Promotion Effect of Ce Doping on Catalytic Performance of LaMnO3 for CO Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance
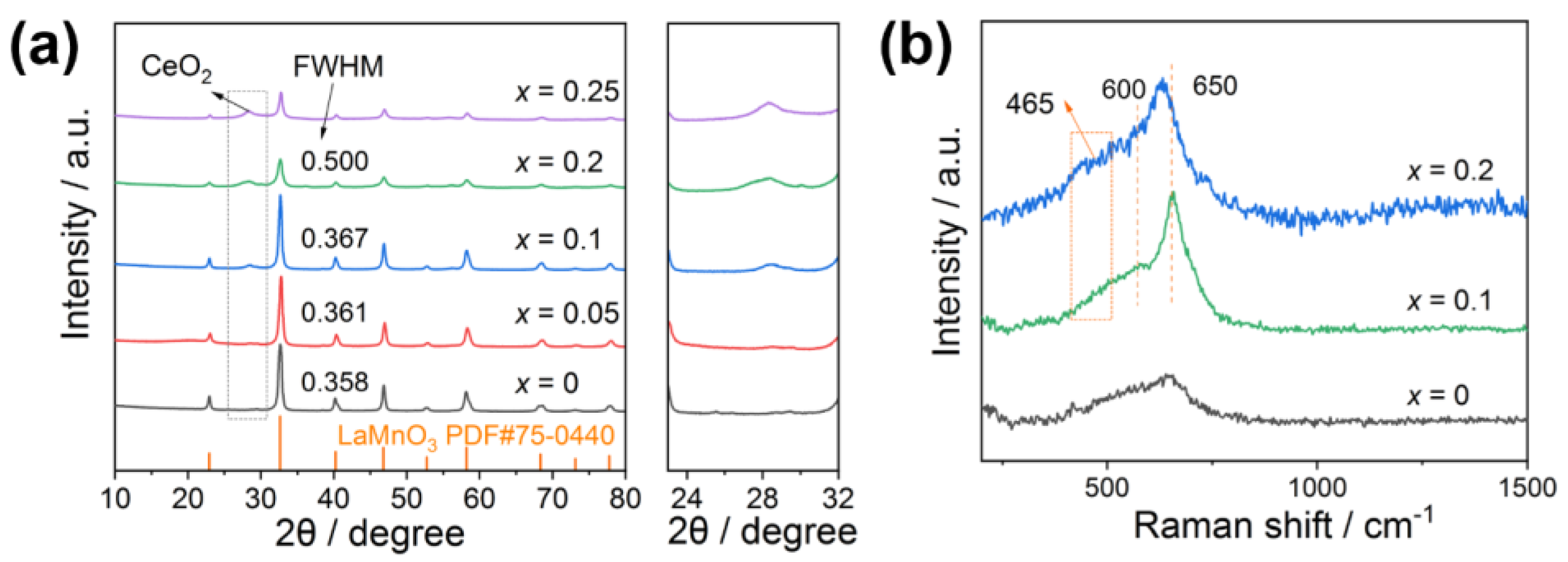
2.2. XRD and Raman Results
2.3. N2 Physisorption Isotherms
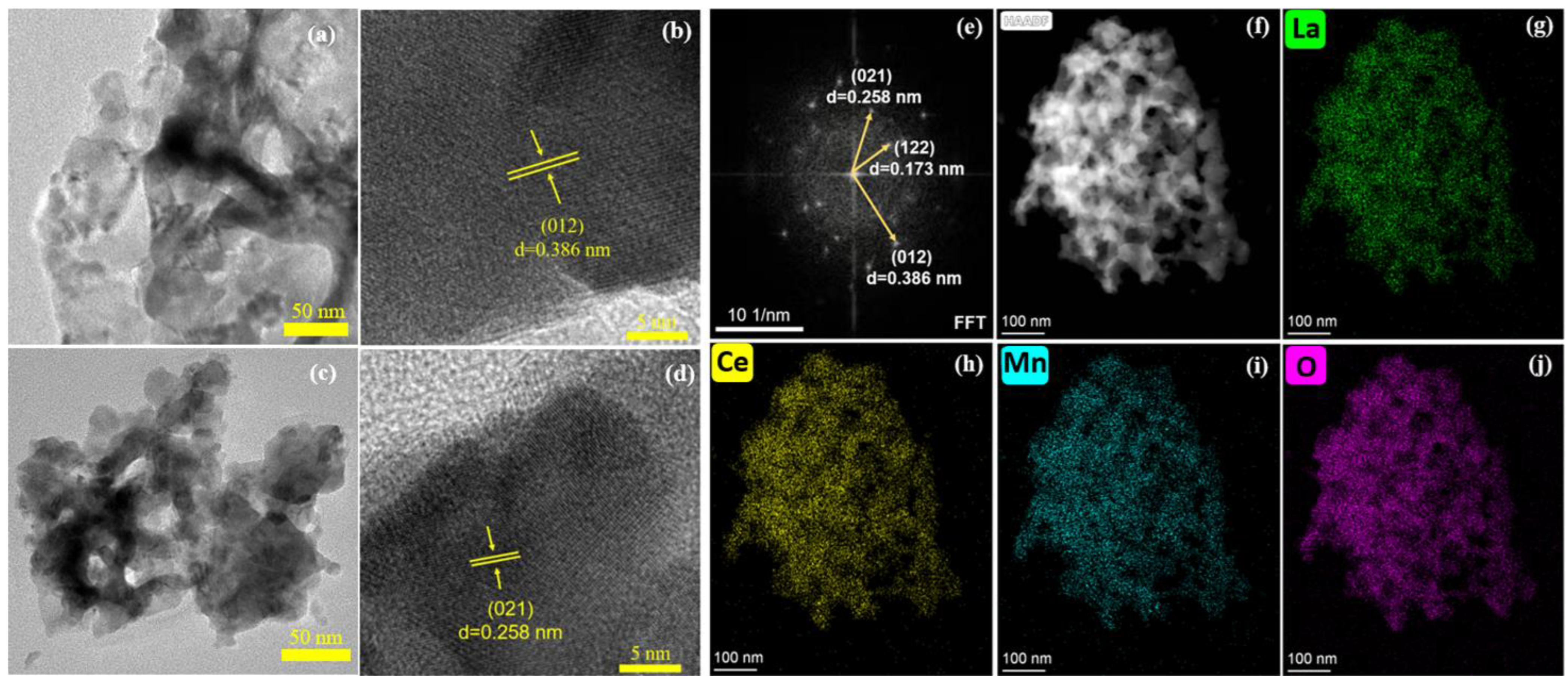
2.4. TEM Results
2.5. XPS Results
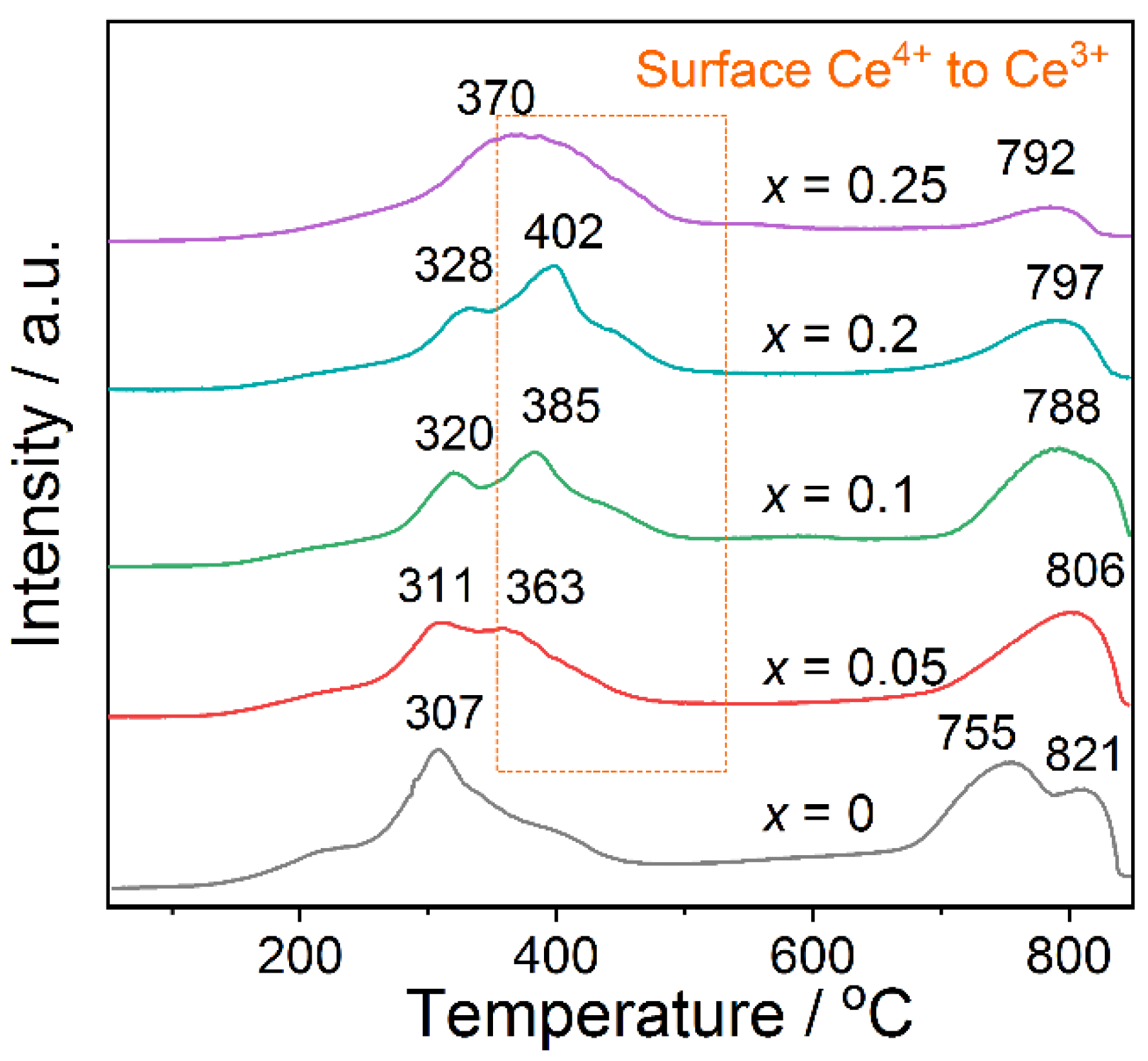
2.6. H2-TPR Results
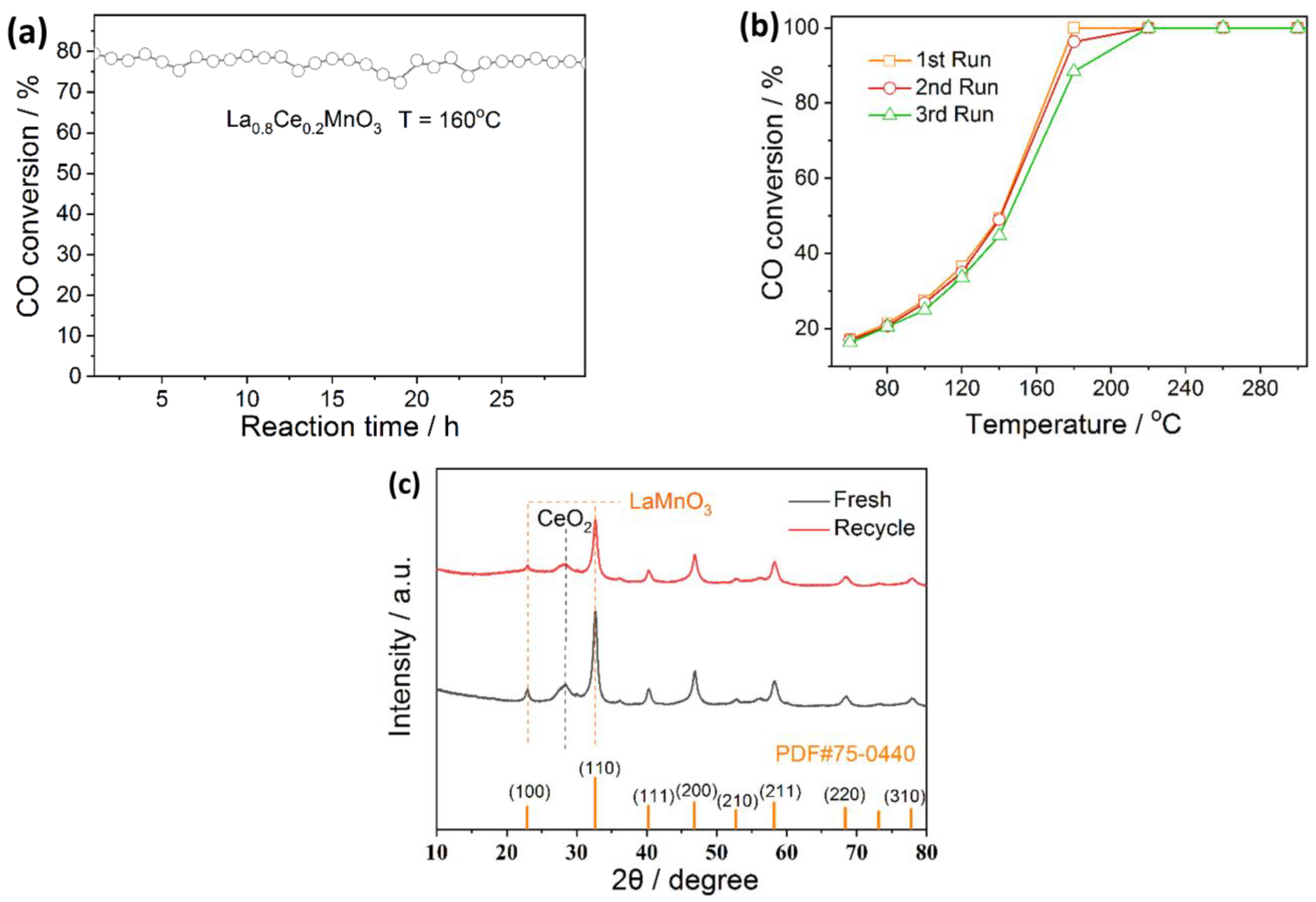
2.7. Stability Test of La0.8Ce0.2MnO3
3. Experimental
3.1. Catalyst’s Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Konsolakis, M.; Yentekakis, I.V. Insight into the role of electropositive promoters in emission control catalysis: An in situ drifts study of NO reduction by C3H6 over Na-promoted Pt/Al2O3 catalysts. Top. Catal. 2013, 56, 165–171. [Google Scholar] [CrossRef]
- Altass, H.M.; Morad, M.; Khder, A.E.-R.S.; Mannaa, M.A.; Jassas, R.S.; Alsimaree, A.A.; Ahmed, S.A.; Salama, R.S. Enhanced catalytic activity for CO oxidation by highly active Pd nanoparticles supported on reduced graphene oxide/copper metal organic framework. J. Taiwan Inst. Chem. Eng. 2021, 128, 194–208. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.L.; Xiao, P.; Zhu, J.J.; Liu, X.Y. Cooperative effect between copper species and oxygen vacancy in Ce0.7–xZrxCu0.3O2 catalysts for carbon monoxide oxidation. Front. Chem. Sci. Eng. 2021, 15, 1524–1536. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional synergistic effect of Cs-Au NPs on the performance of Cs-Au/MgFe2O4 catalysts in catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3765–3776. [Google Scholar] [CrossRef]
- Eichhorn, L.; Thudium, M.; Juttner, B. The diagnosis and treatment of carbon monoxide poisoning. Dtsch. Arztebl. Int. 2018, 115, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Wang, L.N.; Mou, L.H.; He, S.G. Catalytic CO oxidation by gas-phase metal oxide clusters. J. Phys. Chem. A 2019, 123, 9257–9267. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Trawczynski, J. Potassium-copper perovskite catalysts for mild temperature diesel soot combustion. J. Mol. Catal. A Chem. 2014, 485, 214–221. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emission over LaMnO3 and LaB0.2Mn0.8O3 (B=Co, Ni, Fe) catalysts. Appl. Catal. B Environ. 2013, 129, 509–516. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen storage capacity of La1−xA′xBO3 perovskites (with A′=Sr, Ce; B=Co, Mn)-relation with catalytic activity in the CH4 oxidation reaction. Appl. Catal. B Environ. 2005, 58, 273–288. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Sadovskaya, E.M.; Pinaeva, L.G.; Isupova, L.A. Influence of oxygen mobility on catalytic activity of La–Sr–Mn–O composites in the reaction of high temperature N2O decomposition. J. Catal. 2009, 267, 5–13. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Al Soubaihi, R.; Saoud, K.; Dutta, J. Critical review of low-temperature CO oxidation and hysteresis phenomenon on heterogeneous catalysts. Catalysts 2018, 8, 660. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Misono, M.; Yoneda, Y. Reduction-oxidation and catalytic properties of perovskite-type mixed oxide catalysts (La1-xSrxCoO3). Chem. Lett. 1981, 10, 1589–1592. [Google Scholar] [CrossRef]
- Myung, C.-L.; Jang, W.; Kwon, S.; Ko, J.; Jin, D.; Park, S. Evaluation of the real-time de-NOx performance characteristics of a LNT-equipped Euro-6 diesel passenger car with various vehicle emissions certification cycles. Energy 2017, 132, 356–369. [Google Scholar] [CrossRef]
- Alifanti, M.; Florea, M.; Pârvulescu, V.I. Ceria-based oxides as supports for LaCoO3 perovskite catalysts for total oxidation of VOC. Appl. Catal. B Environ. 2007, 70, 400–405. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Zhu, J.; Tang, D.; Zhao, Z. Effect of preparation method on physicochemical properties and catalytic performances of LaCoO3 perovskite for CO oxidation. J. Rare Earths 2019, 37, 970–977. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Yang, Y.; Zheng, C.; Zhou, J.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1-xCexCoO3+δ catalysts. Appl. Catal. B Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Xiang, X.P.; Zhao, L.H.; Teng, B.T.; Lang, J.J.; Hu, X.; Li, T.; Fang, Y.A.; Luo, M.F.; Lin, J.J. Catalytic combustion of methane on La1−xCexFeO3 oxides. Appl. Surf. Sci. 2013, 276, 328–332. [Google Scholar] [CrossRef]
- Mathieu-Deremince, V.; Nagy, J.B.; Verbist, J.J. Structure and catalytic activity of mixed oxides of perovskite structure. Stud. Surf. Sci. Catal. 1995, 96, 393–404. [Google Scholar]
- Zhou, H.; Wu, Q.; Qi, B. Facile one-step flame synthesis of La1-xSrxMnO3 nanoparticles for CO catalytic oxidation. J. Chem. 2021, 2021, 9472008. [Google Scholar] [CrossRef]
- Huang, X.; Niu, P.; Pan, H.; Shang, X. Micromorphological control of porous LaMnO3 and LaMn0.8Fe0.2O3 and its catalytic oxidation performance for CO. J. Solid State Chem. 2018, 265, 218–226. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; De Rossi, S.; Faticanti, M.; Porta, P. Methane combustion and CO oxidation on LaAl1-xMnxO3 perovskite-type oxide solid solutions. Appl. Catal. B Environ. 2003, 43, 397–406. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. Promotion effect of Au on perovskite catalysts for the regeneration of diesel particulate filters. Catal. Today 2008, 137, 306–311. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Litvinchuk, A.P.; Iliev, M.N.; Meng, R.L.; Popov, V.N.; Ivanov, V.G.; Chakalov, R.A.; Thomsen, C. Comparative study of optical phonons in the rhombohedrally distorted perovskites LaAlO3 and LaMnO3. Phys. Rev. B 1999, 59, 4146–4153. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ren, S.; Yang, J.; Liu, W.; Su, Z.; Chen, Z.; Wang, M.; Chen, L. Effect of oxygen vacancies on improving NO oxidation over CeO2 [32] and [17] facets for fast SCR reaction. J. Environ. Chem. Eng. 2021, 9, 106218. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Dai, F.; Li, Q.; Zhang, Z.; Jiang, Z.; Zhang, S.; Huang, Y. NOx-assisted soot combustion over dually substituted perovskite catalysts La1-xKxCo1-yPdyO3-δ. Appl. Catal. B Environ. 2013, 142–143, 278–289. [Google Scholar] [CrossRef]
- Alifanti, M.; Kirchnerova, J.; Delmon, B. Effect of substitution by cerium on the activity of LaMnO3 perovskite in methane combustion. J. Mol. Catal. A Chem. 2003, 245, 231–244. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure-activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Sakthivel, T.S.; Reid, D.L.; Bhatta, U.M.; Möbus, G.; Sayle, D.C.; Seal, S. Engineering of nanoscale defect patterns in CeO2 nanorods via ex situ and in situ annealing. Nanoscale 2015, 7, 5169–5177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgado, J.P.; Munuera, G.; Espinós, J.P.; González-Elipe, A.R. XPS study of oxidation processes of CeOx defective layers. Appl. Surf. Sci. 2000, 158, 164–171. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef]
- Hammami, R.; Aïssa, S.B.; Batis, H. Effects of thermal treatment on physico-chemical and catalytic properties of lanthanum manganite LaMnO3+y. J. Mol. Catal. A Chem. 2009, 353, 145–153. [Google Scholar] [CrossRef]
- Magkoev, T.T.; Zaalishvili, V.B.; Burdzieva, O.G.; Tuaev, G.E.; Grigorkina, G.S. Interaction of Co, Mn, and Fe atoms with calcite: An X-ray photoelectron spectroscopy study. Geochem. Int. 2019, 57, 98–103. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Tejuca, L.G. Non-stoichiometric surface behaviour of LaMO3 oxides as evidenced by XPS. Appl. Surf. Sci. 1987, 27, 453–457. [Google Scholar] [CrossRef]
- Lisi, L.; Bagnasco, G.; Ciambelli, P.; De Rossi, S.; Porta, P.; Russo, G.; Turco, M. Perovskite-type oxides: II. redox properties of LaMn1-xCuxO3 and LaCo1-xCuxO3 and methane catalytic combustion. J. Solid State Chem. 1999, 146, 176–183. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Tang, D.; Zhao, Z.; Carabineiro, S.A.C. Aerobic selective oxidation of alcohols using La1-xCexCoO3 perovskite catalysts. J. Catal. 2016, 340, 41–48. [Google Scholar] [CrossRef]



| Catalyst | BET Surface Area (m2/g) | Reaction Conditions | CO Conversion | Reference | |
|---|---|---|---|---|---|
| T50 (°C) | T100 (°C) | ||||
| La0.8Ce0.2MnO3 | 25.8 | 0.5% CO and 6.5% O2; WHSV = 30,000 mL/(g·h) | 140 | 180 | This work |
| La0.4Sr0.6MnO3 | 12.0 | 1.0% CO and 10.0% O2; WHSV = 90,000 mL/(g·h) | 135 | 163 | [22] |
| LaMn0.8Fe0.2O3 | 55.7 | 1.0% CO and 1.25%/O2; GHSV = 12,000 h–1 | 187 | 200 | [23] |
| LaAl0.8Mn0.2O3 | 25.0 | 1% CO and 20% O2; GHSV = 12,000 h−1 | 127 | 227 | [24] |
| 2 wt% Au-LaMnO3 | 17.7 | 2300 ppm CO and 7% O2; WHSV = 30,000 mL/(g·h) | 234 | 290 | [25] |
| Catalysts | Ce3+/(Ce3+ + Ce4+) | Mn4+/Mn3+ | Oβ/Oγ |
|---|---|---|---|
| LaMnO3 | - | 0.55 | 0.62 |
| La0.9Ce0.1MnO3 | 0.23 | 0.29 | 0.65 |
| La0.8Ce0.2MnO3 | 0.27 | 0.35 | 0.81 |
| La0.75Ce0.25MnO3 | 0.20 | 0.82 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wang, S.; Yang, J.; Xiao, P.; Zhu, J. Promotion Effect of Ce Doping on Catalytic Performance of LaMnO3 for CO Oxidation. Catalysts 2022, 12, 1409. https://doi.org/10.3390/catal12111409
Wang N, Wang S, Yang J, Xiao P, Zhu J. Promotion Effect of Ce Doping on Catalytic Performance of LaMnO3 for CO Oxidation. Catalysts. 2022; 12(11):1409. https://doi.org/10.3390/catal12111409
Chicago/Turabian StyleWang, Nan, Shan Wang, Jie Yang, Ping Xiao, and Junjiang Zhu. 2022. "Promotion Effect of Ce Doping on Catalytic Performance of LaMnO3 for CO Oxidation" Catalysts 12, no. 11: 1409. https://doi.org/10.3390/catal12111409





