; Wu, K.; Zhu, J.-Z.; Zhao, H.-R.; Yan, C.-H.; Jia, C.-J. Very High Loading Oxidized Copper Supported on Ceria to Catalyze the Water-Gas Shift Reaction. J. Catal. 2021, 402, 83–93. [Google Scholar] [CrossRef]
- Curran, C.D.; Lu, L.; Kiely, C.J.; McIntosh, S. Ambient Temperature Aqueous Synthesis of Ultrasmall Copper Doped Ceria Nanocrystals for the Water Gas Shift and Carbon Monoxide Oxidation Reactions. J. Mater. Chem. A 2018, 6, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.A.; Evans, J.; Graciani, J.; Park, J.-B.; Liu, P.; Hrbek, J.; Sanz, J.F. High Water−Gas Shift Activity in TiO2(110) Supported Cu and Au Nanoparticles: Role of the Oxide and Metal Particle Size. J. Phys. Chem. C 2009, 113, 7364–7370. [Google Scholar] [CrossRef]
- Boisen, A.; Janssens, T.V.W.; Schumacher, N.; Chorkendorff, I.; Dahl, S. Support Effects and Catalytic Trends for Water Gas Shift Activity of Transition Metals. J. Mol. Catal. A Chem. 2010, 315, 163–170. [Google Scholar] [CrossRef]
- Franchini, C.A.; de Farias, A.M.D.; Albuquerque, E.M.; dos Santos, R.; Fraga, M.A. Single-Stage Medium Temperature Water-Gas Shift Reaction over Pt/ZrO2—Support Structural Polymorphism and Catalyst Deactivation. Appl. Catal. B Environ. 2012, 117–118, 302–309. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. Calcination Temperature and CuO Loading Dependence on CuO-CeO2 Catalyst Activity for Water-Gas Shift Reaction. Appl. Catal. A Gen. 2008, 347, 23–33. [Google Scholar] [CrossRef]
- Si, R.; Raitano, J.; Yi, N.; Zhang, L.; Chan, S.-W.; Flytzani-Stephanopoulos, M. Structure Sensitivity of the Low-Temperature Water-Gas Shift Reaction on Cu–CeO2 Catalysts. Catal. Today 2012, 180, 68–80. [Google Scholar] [CrossRef]
- Tanaka, Y.; Utaka, T.; Kikuchi, R.; Sasaki, K.; Eguchi, K. Water Gas Shift Reaction over Cu-Based Mixed Oxides for CO Removal from the Reformed Fuels. Appl. Catal. A Gen. 2003, 242, 287–295. [Google Scholar] [CrossRef]
- Tabakova, T.; Idakiev, V.; Avgouropoulos, G.; Papavasiliou, J.; Manzoli, M.; Boccuzzi, F.; Ioannides, T. Highly Active Copper Catalyst for Low-Temperature Water-Gas Shift Reaction Prepared via a Cu-Mn Spinel Oxide Precursor. Appl. Catal. A Gen. 2013, 451, 184–191. [Google Scholar] [CrossRef]
- Ávila-Neto, C.N.; Zanchet, D.; Hori, C.E.; Ribeiro, R.U.; Bueno, J.M.C. Interplay between Particle Size, Composition, and Structure of MgAl2O4-Supported Co–Cu Catalysts and Their Influence on Carbon Accumulation during Steam Reforming of Ethanol. J. Catal. 2013, 307, 222–237. [Google Scholar] [CrossRef]
- White, E.C.; Shultz, J.F. Fused Cobalt Oxide as a Water Gas Catalyst. Ind. Eng. Chem. 1934, 26, 95–97. [Google Scholar] [CrossRef]
- Wang, J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and Catalytic Performance of Alumina-Supported Copper–Cobalt Catalysts for Carbon Monoxide Hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Bakhmutsky, K.; Wieder, N.L.; Baldassare, T.; Smith, M.A.; Gorte, R.J. A Thermodynamic Study of the Redox Properties of Supported Co Particles. Appl. Catal. A Gen. 2011, 397, 266–271. [Google Scholar] [CrossRef]
- Ro, I.; Resasco, J.; Christopher, P. Approaches for Understanding and Controlling Interfacial Effects in Oxide-Supported Metal Catalysts. ACS Catal. 2018, 8, 7368–7387. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Chorkendorff, I.; Knudsen, I.; Balmes, O.; Nerlov, J.; Molenbroek, A.M.; Clausen, B.S.; Helveg, S. Transient Behavior of Cu/ZnO-Based Methanol Synthesis Catalysts. J. Catal. 2009, 262, 65–72. [Google Scholar] [CrossRef]
- Chen, C.S.; Lai, Y.T.; Lai, T.W.; Wu, J.H.; Chen, C.H.; Lee, J.F.; Kao, H.M. Formation of Cu Nanoparticles in SBA-15 Functionalized with Carboxylic Acid Groups and Their Application in the Water–Gas Shift Reaction. ACS Catal. 2013, 3, 667–677. [Google Scholar] [CrossRef]
- Roberts, S.; Gorte, R.J. A Comparison of Pt Overlayers on α-Al2O3(0001), ZnO(0001)Zn, and ZnO(0001−)O. J. Chem. Phys. 1990, 93, 5337–5344. [Google Scholar] [CrossRef]
- Onn, T.M.; Zhang, S.; Arroyo-Ramirez, L.; Xia, Y.; Wang, C.; Pan, X.; Graham, G.W.; Gorte, R.J. High-Surface-Area Ceria Prepared by ALD on Al2O3 Support. Appl. Catal. B Environ. 2017, 201, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Foucher, A.; Stach, E.A.; Gorte, R.J. A Study of Support Effects for CH4 and CO Oxidation over Pd Catalysts on ALD-Modified Al2O3. Catal. Lett. 2019, 149, 905–915. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of Inorganic Films Grown by Atomic Layer Deposition: Overview and General Trends. J. Appl. Phys. 2013, 113, 21301. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.; Curran, C.D.; Stach, E.A.; McIntosh, S.; Gorte, R.J. “Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films. ACS Catal. 2019, 9, 7318–7327. [Google Scholar] [CrossRef]
- Wang, C.; Mao, X.; Lee, J.D.; Onn, T.M.; Yeh, Y.-H.; Murray, C.B.; Gorte, R.J. A Characterization Study of Reactive Sites in ALD-Synthesized WOx/ZrO2 Catalysts. Catalysts 2018, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Ginés, M.J.L.; Amadeo, N.; Laborde, M.; Apesteguía, C.R. Activity and Structure-Sensitivity of the Water-Gas Shift Reaction over CuZnAl Mixed Oxide Catalysts. Appl. Catal. A Gen. 1995, 131, 283–296. [Google Scholar] [CrossRef]
- Chinchen, G.C.; Spencer, M.S. Sensitive and Insensitive Reactions on Copper Catalysts: The Water-Gas Shift Reaction and Methanol Synthesis from Carbon Dioxide. Catal. Today 1991, 10, 293–301. [Google Scholar] [CrossRef]
- Cao, T.; Kwon, O.; Lin, C.; Vohs, J.M.; Gorte, R.J. Two-Dimensional Perovskite Crystals Formed by Atomic Layer Deposition of CaTiO3 on γ-Al2O3. Nanomaterials 2021, 11, 2207. [Google Scholar] [CrossRef]
- Huang, R.; Cheng, Y.; Ji, Y.; Gorte, R.J. Atomic Layer Deposition for Preparing Isolated Co Sites on SiO2 for Ethane Dehydrogenation Catalysis. Nanomaterials 2020, 10, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandekar, A.; Vannice, M.A. Determination of the Dispersion and Surface Oxidation States of Supported Cu Catalysts. J. Catal. 1998, 178, 621–639. [Google Scholar] [CrossRef]
- Onn, T.M.; Küngas, R.; Fornasiero, P.; Huang, K.; Gorte, R.J. Atomic Layer Deposition on Porous Materials: Problems with Conventional Approaches to Catalyst and Fuel Cell Electrode Preparation. Inorganics 2018, 6, 34. [Google Scholar] [CrossRef]
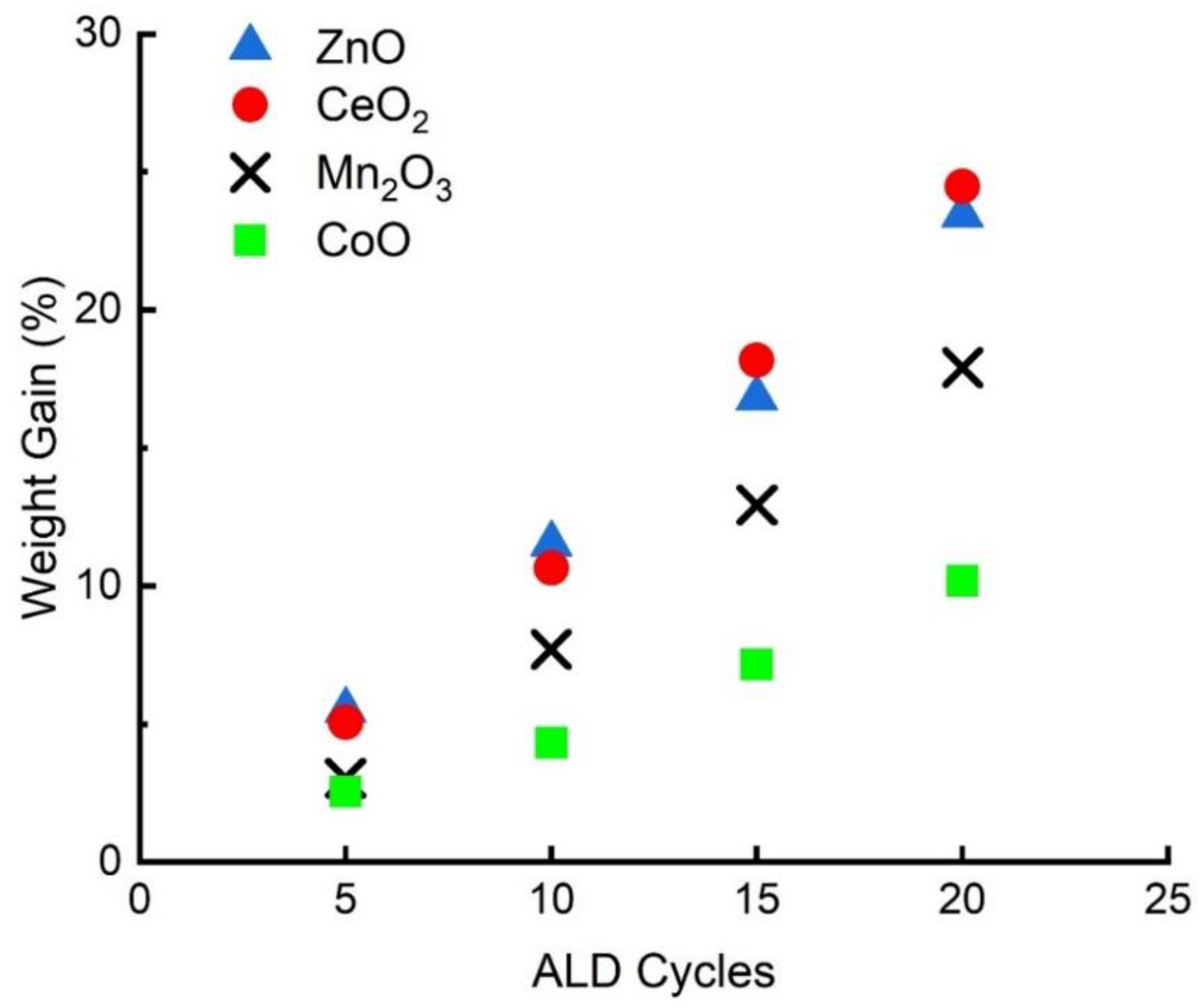


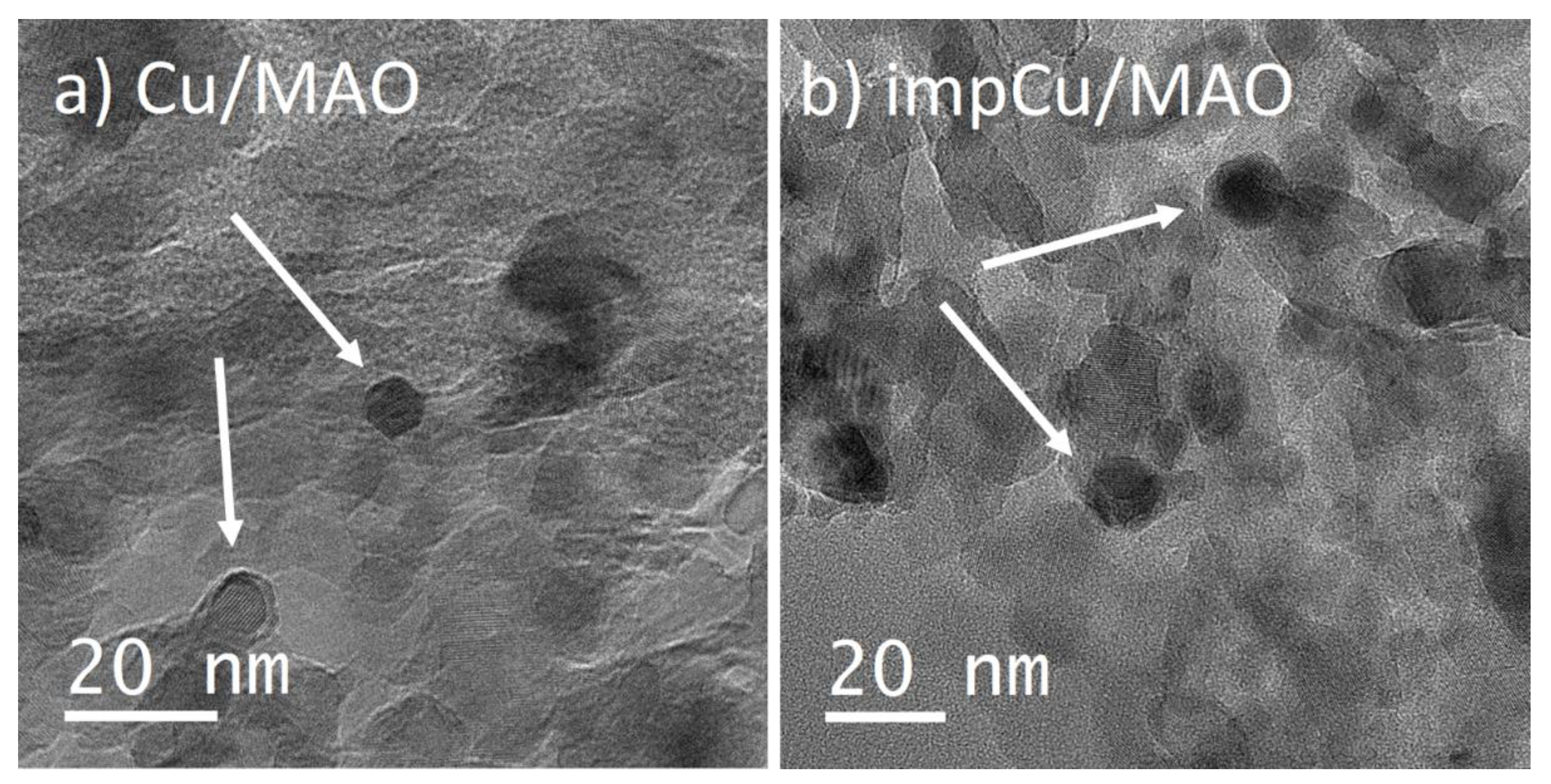

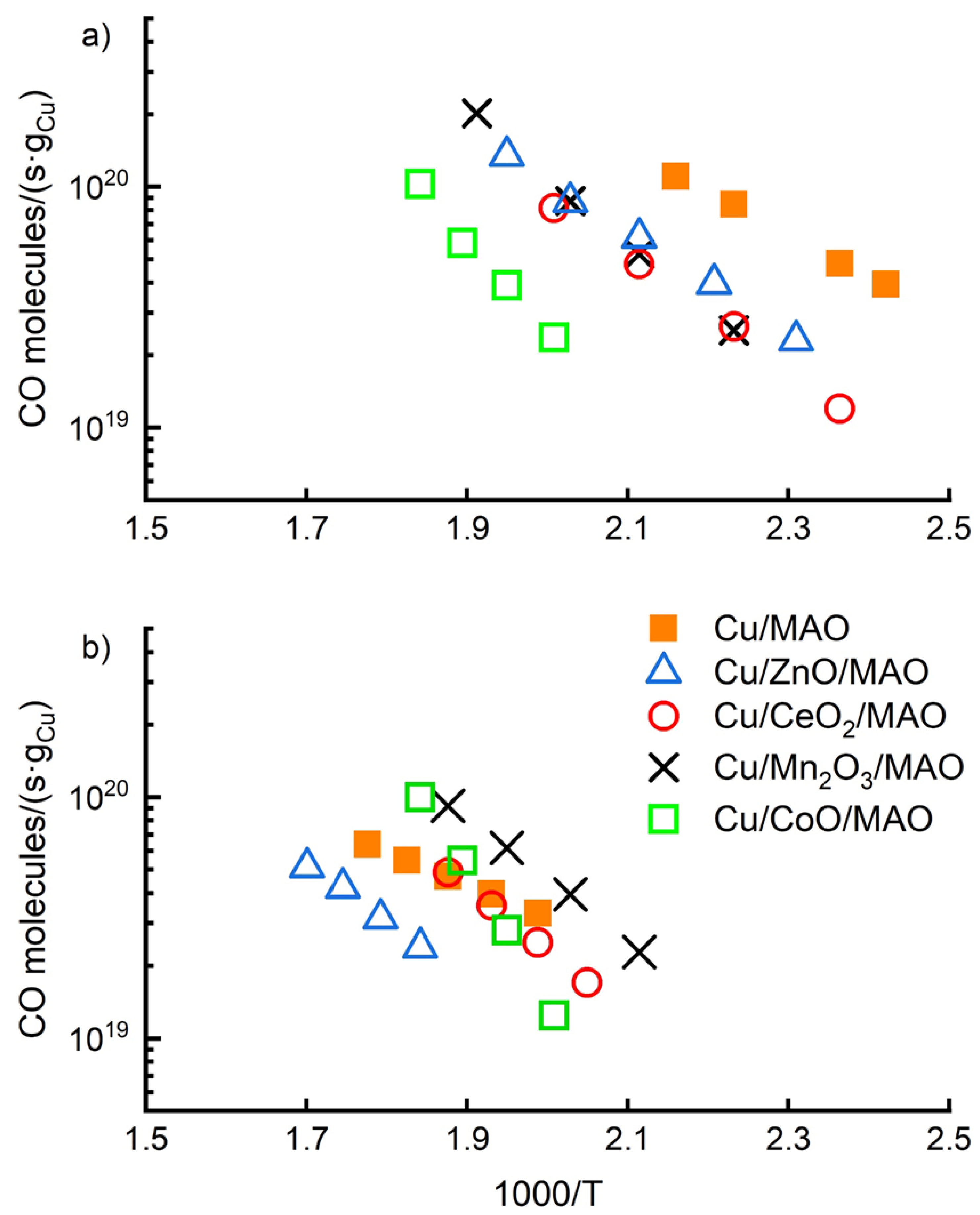
| Sample | Thickness Growth Rate (nm/cycle) | Final Metal Oxide Weight Loading (%) | Final Film Thickness (nm) | BET Surface Area (m2/g) |
|---|---|---|---|---|
| MgAl2O4 (MAO) * | – | – | – | 95 |
| ZnO/MAO | 0.020 | 23 | 0.41 | 60 |
| CeO2/MAO | 0.018 | 24 | 0.36 | 77 |
| Mn2O3/MAO | 0.018 | 18 | 0.35 | 86 |
| CoO/MAO | 0.009 | 10 | 0.19 | 74 |
| Sample | Weight Loading (%) | Dispersion (%) | Particle Size (nm) |
|---|---|---|---|
| Cu/MAO | 1.1 | 15 | 7 |
| impCu/MAO | 1.0 | 8 | 13 |
| Cu/MAO (1073K) * | 1.1 | 6.5 | 17 |
| Cu/ZnO/MAO | 1.0 | – | – |
| Cu/CeO2/MAO | 0.9 | – | – |
| Cu/Mn2O3/MAO | 1.0 | – | – |
| Cu/CoO/MAO | 1.1 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Feng, Z.; Vohs, J.M.; Gorte, R.J. A Study of Support Effects for the Water-Gas-Shift Reaction over Cu. Catalysts 2022, 12, 1364. https://doi.org/10.3390/catal12111364
Chang J, Feng Z, Vohs JM, Gorte RJ. A Study of Support Effects for the Water-Gas-Shift Reaction over Cu. Catalysts. 2022; 12(11):1364. https://doi.org/10.3390/catal12111364
Chicago/Turabian StyleChang, Jian, Zhuoming Feng, John M. Vohs, and Raymond J. Gorte. 2022. "A Study of Support Effects for the Water-Gas-Shift Reaction over Cu" Catalysts 12, no. 11: 1364. https://doi.org/10.3390/catal12111364








