Enhanced Catalytic Performance and Sulfur Dioxide Resistance of Reduced Graphene Oxide-Promoted MnO2 Nanorods-Supported Pt Nanoparticles for Benzene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
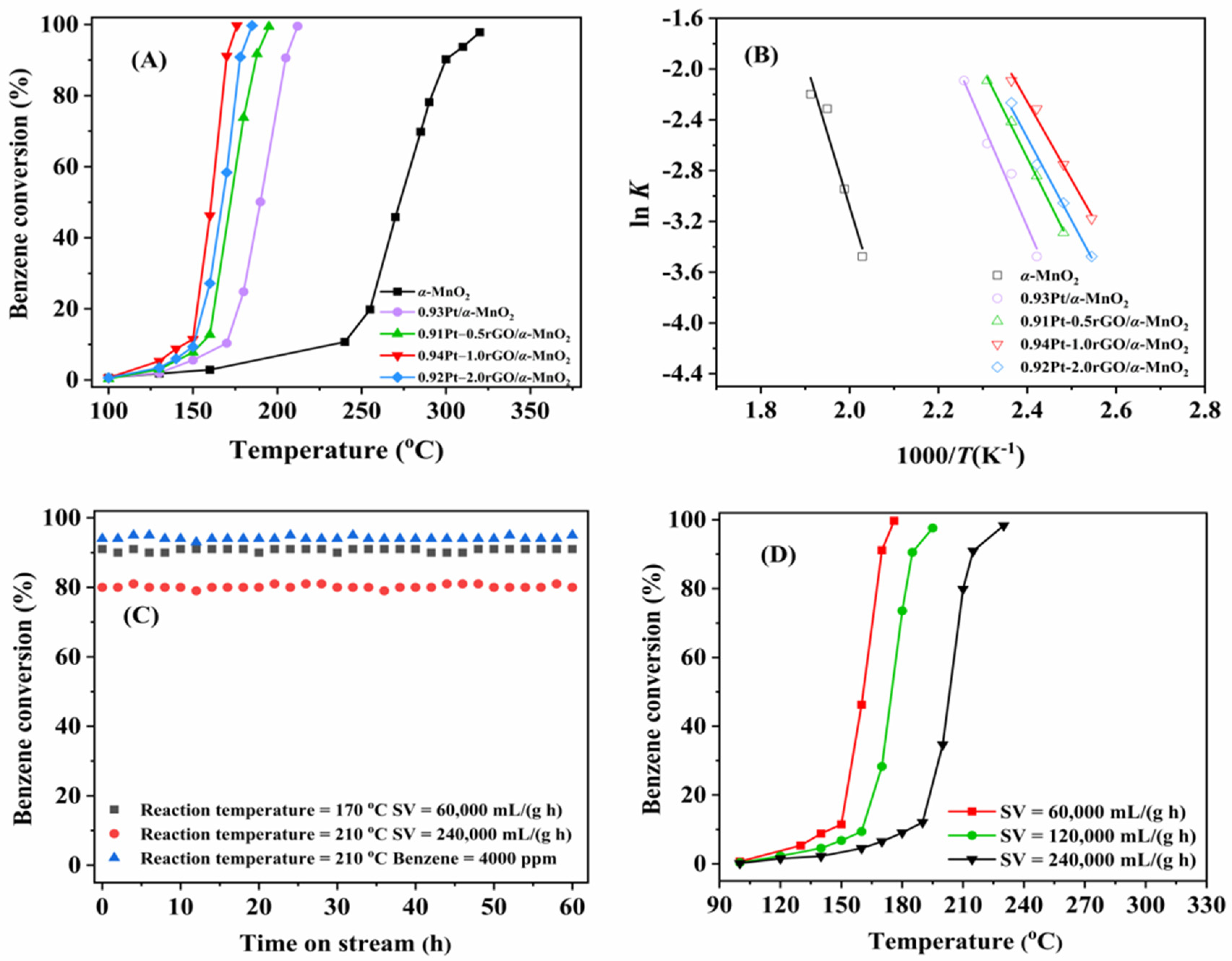
2.1. Catalyst Performance and Stability
2.1.1. Catalyst Performance
2.1.2. Catalytic Stability and Effects of GHSV and Benzene Concentration on Catalytic Activity
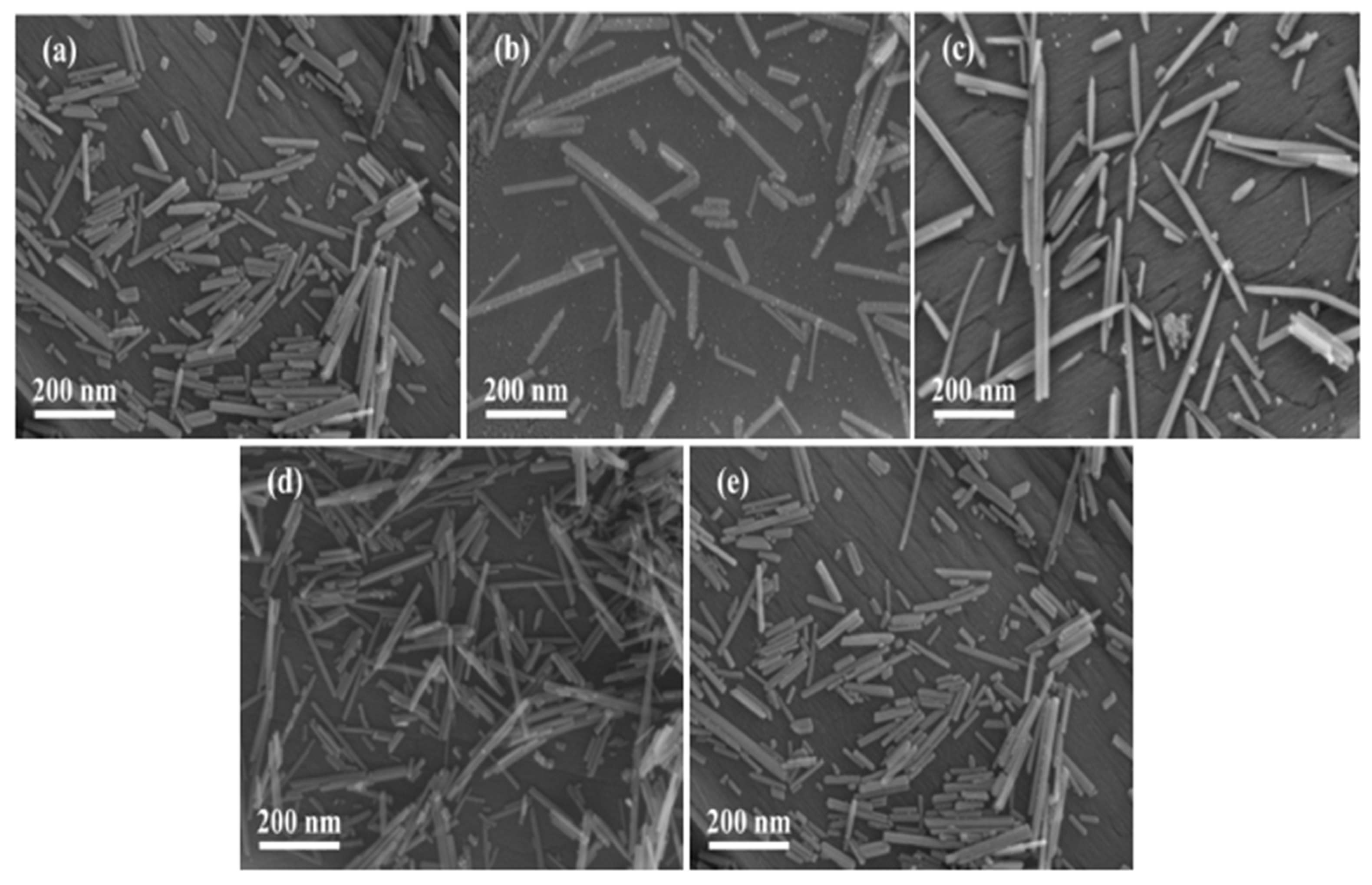
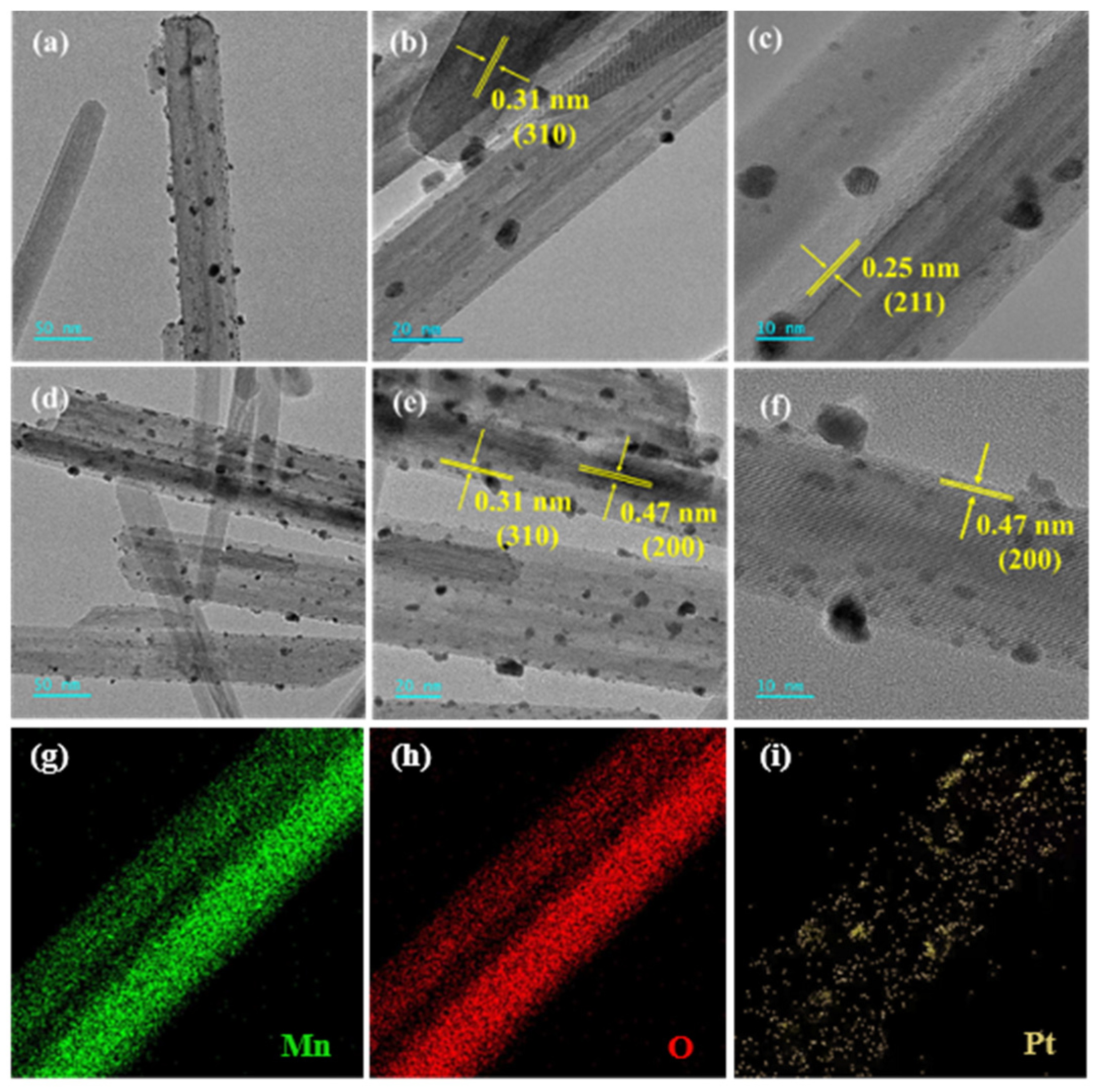
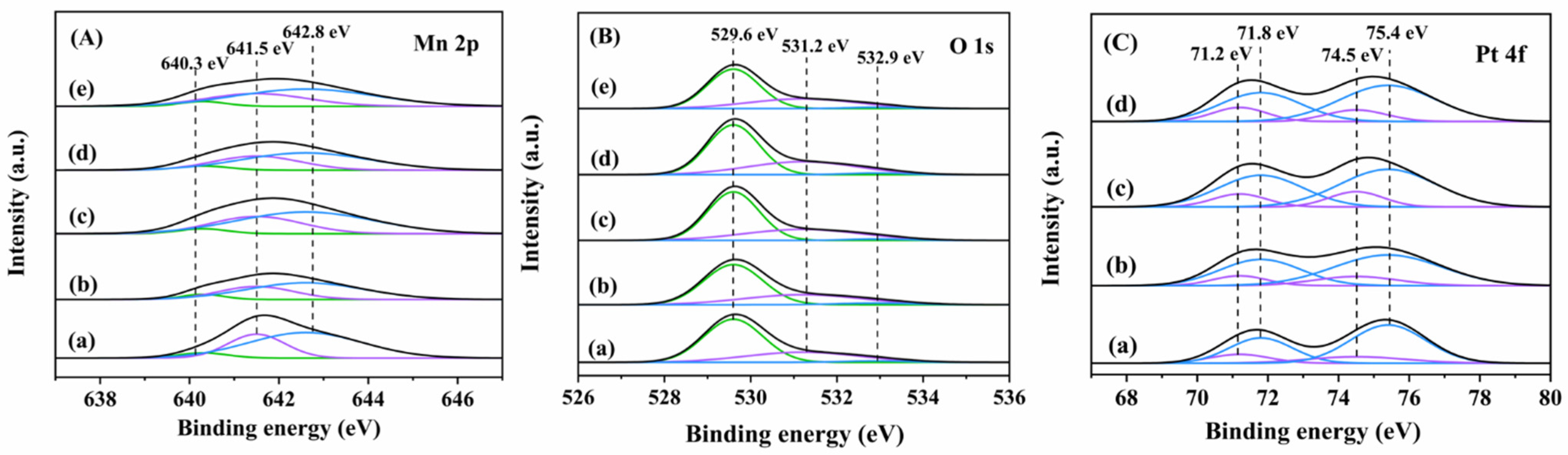
2.2. Catalyst Characterization
2.2.1. Structure and Morphology
2.2.2. Surface Property
2.2.3. Low-Temperature Reducibility and Adsorption Ability
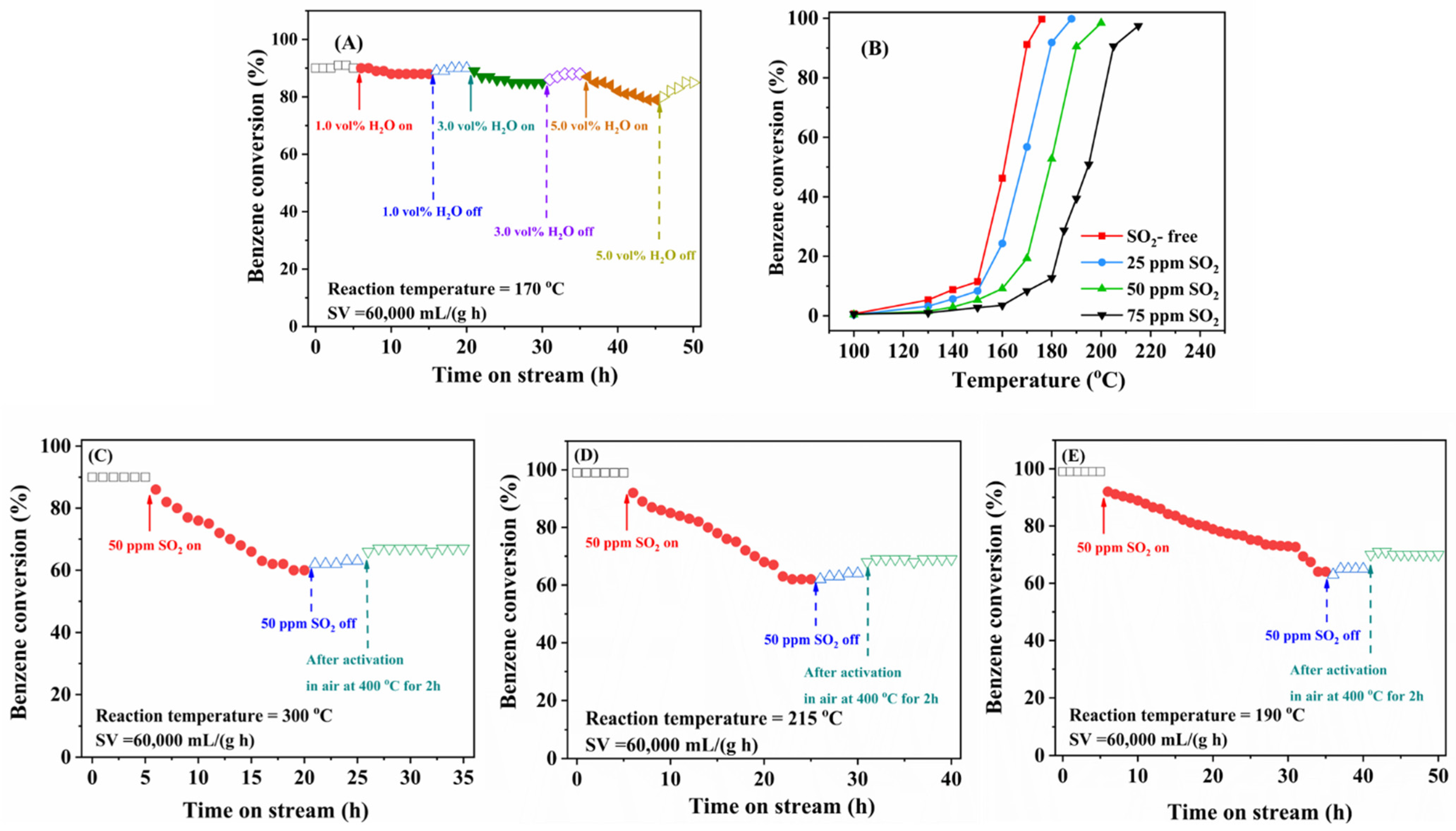
2.3. Effects of Water Vapor and SO2 on Catalytic Activity
2.4. Physicochemical Characterization Analysis of the Catalyst after SO2 Poisoning
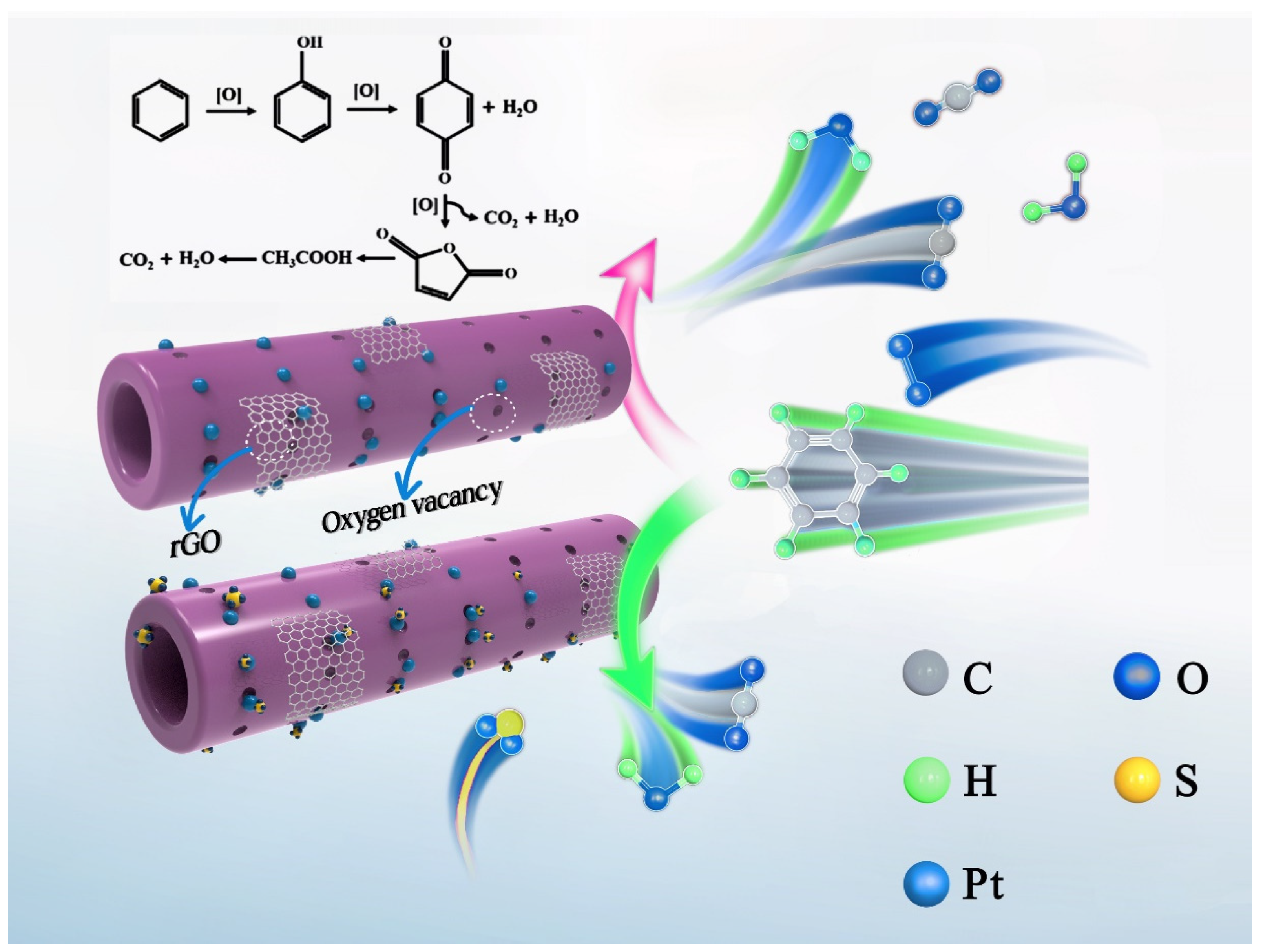
2.5. In Situ DRIFTS Studies
3. Experimental Section
3.1. Preparation of Catalysts
3.1.1. Synthesis of α-MnO2 Nanorods
3.1.2. Preparation of xPt-yrGO/α-MnO2
3.2. Characterization
3.3. Catalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Chai, X.; Xu, L.; Zhang, L.; Ning, P.; Huang, J.; Tian, S. Interaction of inhalable volatile organic compounds and pulmonary surfactant: Potential hazards of VOCs exposure to lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Dong, N.; Ye, Q.; Chen, M.; Cheng, S.; Kang, T.; Dai, H. Sodium-treated sepiolite-supported transition metal (Cu, Fe, Ni, Mn, or Co) catalysts for HCHO oxidation. Chin. J. Catal. 2020, 41, 1734–1744. [Google Scholar] [CrossRef]
- Yi, H.; Song, L.; Tang, X.; Zhao, S.; Yang, Z.; Xie, X.; Ma, C.; Zhang, Y.; Zhang, X. Effect of microwave absorption properties and morphology of manganese dioxide on catalytic oxidation of toluene under microwave irradiation. Ceram. Int. 2020, 46, 3166–3176. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.A.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B 2020, 260, 118150. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Liu, R.; Li, X.; Zhu, T. Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. 2020, 384, 123362. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Zhu, Q.; Li, K.; Zhou, X.; Lu, S.; Fan, Z.; He, L.; Liu, Y.; Pang, F. Efficient formaldehyde elimination over Ag/MnO2 nanorods: Influence of the Ag loading. Catal. Surv. Asia 2019, 23, 33–40. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Yang, J.; Zhao, X.; Han, Z.; Zhang, K.; Lu, Y.; Liu, F.; Dai, H. Carbon monoxide oxidation over rGO-mediated gold/cobalt oxide catalysts with strong metal–support interaction. ACS Appl. Mater. Interfaces 2020, 12, 31467–31476. [Google Scholar] [CrossRef]
- Lu, L.; Tian, H.; He, J.; Yang, Q. Graphene–MnO2 hybrid nanostructure as a new catalyst for formaldehyde oxidation. J. Phys. Chem. C 2016, 120, 23660–23668. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Zhou, C.; Qi, X.; Huang, X.; Yin, Z.; Yang, Y.; Zhang, H. Synthesis of Fe3O4 and Pt nanoparticles on reduced graphene oxide and their use as a recyclable catalyst. Nanoscale 2012, 4, 2478. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, S.; Shigenobu, S.; Hojo, H.; Einaga, H. Catalyst design of Pt/TiO2 microsphere for benzene oxidation under microwave irradiation. Catal. Today 2021, 376, 285–291. [Google Scholar] [CrossRef]
- Wu, B.; Chen, B.; Zhu, X.; Yu, L.; Shi, C. Lower loading of Pt on hydrophobic Ts-1 zeolite: A high-efficiency catalyst for benzene oxidation at low temperature. Catal. Today 2020, 355, 512–517. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Y.; Yang, D.; Dai, J.; Liu, Z.; Chen, Y.; Huang, J.; Li, Q. Biogenic Pt/CaCO3 nanocomposite as a robust catalyst toward benzene oxidation. ACS Appl. Mater. Interfaces 2020, 12, 2469–2480. [Google Scholar] [CrossRef]
- Hao, X.; Dai, L.; Deng, J.; Liu, Y.; Jing, L.; Wang, J.; Pei, W.; Zhang, X.; Hou, Z.; Dai, H. Nanotubular OMS-2 supported single-atom platinum catalysts highly active for benzene oxidation. J. Phys. Chem. C 2021, 125, 17696–17708. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Han, Z.; Hou, Z.; Dai, H. Three-dimensionally ordered mesoporous iron oxide-supported single-atom platinum: Highly active catalysts for benzene combustion. Appl. Catal. B 2019, 244, 650–659. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Chen, Z.; Zuo, S. Mechanism of CeO2 synthesized by thermal decomposition of Ce-MOF and its performance of benzene catalytic combustion. J. Rare Earths 2021, 39, 790–796. [Google Scholar] [CrossRef]
- Mori, K.; Shimada, M.; Horiuchi, Y.; Ohmichi, T.; Nishiyama, N.; Fujii, H.; Yamashita, H. Preparation of nano-sized Pt metal particles by photo-assisted deposition (PAD) on transparent Ti-containing mesoporous silica thin film. Res. Chem. Intermed. 2008, 34, 495–505. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, L.; Liu, Y.; Deng, J.; Jing, L.; Zhang, K.; Hou, Z.; Zhang, X.; Wang, J.; Feng, Y.; et al. Insights into the active sites of chlorine-resistant Pt-based bimetallic catalysts for benzene oxidation. Appl. Catal. B 2020, 279, 119372. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, J.; Zhou, R. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature. Appl. Surf. Sci. 2019, 465, 15–22. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Liu, Y.; Yang, T.; Deng, J.; Dai, H. Concurrent catalytic removal of typical volatile organic compound mixtures over Au–Pd/α-MnO2 nanotubes. J. Environ. Sci. 2018, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, J.; Zhang, C.; Jin, B.; Men, Y. Boosting acetone oxidation efficiency over MnO2 nanorods by tailoring crystal phases. New J. Chem. 2019, 43, 19126–19136. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, G.; Wang, M.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt/MnO2 nanoflowers anchored to boron nitride aerogels for highly efficient enrichment and catalytic oxidation of formaldehyde at room temperature. Angew. Chem. Int. Ed. 2021, 60, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Zhai, C.; Ren, F.; Zhu, M.; Yang, P.; Du, Y. Three-dimensional Au0.5/reduced graphene oxide/carbon fiber electrode and its high catalytic performance toward ethanol electrooxidation in alkaline media. J. Mater. Chem. A 2015, 3, 4389–4398. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Li, J.; Qu, Z. Effect of Ag on toluene oxidation over Ag supported wire-like MnO2 catalysts. Surf. Interf. 2020, 21, 100657. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Li, R.; Deng, W.; Hong, C.; Liu, D.; Guo, L. Ag-doped δ-MnO2 nanosheets as robust catalysts for toluene combustion. ACS Appl. Nano Mater. 2020, 3, 11869–11880. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, Q.; Dong, Y.; Zheng, Y.; Wang, X.; Li, K.; Huang, F.; Peng, B.; Chen, Y. Influence of MnO2 morphology on the catalytic performance of Ag/MnO2 for the HCHO oxidation. Catal. Surv. Asia 2019, 23, 210–218. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Luo, M.; Lu, J. Remarkable enhancement of dichloromethane oxidation over potassium-promoted Pt/Al2O3 catalysts. J. Catal. 2014, 311, 314–324. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Deng, W.; Han, J.; Qin, L.; Zhao, B.; Guo, L.; Xing, F. Study on the structure-activity relationship of Fe-Mn oxide catalysts for chlorobenzene catalytic combustion. Chem. Eng. J. 2020, 395, 125172. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yang, Z.; Yue, L.; Huang, L. Graphene oxide/Fe2O3 nanoplates supported Pt for enhanced room-temperature oxidation of formaldehyde. Appl. Surf. Sci. 2019, 467–468, 277–285. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Sun, P.; Long, Y.; Long, Y.; Cao, S.; Weng, X.; Wu, Z. Deactivation effects of Pb(ii) and sulfur dioxide on a γ-MnO2 catalyst for combustion of chlorobenzene. J. Colloid Interf. Sci. 2020, 559, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Li, B.; Niu, Q.; Li, L.; Kan, J.W.; Zhu, S.M.; Shen, S.B. Combined promoting effects of low-Pd-containing and Cu-doped LaCoO3 perovskite supported on cordierite for the catalytic combustion of benzene. Environ. Sci. Pollut. Res. 2016, 23, 15193–15201. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhao, S.; Yu, J.; Jin, X.; Ye, D.; Yang, S.; Qiu, Y. Effect of absorbed sulfate poisoning on the performance of catalytic oxidation of VOCs over MnO2. ACS Appl. Mater. Interfaces 2020, 12, 50566–50572. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. SO2 adsorption and desorption characteristics of Pd and Pt catalysts: Precious metal crystallite size dependence. Appl. Catal. A 2017, 534, 85–93. [Google Scholar] [CrossRef]
- Li, J.; Cai, S.; Yu, E.; Weng, B.; Chen, X.; Chen, J.; Jia, H.; Xu, Y. Efficient infrared light promoted degradation of volatile organic compounds over photo-thermal responsive Pt-rGO-TiO2 composites. Appl. Catal. B 2018, 233, 260–271. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, P.; Liu, F.; Yang, Y. Engineering crystal facet of α-MnO2 nanowire for highly efficient catalytic oxidation of carcinogenic airborne formaldehyde. ACS Catal. 2018, 8, 3435–3446. [Google Scholar] [CrossRef]
- Wu, X.; Lee, H.; Liu, S.; Weng, D. Sulfur poisoning and regeneration of MnOx-CeO2-Al2O3 catalyst for soot oxidation. J. Rare Earths 2012, 30, 659–664. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, T.; Luo, Y.; Lan, Z.; Zhang, K.; Zuo, J.; Jiang, L.; Wang, R. Geometrical-site-dependent catalytic activity of ordered mesoporous Co-based spinel for benzene oxidation: In situ Drifts study coupled with Raman and EXAFS spectroscopy. ACS Catal. 2017, 7, 1626–1636. [Google Scholar] [CrossRef]
- Sivasankar, N.; Vasudevan, S. Temperature-programmed desorption and infrared spectroscopic studies of benzene adsorption in zeolite ZSM-5. J. Phys. Chem. B 2004, 108, 11585–11590. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Wu, X.; Zhang, T.; Liu, Y.; Zhang, K.; Xiao, Y.; Jiang, L. Total oxidation of benzene over ACo2O4 (A = Cu, Ni and Mn) catalysts: In situ drifts account for understanding the reaction mechanism. Appl. Surf. Sci. 2017, 426, 1198–1205. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, M.; Yang, X.; Yu, X.; Ge, M. Boosting benzene combustion by engineering oxygen vacancy-mediated Ag/CeO2-Co3O4 catalyst via interfacial electron transfer. J. Colloid Interf. Sci. 2021, 594, 882–890. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.; Chen, Y.; Du, Z.; Guo, Y.; Huang, J.; Li, Q. Waste eggshells to valuable CO3O4/CaCO3 materials as efficient catalysts for VOCs oxidation. Mol. Catal. 2020, 483, 110766. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, D.; Wang, Y.; Zheng, Z.; Li, K.; Zhang, Y.; Zhan, R.; Lin, H. Electric field assisted benzene oxidation over Pt-Ce-Zr nano-catalysts at low temperature. J. Hazard. Mater. 2021, 407, 124349. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Wang, D.; Peng, Y.; Yan, J.; Li, J.; Crittenden, J. Facile synthesis λ-MnO2 spinel for highly effective catalytic oxidation of benzene. Chem. Eng. J. 2021, 421, 127828. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Liu, R.; Xie, S.; Liu, Y.; Dai, H.; Huang, H.; Deng, J. Probing toluene catalytic removal mechanism over supported Pt nano- and single-atom-catalyst. J. Hazard. Mater. 2020, 392, 122258. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Jiang, Y.; Lv, H.; Pan, M.; Mu, S. Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl. Catal. B 2013, 132–133, 379–388. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J. Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation. Appl. Catal. B 2017, 203, 416–427. [Google Scholar] [CrossRef]





| Sample | Benzene Oxidation Activity | Ea (kJ/mol) | TOFPt (×10−2 s−1) | rcat (mol/(g s)) | ||
|---|---|---|---|---|---|---|
| T10% (°C) | T50% (°C) | T90% (°C) | ||||
| α-MnO2 | 240 | 275 | 300 | 96.1 | − | 1.91 × 10−5 |
| 0.93Pt/α-MnO2 | 170 | 190 | 205 | 67.0 | 0.28 | 5.11 × 10−5 |
| 0.91Pt-0.5rGO/α-MnO2 | 158 | 176 | 188 | 58.4 | 0.47 | 8.18 × 10−5 |
| 0.94Pt-1.0rGO/α-MnO2 | 149 | 163 | 169 | 51.3 | 1.54 | 3.14 × 10−4 |
| 0.92Pt-2.0rGO/α-MnO2 | 152 | 168 | 178 | 54.3 | 0.85 | 1.84 × 10−4 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Da (nm) |
|---|---|---|---|---|
| α-MnO2 | 36.6 | 0.067 | 8.9 | 27.2 |
| 0.93Pt/α-MnO2 | 37.1 | 0.060 | 83 | 27.9 |
| 0.91Pt-0.5rGO/α-MnO2 | 37.9 | 0.071 | 9.0 | 28.7 |
| 0.94Pt-1.0rGO/α-MnO2 | 38.7 | 0.077 | 8.5 | 29.1 |
| 0.92Pt-2.0rGO/α-MnO2 | 40.7 | 0.087 | 8.1 | 33.9 |
| Sample | Surface Element Composition (Molar Ratio) | H2 Consumption (mmol/g) | |||||
|---|---|---|---|---|---|---|---|
| Mn3+/Mn4+ | Pt2+/Pt0 | Oads/Olatt | α | β | γ | Total | |
| α-MnO2 | 0.48 | – | 0.45 | 16.3 | 18.5 | 6.7 | 41.5 |
| 0.93Pt/α-MnO2 | 0.50 | 0.50 | 0.50 | 20.9 | 14.3 | 8.6 | 43.8 |
| 0.91Pt-0.5rGO/α-MnO2 | 0.55 | 0.57 | 0.53 | 21.1 | 13.1 | 9.9 | 44.1 |
| 0.94Pt-1.0rGO/α-MnO2 | 0.60 | 0.61 | 0.57 | 23.3 | 14.2 | 9.8 | 47.3 |
| 0.92Pt-2.0rGO/α-MnO2 | 0.58 | 0.59 | 0.55 | 29.8 | 12.8 | 10.5 | 46.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Ye, Q.; Dong, N.; Wang, W.; Xiao, Y.; Dai, H. Enhanced Catalytic Performance and Sulfur Dioxide Resistance of Reduced Graphene Oxide-Promoted MnO2 Nanorods-Supported Pt Nanoparticles for Benzene Oxidation. Catalysts 2022, 12, 1426. https://doi.org/10.3390/catal12111426
Zhang D, Ye Q, Dong N, Wang W, Xiao Y, Dai H. Enhanced Catalytic Performance and Sulfur Dioxide Resistance of Reduced Graphene Oxide-Promoted MnO2 Nanorods-Supported Pt Nanoparticles for Benzene Oxidation. Catalysts. 2022; 12(11):1426. https://doi.org/10.3390/catal12111426
Chicago/Turabian StyleZhang, Dan, Qing Ye, Ning Dong, Wenjin Wang, Yang Xiao, and Hongxing Dai. 2022. "Enhanced Catalytic Performance and Sulfur Dioxide Resistance of Reduced Graphene Oxide-Promoted MnO2 Nanorods-Supported Pt Nanoparticles for Benzene Oxidation" Catalysts 12, no. 11: 1426. https://doi.org/10.3390/catal12111426






