Optimization of SnPd Shell Configuration to Boost ORR Performance of Pt-Clusters Decorated CoOx@SnPd Core-Shell Nanocatalyst
Abstract
:1. Introduction
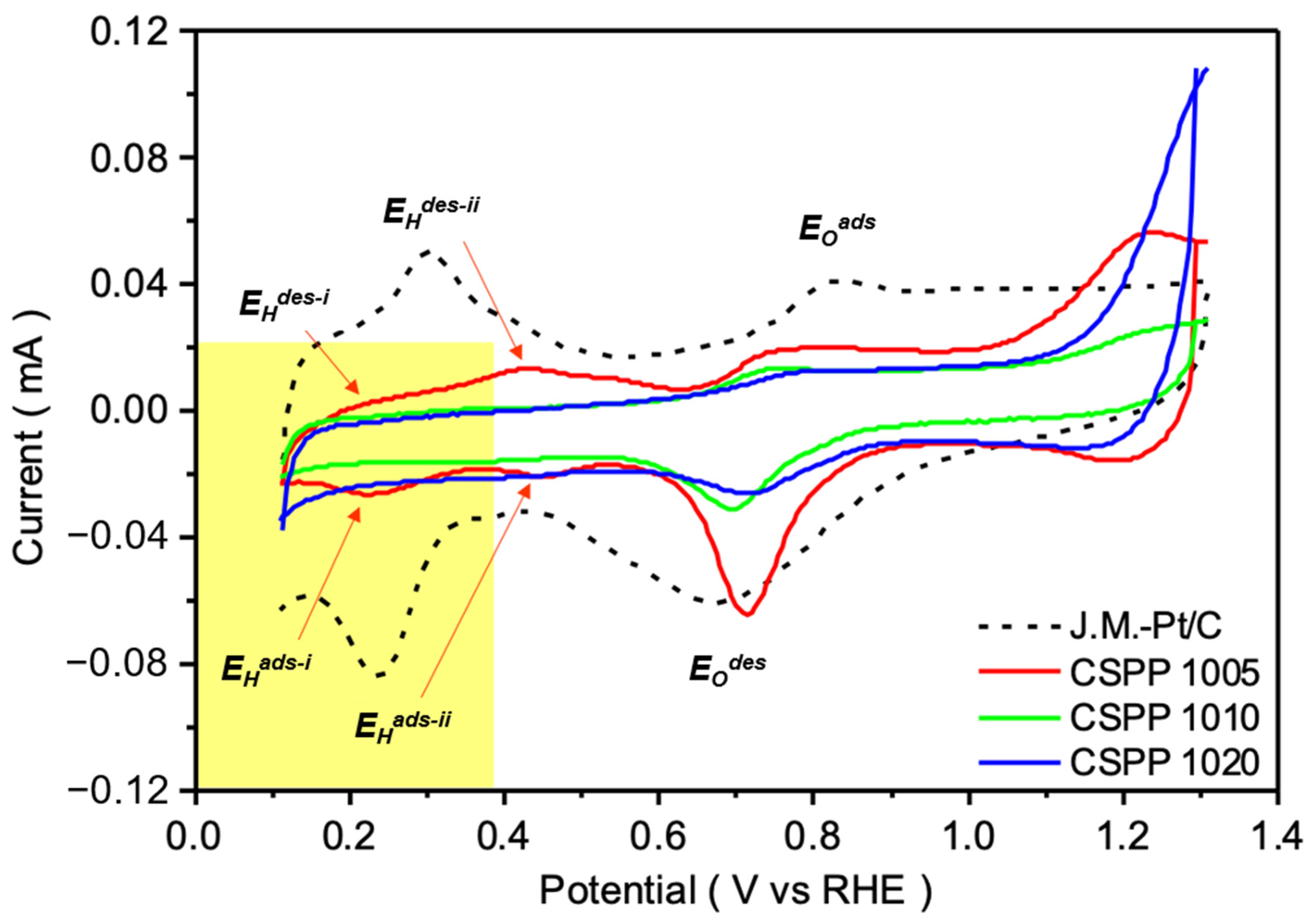
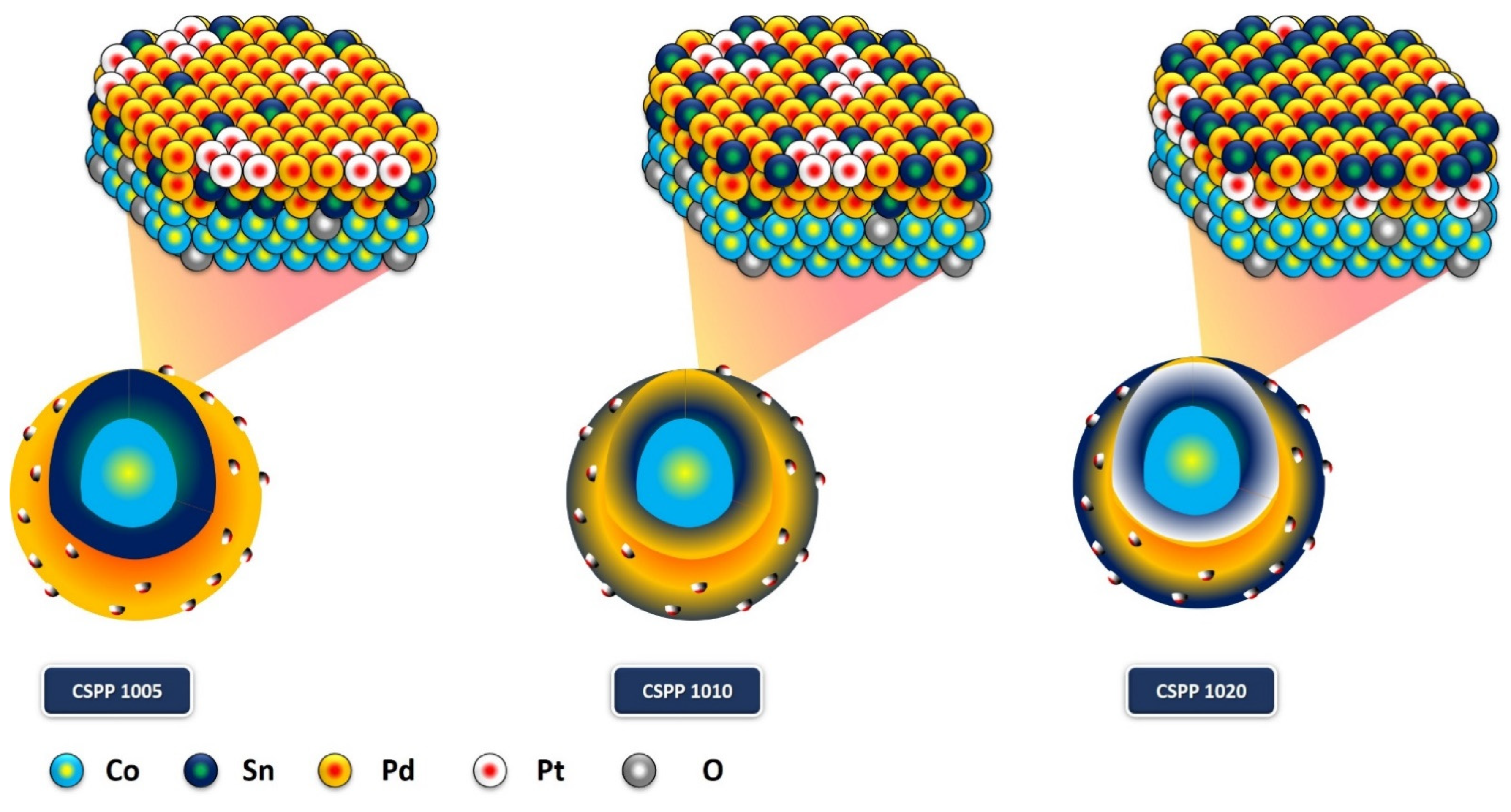
2. Results and Discussion
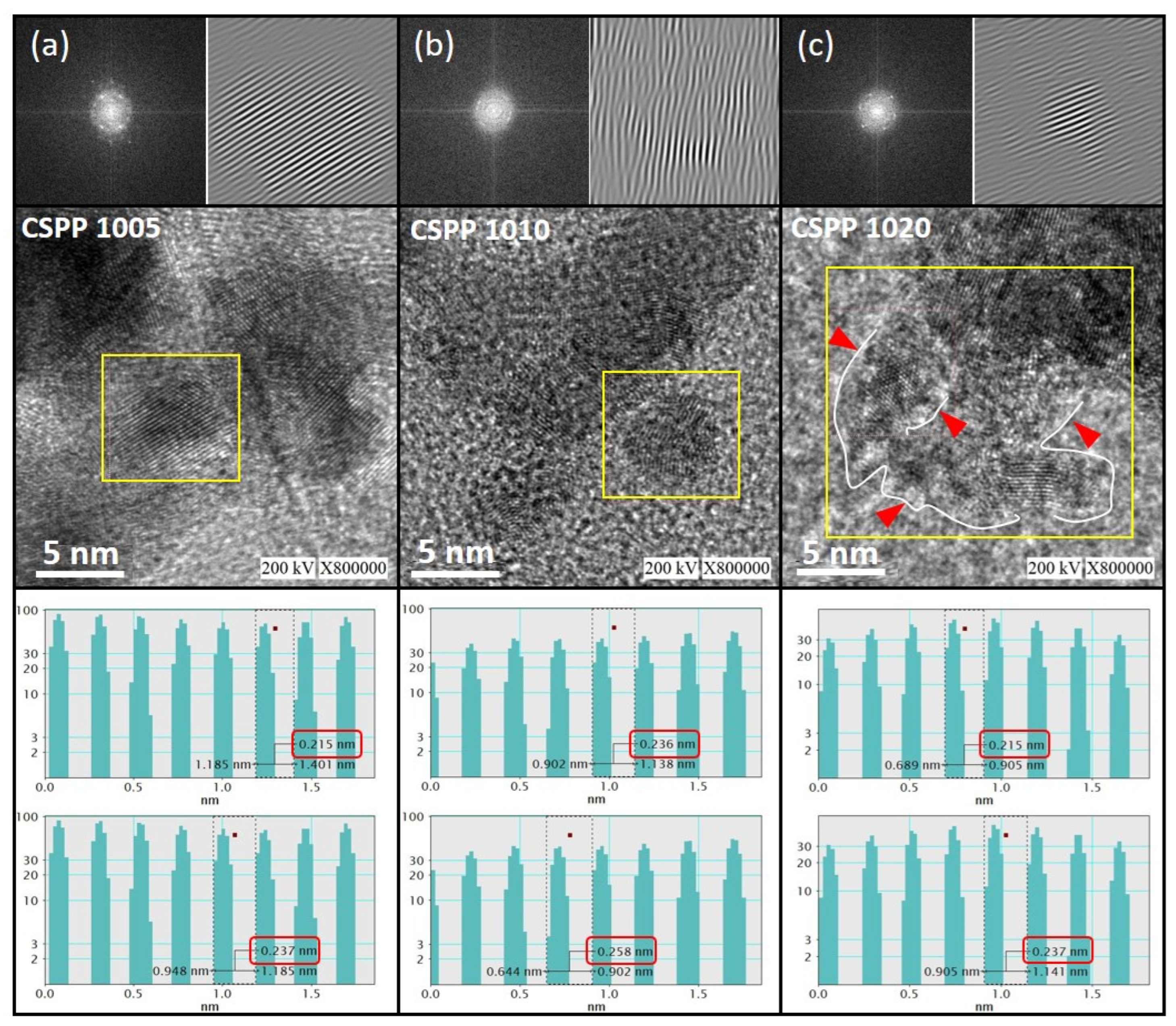
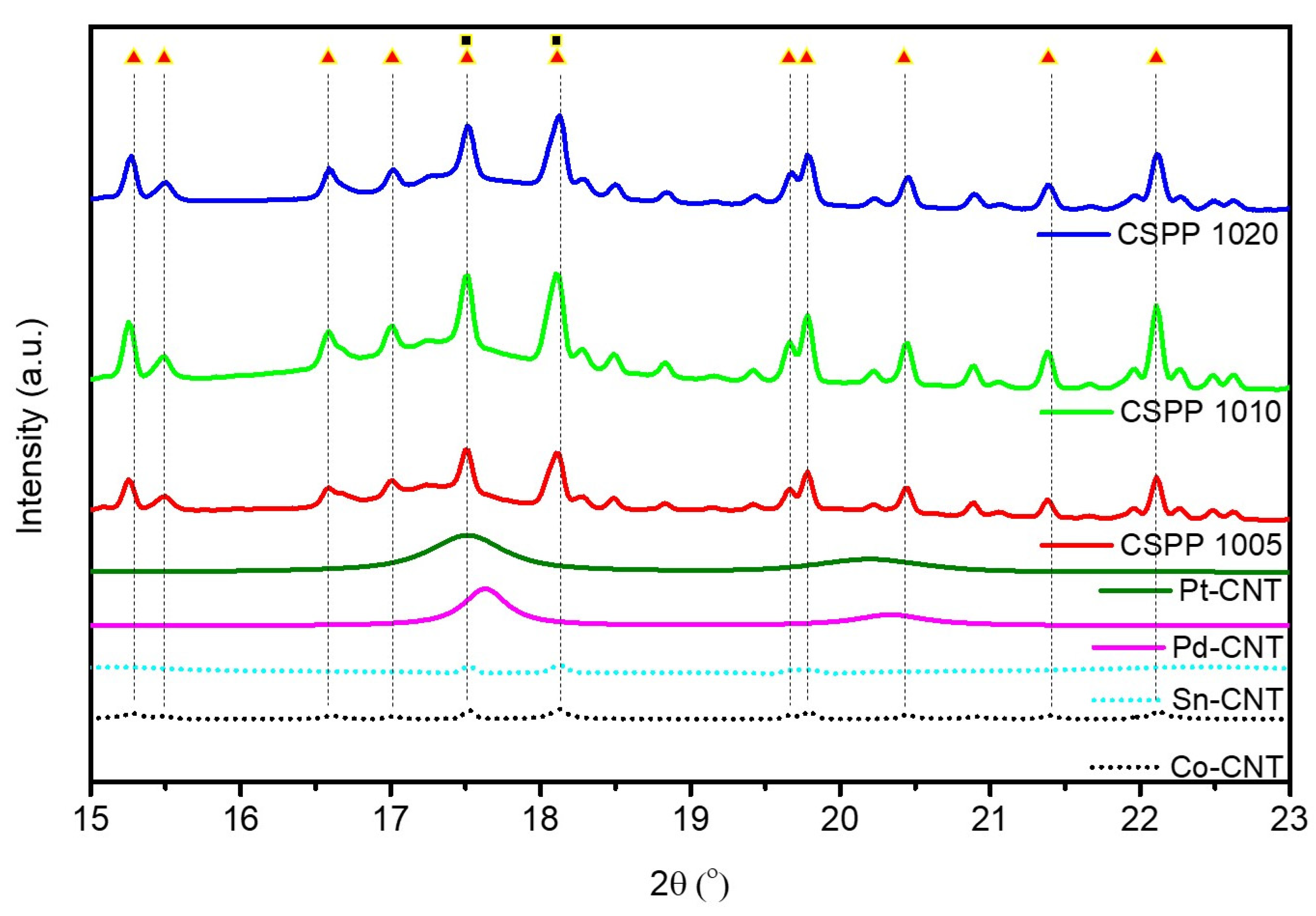
Surface Morphology and Crystal Structure
3. Experimental Section
3.1. Materials and Methods
3.2. Physical Characterization
3.3. Electrochemical Analysis
3.4. Calculation for the ORR Activity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, N.; Chung, D.Y.; Ryu, J.; Yoo, S.J.; Sung, Y.-E. Pt-based nanoarchitecture and catalyst design for fuel cell applications. Nano Today 2014, 9, 433–456. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-j. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Ortiz-Herrera, J.C.; Cruz-Martínez, H.; Solorza-Feria, O.; Medina, D.I. Recent progress in carbon nanotubes support materials for Pt-based cathode catalysts in PEM fuel cells. Int. J. Hydrog. Energy 2022, 47, 30213–30224. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-Based Core-shell Electrocatalysts for Fuel Cell Cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, Y. A fundamental comprehension and recent progress in advanced Pt-based ORR nanocatalysts. SmartMat 2021, 2, 56–75. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Leng, D.; Yin, F. The enhanced activity of Pt-Ce nanoalloy for oxygen electroreduction. Sci. Rep. 2020, 10, 14837. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Ott, S.; Dionigi, F.; Strasser, P. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2019, 18, 61–71. [Google Scholar] [CrossRef]
- Kim, J.; Hong, Y.; Lee, K.; Kim, J.Y. Highly Stable Pt-Based Ternary Systems for Oxygen Reduction Reaction in Acidic Electrolytes. Adv. Energy Mater. 2020, 10, 2002049. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Bhalothia, D.; Chou, J.-P.; Hu, A.; Chen, T.-Y. Collaboration between a Pt-dimer and neighboring Co-Pd atoms triggers efficient pathways for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2021, 23, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Weber, D.J.; Dosche, C.; Oezaslan, M. Highly Durable Pt-Based Core-Shell Catalysts with Metallic and Oxidized Co Species for Boosting the Oxygen Reduction Reaction. ACS Catal. 2022, 12, 6394–6408. [Google Scholar] [CrossRef]
- Strasser, P.; Kühl, S. Dealloyed Pt-based core-shell oxygen reduction electrocatalysts. Nano Energy 2016, 29, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Maiti, S.; Maiti, K.; Curnan, M.T.; Kim, K.; Noh, K.-J.; Han, J.W. Engineering electrocatalyst nanosurfaces to enrich the activity by inducing lattice strain. Energy Environ. Sci. 2021, 14, 3717–3756. [Google Scholar] [CrossRef]
- Deng, Y.-J.; Tripkovic, V.; Rossmeisl, J.; Arenz, M. Oxygen Reduction Reaction on Pt Overlayers Deposited onto a Gold Film: Ligand, Strain, and Ensemble Effect. ACS Catal. 2016, 6, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Heterogeneous NiO2-to-Pd Epitaxial Structure Performs Outstanding Oxygen Reduction Reaction Activity. J. Phys. Chem. C 2020, 124, 2295–2306. [Google Scholar] [CrossRef]
- Bhalothia, D.; Wang, S.-P.; Lin, S.; Yan, C.; Wang, K.-W.; Chen, P.-C. Atomic Pt-Clusters Decoration Triggers a High-Rate Performance on Ni@Pd Bimetallic Nanocatalyst for Hydrogen Evolution Reaction in Both Alkaline and Acidic Medium. Appl. Sci. 2020, 10, 5155. [Google Scholar] [CrossRef]
- Beniwal, A.; Bhalothia, D.; Yeh, W.; Cheng, M.; Yan, C.; Chen, P.-C.; Wang, K.-W.; Chen, T.-Y. Co-Existence of Atomic Pt and CoPt Nanoclusters on Co/SnOx Mix-Oxide Demonstrates an Ultra-High-Performance Oxygen Reduction Reaction Activity. Nanomaterials 2022, 12, 2804. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Yeh, W.; Tsai, D.-L.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y. Heterogeneous assembly of Pt-clusters on hierarchically structured CoOx@SnPd2@SnO2 quaternary nanocatalysts manifesting oxygen reduction reaction performance. New J. Chem. 2020, 44, 9712–9724. [Google Scholar] [CrossRef]
- Bhalothia, D.; Dai, S.; Wang, S.-P.; Yan, C.; Huang, T.-H.; Chen, P.-C.; Hiraoka, N.; Wang, K.-W.; Chen, T.-Y. Sub-nanometer Pt cluster decoration enhances the oxygen reduction reaction performances of NiOx supported Pd nano-islands. Sustain. Energy Fuels 2020, 4, 809–823. [Google Scholar] [CrossRef]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. H2 Reduction Annealing Induced Phase Transition and Improvements on Redox Durability of Pt Cluster-Decorated Cu@Pd Electrocatalysts in Oxygen Reduction Reaction. ACS Omega 2019, 4, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. Effects of Pt metal loading on the atomic restructure and oxygen reduction reaction performance of Pt-cluster decorated Cu@Pd electrocatalysts. Sustain. Energy Fuels 2019, 3, 1668–1681. [Google Scholar] [CrossRef]
- Bhalothia, D.; Hsiung, W.-H.; Yang, S.-S.; Yan, C.; Chen, P.-C.; Lin, T.-H.; Wu, S.-C.; Chen, P.-C.; Wang, K.-W.; Lin, M.-W.; et al. Submillisecond Laser Annealing Induced Surface and Subsurface Restructuring of Cu–Ni–Pd Trimetallic Nanocatalyst Promotes Thermal CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 14043–14058. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chou, J.-P.; Yan, C.; Hu, A.; Yang, Y.-T.; Chen, T.-Y. Programming ORR Activity of Ni/NiOx@Pd Electrocatalysts via Controlling Depth of Surface-Decorated Atomic Pt Clusters. ACS Omega 2018, 3, 8733–8744. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Hwang, H.; Hwang, J.P.; Kim, J.W.; Lee, C.H.; Lee, J. The effect of morphological difference and hydride incorporation on the activity of Pd/C catalysts in direct alkaline formate fuel cell. Catal. Today 2021, 359, 28–34. [Google Scholar] [CrossRef]
- Dai, S.; Chou, J.-P.; Wang, K.-W.; Hsu, Y.-Y.; Hu, A.; Pan, X.; Chen, T.-Y. Platinum-trimer decorated cobalt-palladium core-shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 2019, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-H.; Bhalothia, D.; Dai, S.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Bifunctional Pt-SnOx nanorods for enhanced oxygen reduction and hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 2960–2971. [Google Scholar] [CrossRef]
- Bhalothia, D.; Fan, Y.-J.; Lai, Y.-C.; Yang, Y.-T.; Yang, Y.-W.; Lee, C.-H.; Chen, T.-Y. Conformational Effects of Pt-Shells on Nanostructures and Corresponding Oxygen Reduction Reaction Activity of Au-Cluster-Decorated NiOx@Pt Nanocatalysts. Nanomaterials 2019, 9, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalothia, D.; Tsai, D.-L.; Wang, S.-P.; Yan, C.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y.; Chen, P.-C. Ir-oxide mediated surface restructure and corresponding impacts on durability of bimetallic NiOx@Pd nanocatalysts in oxygen reduction reaction. J. Alloys Compd. 2020, 844, 156160. [Google Scholar] [CrossRef]
- Luo, L.-M.; Zhan, W.; Zhang, R.-H.; Chen, D.; Hu, Q.-Y.; Guo, Y.F.; Zhou, X.-W. Ternary CoAuPd and binary AuPd electrocatalysts for methanol oxidation and oxygen reduction reaction: Enhanced catalytic performance by surface reconstruction. J. Power Sources 2019, 412, 142–152. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Q.; Ji, Y.; Cui, X.; Li, Y.; Zhu, X.; Huang, X. Surface-modulated palladium-nickel icosahedra as high-performance non-platinum oxygen reduction electrocatalysts. Sci. Adv. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-Y.T.; Chou, J.-P.; Lin, C.-Y.; Hu, C.-W.; Yang, Y.-T.; Chen, T.-Y. Heterogeneous Cu-Pd binary interface boosts stability and mass activity of atomic Pt clusters in the oxygen reduction reaction. Nanoscale 2017, 9, 7207–7216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gao, H.; Lu, S.; Zhang, X.; Wang, C.-Y.; Wang, W.-K.; Yu, H.-Q. Ni-Pd core-shell nanoparticles with Pt-like oxygen reduction electrocatalytic performance in both acidic and alkaline electrolytes. J. Mater. Chem. A 2017, 5, 9233–9240. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Zou, L.; Huang, Q.; Yang, H. Incorporation of cobalt into Pd2Sn intermetallic nanoparticles as durable oxygen reduction electrocatalyst. J. Electroanal. Chem. 2017, 789, 167–173. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, H.; Zhao, M.; DiSalvo, F.J. High-Performance Pd3Pb Intermetallic Catalyst for Electrochemical Oxygen Reduction. Nano Lett. 2016, 16, 2560–2566. [Google Scholar] [CrossRef]
- Holade, Y.; da Silva, R.G.; Servat, K.; Napporn, T.W.; Canaff, C.; de Andrade, A.R.; Kokoh, K.B. Facile synthesis of highly active and durable PdM/C (M = Fe, Mn) nanocatalysts for the oxygen reduction reaction in an alkaline medium. J. Mater. Chem. A 2016, 4, 8337–8349. [Google Scholar] [CrossRef]
- Kuttiyiel, K.A.; Sasaki, K.; Su, D.; Wu, L.; Zhu, Y.; Adzic, R.R. Gold-promoted structurally ordered intermetallic palladium cobalt nanoparticles for the oxygen reduction reaction. Nat. Commun. 2014, 5, 5185. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly Coupled Pd Nanotetrahedron/Tungsten Oxide Nanosheet Hybrids with Enhanced Catalytic Activity and Stability as Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef] [PubMed]
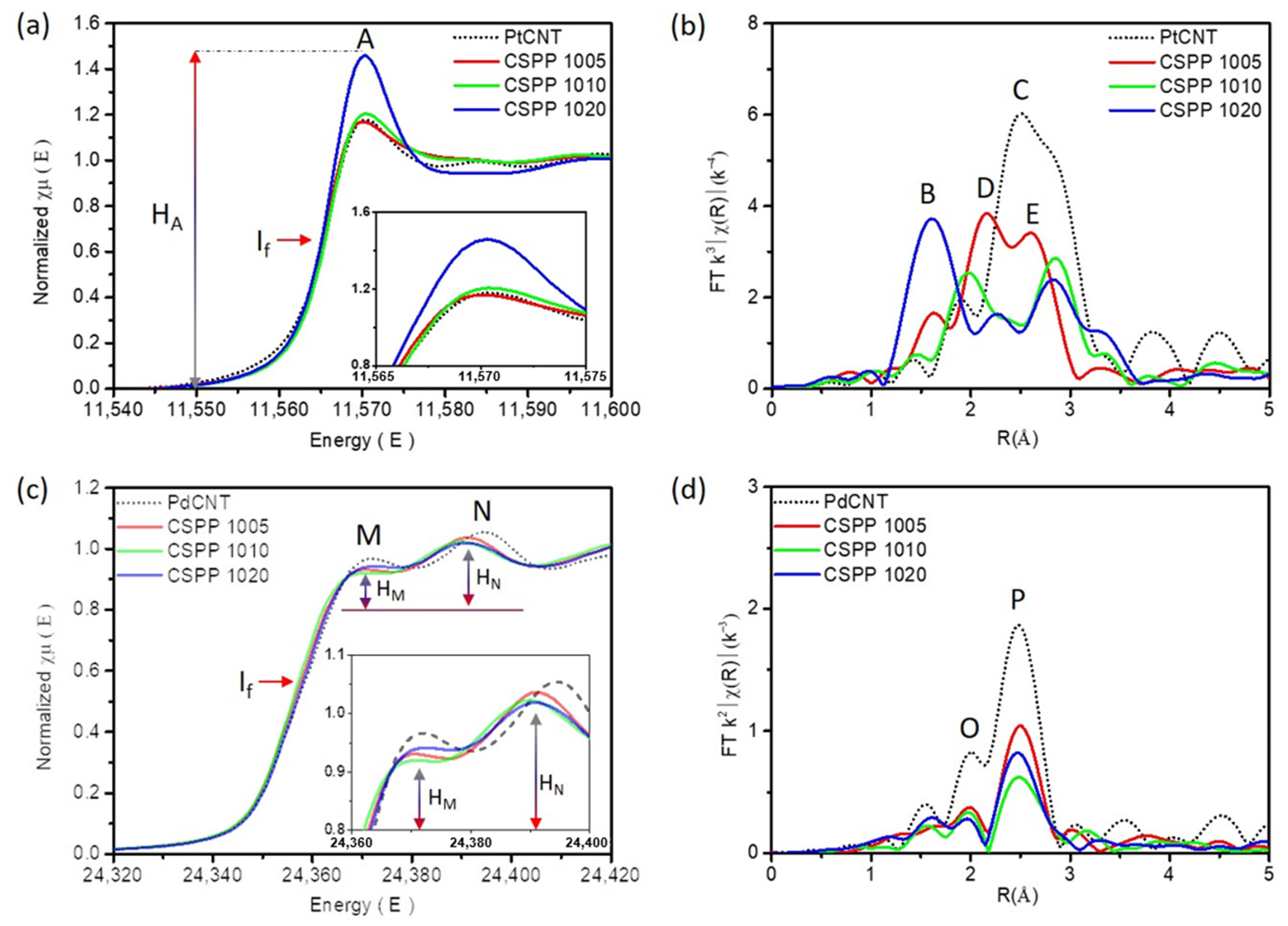

| Sample | PtL3-Edge | |||||
| Bond Pair | CN | R (Å) | R-Factor | ΔE0 | χ | |
| CSPP 1005 | Pt-Pt | 3.86 | 2.672 | 0.0005 | 0.189 | 68.23% |
| Pt-Pd | 0.842 | 2.670 | 14.89% | |||
| Pt-Sn | 0.955 | 2.587 | 16.88% | |||
| CSPP 1010 | Pt-Pt | 4.351 | 2.726 | 0.024 | 2.874 | 74.82% |
| Pt-Pd | 0.286 | 2.689 | 4.92% | |||
| Pt-Sn | 1.178 | 2.630 | 20.26% | |||
| CSPP 1020 | Pt-Pt | 3.906 | 2.727 | 0.004 | 5.385 | 57.07% |
| Pt-Pd | 0.279 | 2.671 | 4.08% | |||
| Pt-Sn | 0.970 | 2.633 | 14.17% | |||
| Pt-O | 1.689 | 1.948 | 24.68% | |||
| Sample | Pd K-Edge | |||||
| Bond Pair | CN | R (Å) | R-Factor | ΔE0 | χ | |
| CSPP 1005 | Pd-Pd | 4.050 | 2.705 | 0.0003 | −1.666 | 43.12% |
| Pd-Pt | 0.234 | 2.746 | 2.49% | |||
| Pd-Sn | 4.588 | 2.795 | 48.85% | |||
| Pd-Co | 0.520 | 2.623 | 5.54% | |||
| CSPP 1010 | Pd-Pd | 3.593 | 2.699 | 0.0023 | −0.790 | 50.85% |
| Pd-Pt | 0.313 | 2.677 | 4.43% | |||
| Pd-Sn | 2.661 | 2.795 | 37.66% | |||
| Pd-Co | 0.499 | 2.713 | 7.06% | |||
| CSPP 1020 | Pd-Pd | 4.662 | 2.697 | 0.0011 | −2.732 | 48.34% |
| Pd-Pt | 1.015 | 2.670 | 10.53% | |||
| Pd-Sn | 3.377 | 2.794 | 35.02% | |||
| Pd-Co | 0.589 | 2.735 | 6.11% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Bhalothia, D.; Yeh, W.; Beniwal, A.; Yan, C.; Wang, K.-W.; Chen, P.-C.; Tu, X.; Chen, T.-Y. Optimization of SnPd Shell Configuration to Boost ORR Performance of Pt-Clusters Decorated CoOx@SnPd Core-Shell Nanocatalyst. Catalysts 2022, 12, 1411. https://doi.org/10.3390/catal12111411
Cheng M, Bhalothia D, Yeh W, Beniwal A, Yan C, Wang K-W, Chen P-C, Tu X, Chen T-Y. Optimization of SnPd Shell Configuration to Boost ORR Performance of Pt-Clusters Decorated CoOx@SnPd Core-Shell Nanocatalyst. Catalysts. 2022; 12(11):1411. https://doi.org/10.3390/catal12111411
Chicago/Turabian StyleCheng, Mingxing, Dinesh Bhalothia, Wei Yeh, Amisha Beniwal, Che Yan, Kuan-Wen Wang, Po-Chun Chen, Xin Tu, and Tsan-Yao Chen. 2022. "Optimization of SnPd Shell Configuration to Boost ORR Performance of Pt-Clusters Decorated CoOx@SnPd Core-Shell Nanocatalyst" Catalysts 12, no. 11: 1411. https://doi.org/10.3390/catal12111411






