Facile Preparation and Promising Hydrothermal Stability of Spherical γ-Alumina Support with High Specific Surface Area
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphologies and Structure of PB Powder
2.2. Pore Structure of PB Powder
2.3. Morphologies and Structure of Spherical γ-Al2O3 Balls
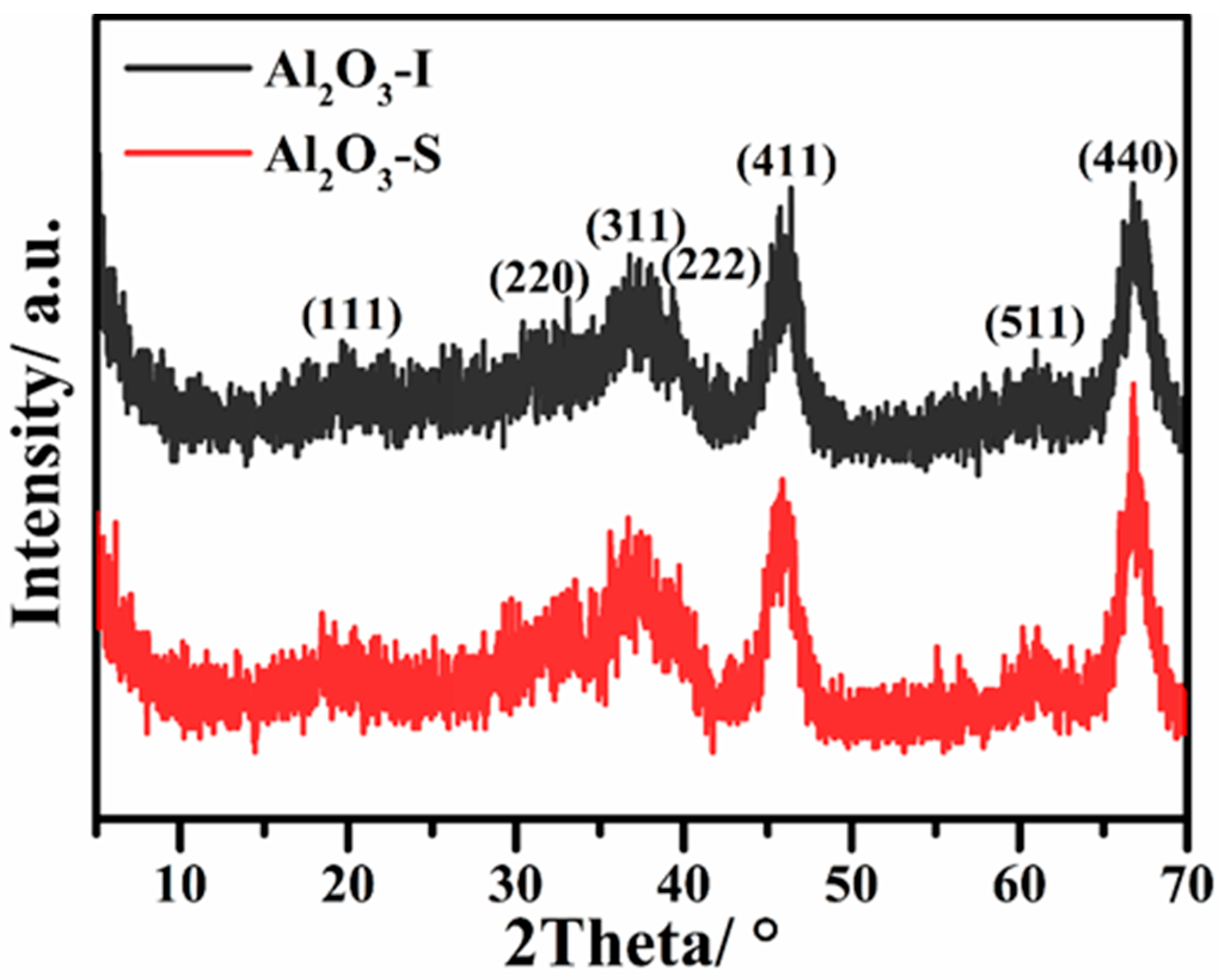
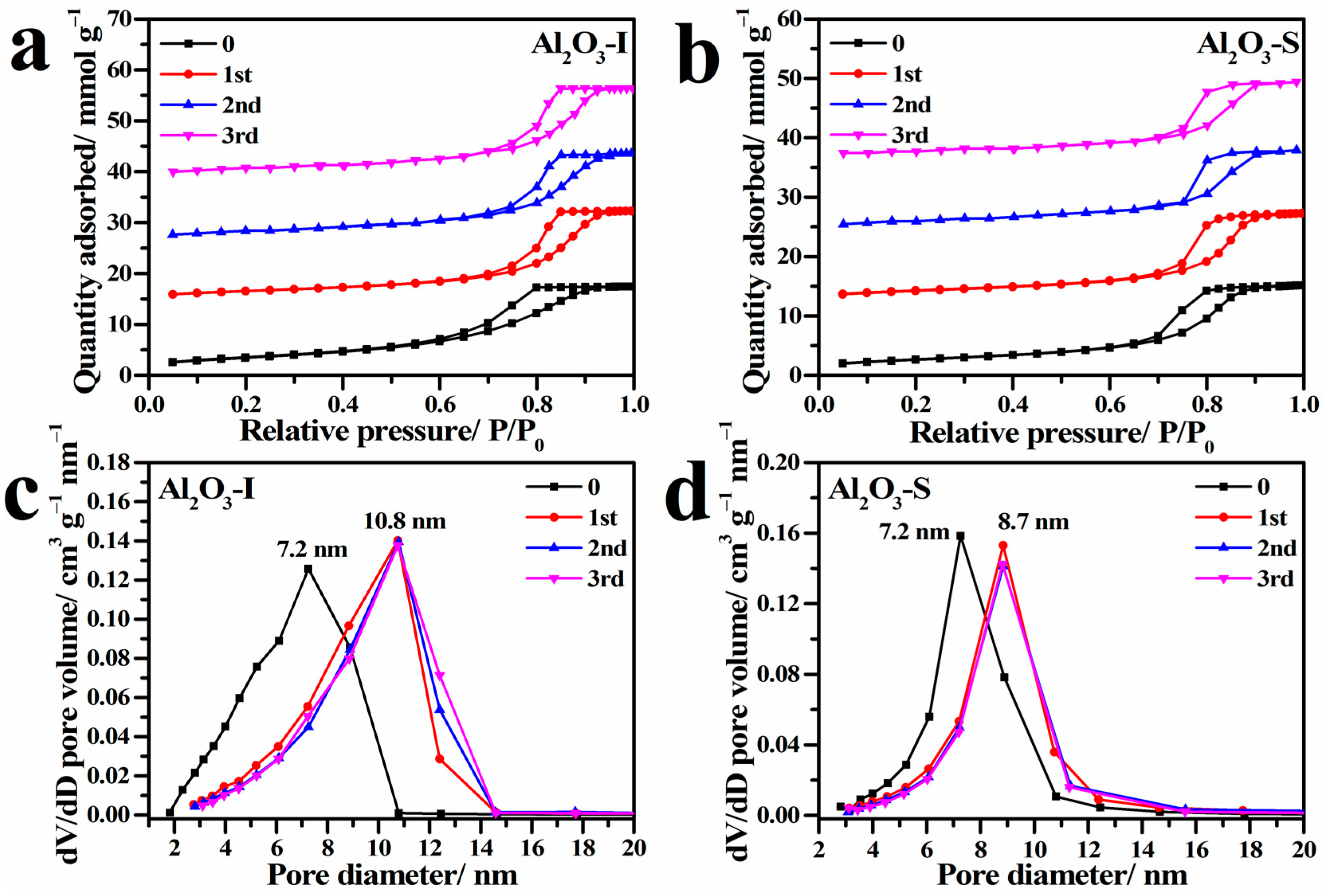
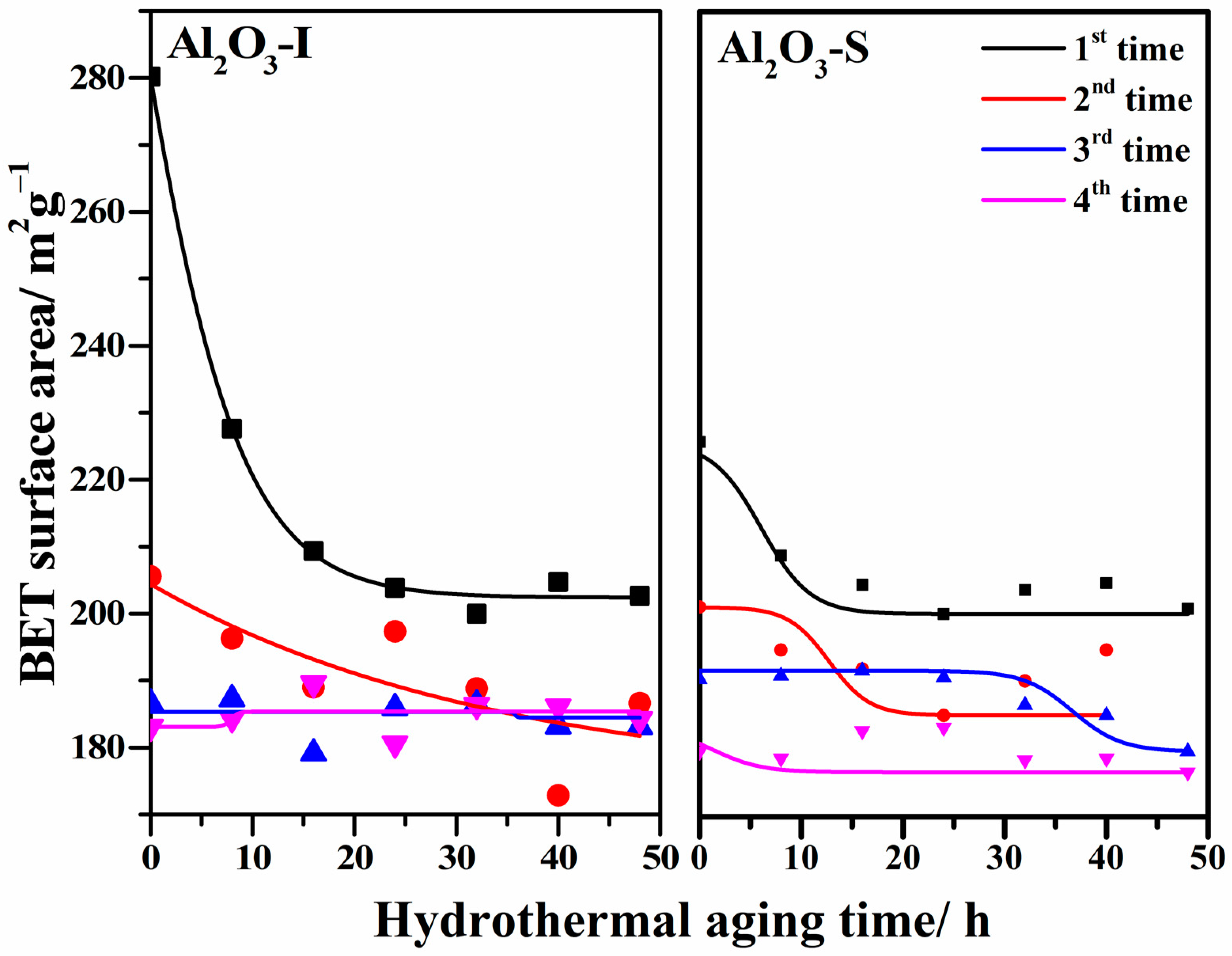
2.4. Hydrothermal Stability of γ-Al2O3
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Pseudo-Boehmite
3.3. Preparation of Spherical γ-Al2O3 Balls
3.4. Hydrothermal Stability Evaluation
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Ibrahim, A.-R.; Hu, X.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Preparation of large pore volume γ-alumina and its performance as catalyst support in phenol hydroxylation. Microporous Mesoporous Mater. 2016, 231, 1–8. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Tao, Z.; Hu, C.; Zhao, C.; Yi, F.; Gao, X.; Wen, X.; Yang, Y.; Li, Y. Synthesis and characterization of amorphous silica-alumina with enhanced acidity and its application in hydro-isomerization/cracking. Fuel 2020, 279, 118487. [Google Scholar] [CrossRef]
- Liu, P.; Feng, J.; Zhang, X.; Lin, Y.; Evans, D.G.; Li, D. Preparation of high purity spherical gamma-alumina using a reduction-magnetic separation process. J. Phys. Chem. Solids 2008, 69, 799–804. [Google Scholar] [CrossRef]
- Delgado, A.D.; Alvarez-Contreras, L.; Beltran, K.A.; Leyva-Porras, C.; Aguilar-Elguezabal, A. Comparison of three-dimensional versus two-dimensional structure of mesoporous alumina as support of (Ni)MoS2 catalysts for HDS. Catal. Today 2021, 360, 165–175. [Google Scholar] [CrossRef]
- Rahmati, M.; Huang, B.; Mortensen, M.K., Jr.; Keyvanloo, K.; Fletcher, T.H.; Woodfield, B.F.; Hecker, W.C.; Argyle, M.D. Effect of different alumina supports on performance of cobalt Fischer-Tropsch catalysts. J. Catal. 2018, 359, 92–100. [Google Scholar] [CrossRef]
- Shan, Y.-L.; Zhao, W.-T.; Zhao, S.-L.; Wang, X.-X.; Sun, H.-L.; Yu, W.-L.; Ding, J.-W.; Feng, X.; Chen, D. Effects of alumina phases on the structure and performance of VOx/Al2O3 catalysts in non-oxidative propane dehydrogenation. Mol. Catal. 2021, 504, 111466. [Google Scholar] [CrossRef]
- Lv, Y.; Li, D.; Tang, P.; Feng, Y. A simple and promoter free way to synthesize spherical γ-alumina with high hydrothermal stability. Mater. Lett. 2015, 155, 75–77. [Google Scholar] [CrossRef]
- Nazer, S.; Dabbagh, H.A.; Najafi Chermahini, A.; Farrokhpour, H. Surface modification of alumina with P2O5 and its application in 2-octanol dehydration. React. Kinet. Mech. Catal. 2020, 129, 265–282. [Google Scholar] [CrossRef]
- Enache, D.; Roy-Auberger, M.; Esterle, K.; Revel, R. Preparation of Al2O3–ZrO2 mixed supports; their characteristics and hydrothermal stability. Colloids Surf. A Physicochem. Eng. Asp. 2003, 220, 223–233. [Google Scholar] [CrossRef]
- Cheng, G.; Shen, G.; Wang, J.; Wang, Y.; Zhang, W.; Wang, J.; Shen, M. The Hydrothermal Stability and the Properties of Non- and Strongly-Interacting Rh Species over Rh/γ, θ-Al2O3 Catalysts. Catalysts 2021, 11, 99. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Mardkhe, M.K.; Woodfield, B.F. Thermal and hydrothermal stability of pure and silica-doped mesoporous aluminas. Microporous Mesoporous Mater. 2019, 284, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Claude, V.; Mahy, J.G.; Wolfs, C.; Lambert, S.D. Physico-chemical properties of alumina supports modified with silicon alkoxides. J. Solid State Chem. 2020, 282, 121102. [Google Scholar] [CrossRef]
- Amrute, A.P.; Jeske, K.; Lodziana, Z.; Prieto, G.; Schueth, F. Hydrothermal Stability of High-Surface-Area alpha-Al2O3 and Its Use as a Support for Hydrothermally Stable Fischer-Tropsch Synthesis Catalysts. Chem. Mater. 2020, 32, 4369–4374. [Google Scholar] [CrossRef]
- Chung, S.; Liu, Q.; Joshi, U.A.; Regalbuto, J.R.; Boateng, A.A.; Smith, M.A.; Coe, C.G. Using polyfurfuryl alcohol to improve the hydrothermal stability of mesoporous oxides for reactions in the aqueous phase. J. Porous Mater. 2018, 25, 407–414. [Google Scholar] [CrossRef]
- Girel, E.; Cabiac, A.; Chaumonnot, A.; Besson, M.; Tuel, A. Selective Carbon Deposition on gamma-Alumina Acid Sites: Toward the Design of Catalyst Supports with Improved Hydrothermal Stability in Aqueous Media. ACS Appl. Mater. Interfaces 2020, 12, 13558–13567. [Google Scholar] [CrossRef]
- Van Cleve, T.; Underhill, D.; Rodrigues, M.V.; Sievers, C.; Medlin, J.W. Enhanced Hydrothermal Stability of gamma-Al2O3 Catalyst Supports with Alkyl Phosphonate Coatings. Langmuir 2018, 34, 3619–3625. [Google Scholar] [CrossRef]
- Pérez, L.L.; Alvarez-Galván, C.; Zarubina, V.; Fernandes, B.O.F.; Melián-Cabrera, I. A hydrothermally stable transition alumina by condensation-enhanced self-assembly and pyrolysis crystallization: Application in the steam reforming of methane. CrystEngComm 2014, 16, 6775–6783. [Google Scholar] [CrossRef]
- Fujisaki, S.; Zahir, M.H.; Ikuhara, Y.H.; Iwamoto, Y.; Kuroda, K. Nanostructural Characterization of Hydrothermally Stable γ-Alumina-Based Composite Materials by Transmission Electron Microscopy. Adv. Mater. Res. 2007, 26–28, 1109–1112. [Google Scholar] [CrossRef]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally stable silica–alumina composite membranes for hydrogen separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Li, Z.J.; He, L.; Tian, W.L.; Huang, R.Y.; Wang, X.P.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Batch and fixed-bed adsorption behavior of porous boehmite with high percentage of exposed (020) facets and surface area towards Congo red. Inorg. Chem. Front. 2021, 8, 735–745. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, D.Q.; Li, C.X.; Wang, Z.H.; Evans, D.G.; Duan, X. Synthesis of Cu-containing layered double hydroxides with a narrow crystallite-size distribution. Clays Clay Miner. 2003, 51, 566–569. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Han, B.; Xu, B.; Liu, X.; Yan, Z. Effects of synthetic conditions on the textural structure of pseudo-boehmite. J. Colloid Interface Sci. 2016, 469, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Coronado, I.; Stekrova, M.; Reinikainen, M.; Simell, P.; Lefferts, L.; Lehtonen, J. A review of catalytic aqueous-phase reforming of oxygenated hydrocarbons derived from biorefinery water fractions. Int. J. Hydrogen Energy 2016, 41, 11003–11032. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Copeland, J.R.; Kim, W.G.; Crittenden, J.C.; Sievers, C. Structural Changes of gamma-Al2O3-Supported Catalysts in Hot Liquid Water. ACS Catal. 2011, 1, 552–561. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lv, Y.; Mo, Y.; Li, H.; Tang, P.; Li, D.; Feng, Y. Facile Preparation and Promising Hydrothermal Stability of Spherical γ-Alumina Support with High Specific Surface Area. Catalysts 2022, 12, 1416. https://doi.org/10.3390/catal12111416
Zhang Y, Lv Y, Mo Y, Li H, Tang P, Li D, Feng Y. Facile Preparation and Promising Hydrothermal Stability of Spherical γ-Alumina Support with High Specific Surface Area. Catalysts. 2022; 12(11):1416. https://doi.org/10.3390/catal12111416
Chicago/Turabian StyleZhang, Yi, Yimin Lv, Yufan Mo, Huiyu Li, Pinggui Tang, Dianqing Li, and Yongjun Feng. 2022. "Facile Preparation and Promising Hydrothermal Stability of Spherical γ-Alumina Support with High Specific Surface Area" Catalysts 12, no. 11: 1416. https://doi.org/10.3390/catal12111416




