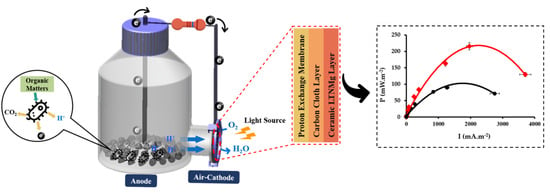
Bioenergy Generation and Wastewater Purification with Li0.95Ta0.76Nb0.19Mg0.15O3 as New Air-Photocathode for MFCs
Abstract
:1. Introduction
2. Results
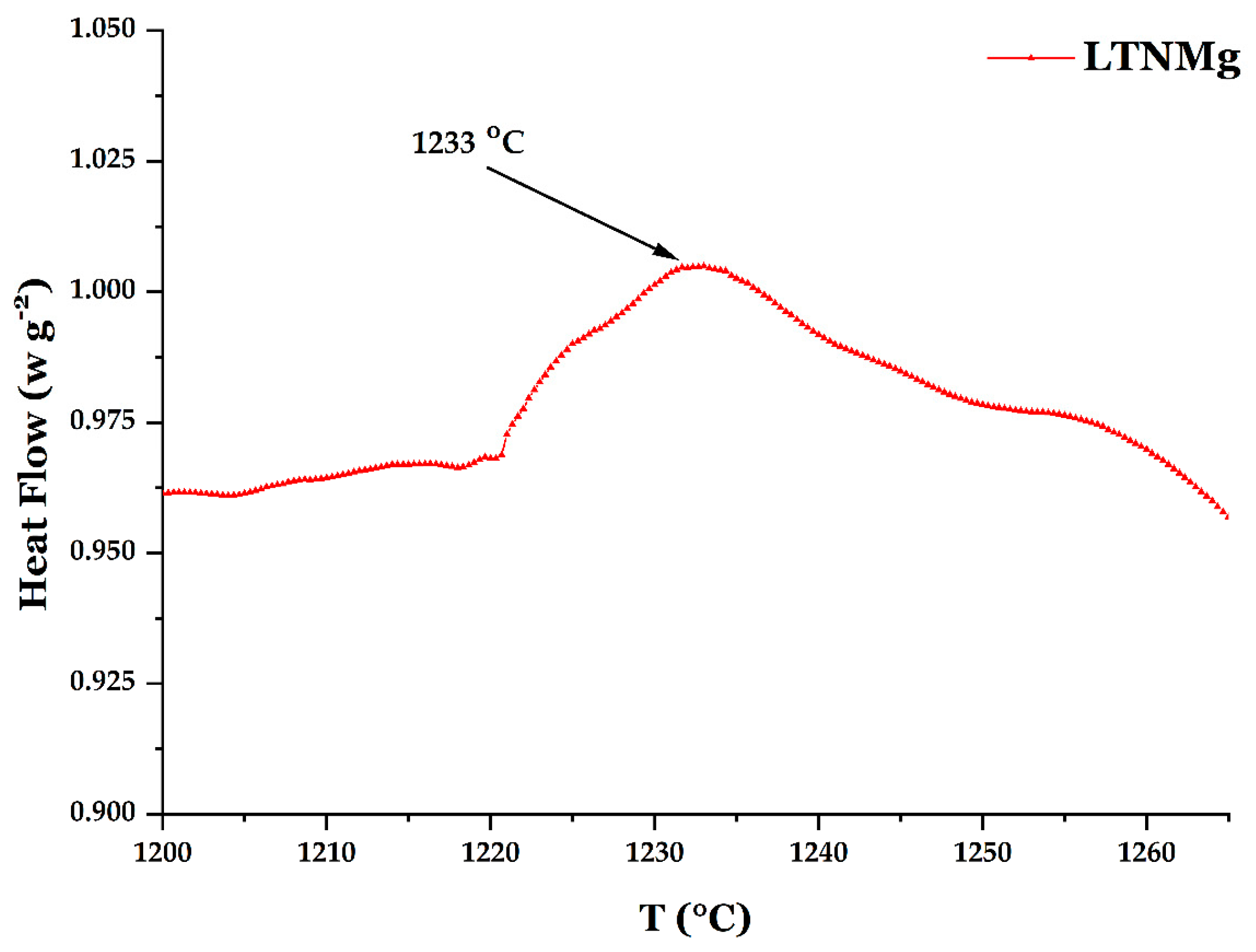
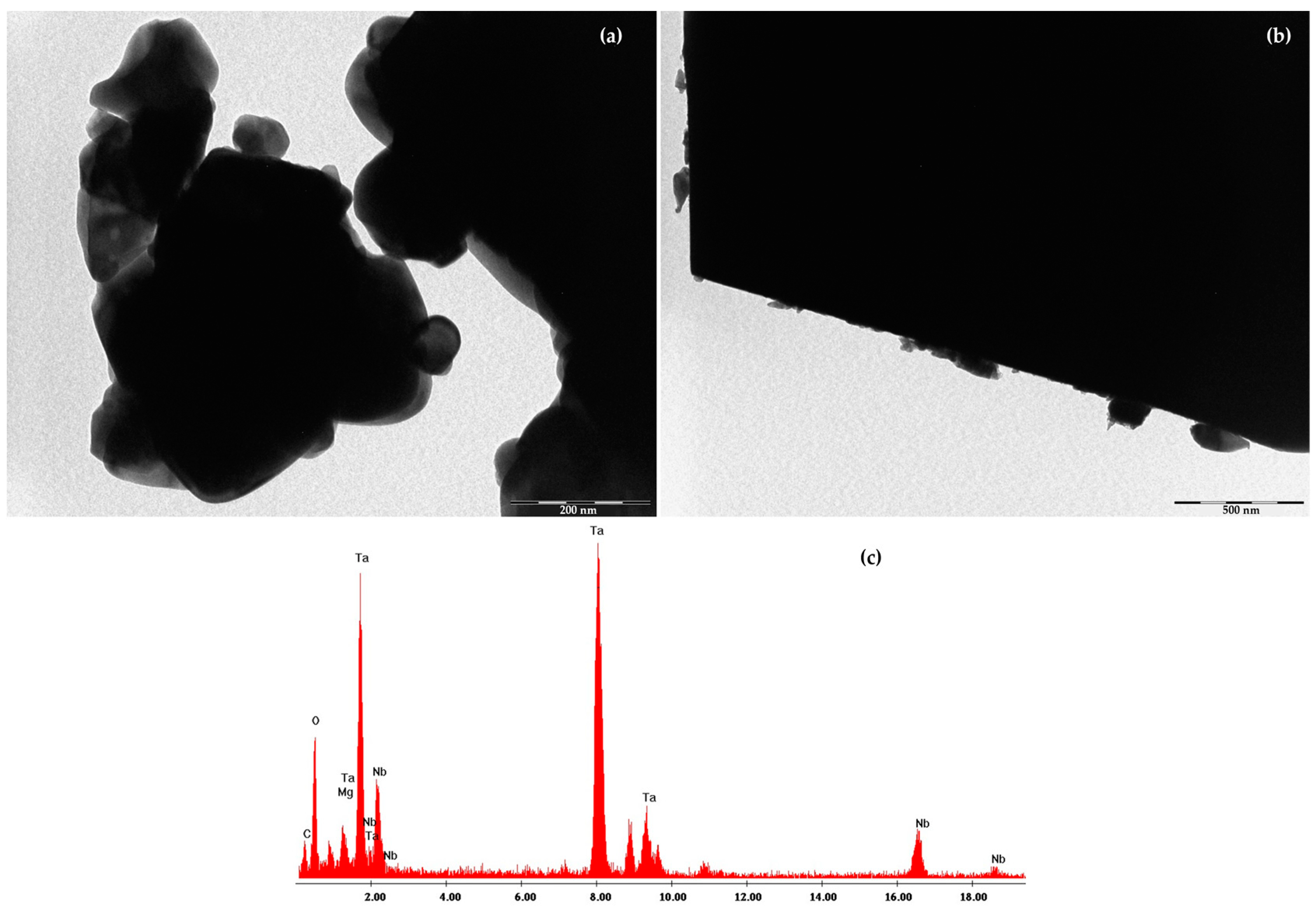
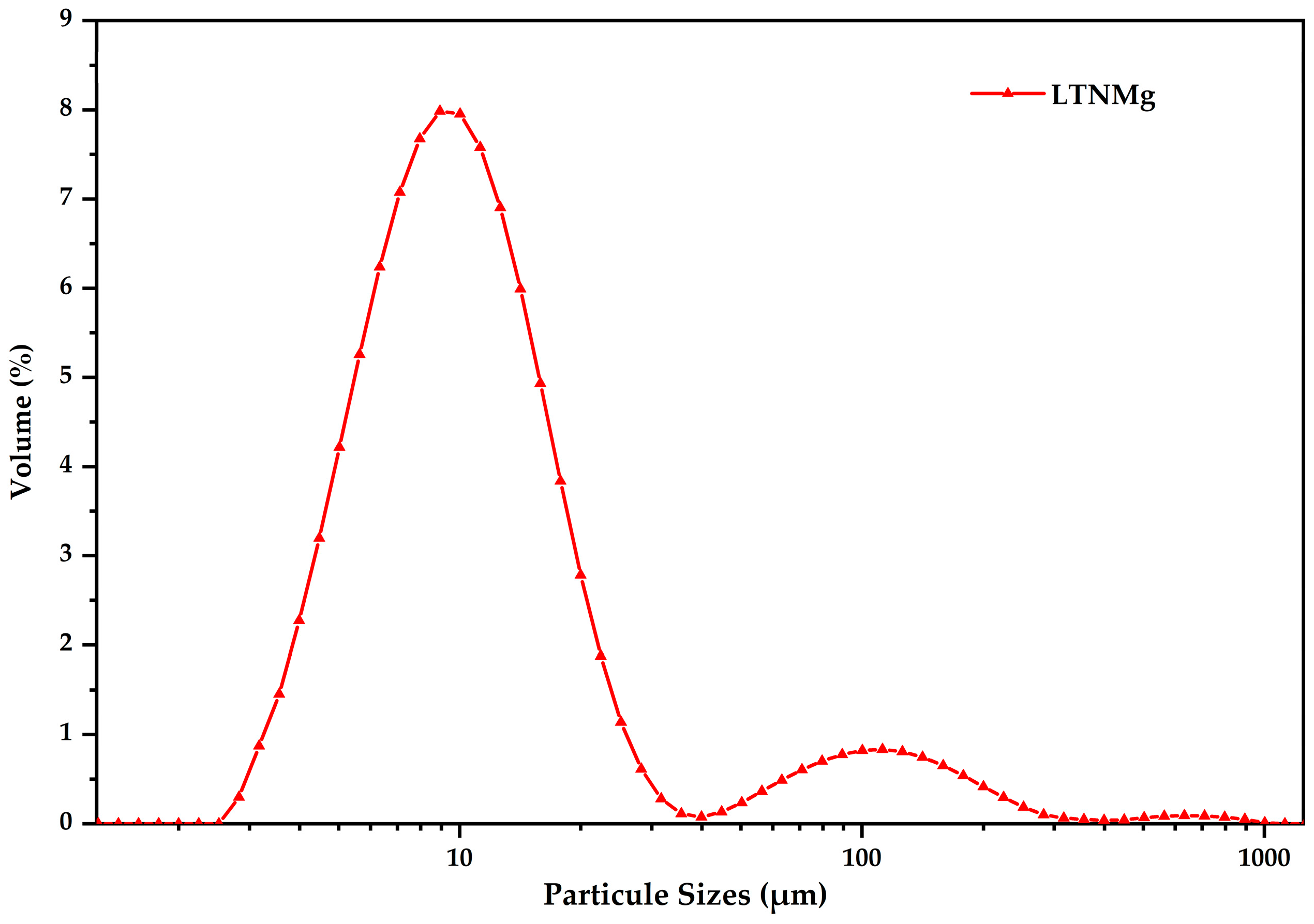
2.1. Cathodic Photocatalyst Characterization
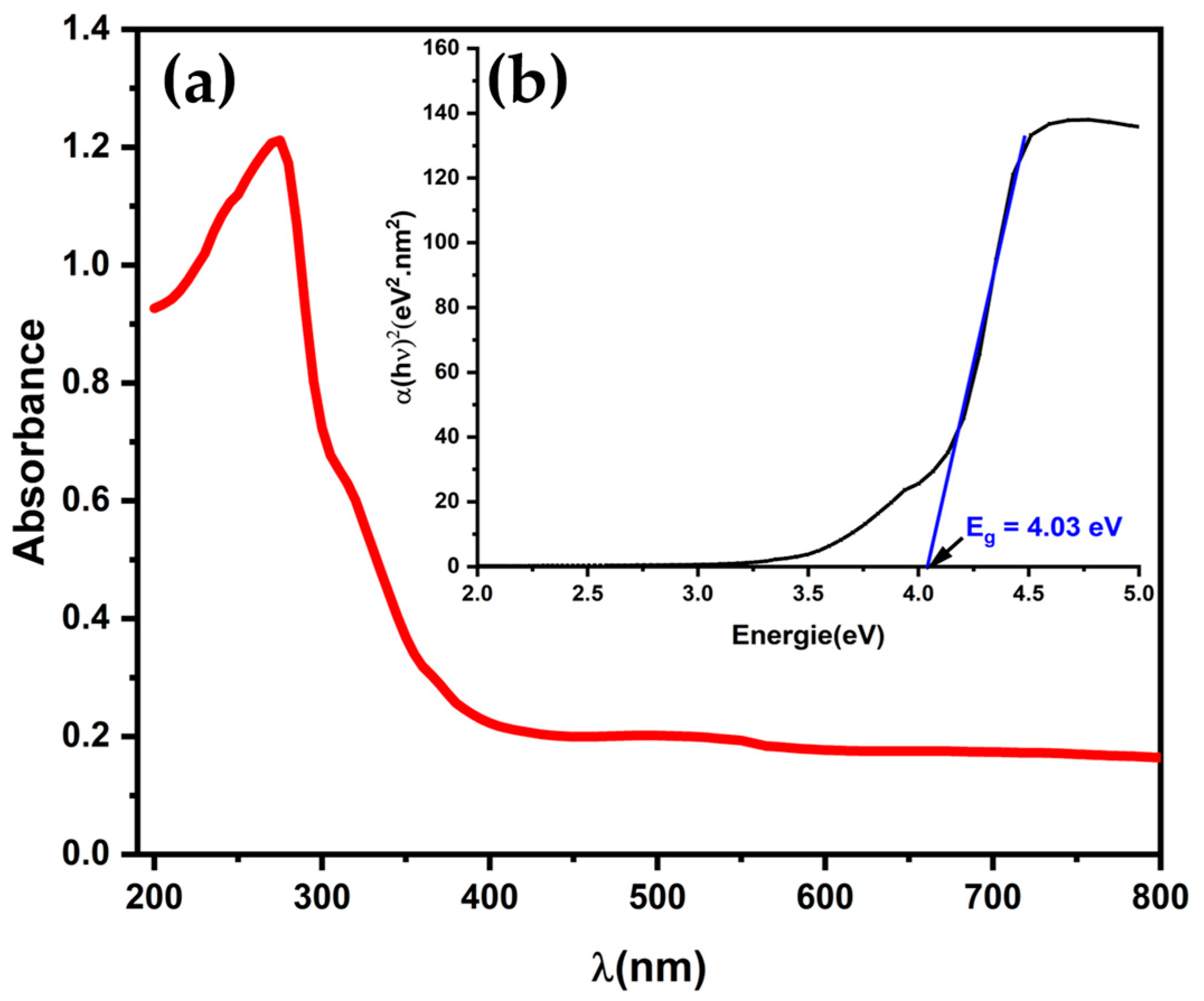
2.2. Optical Properties
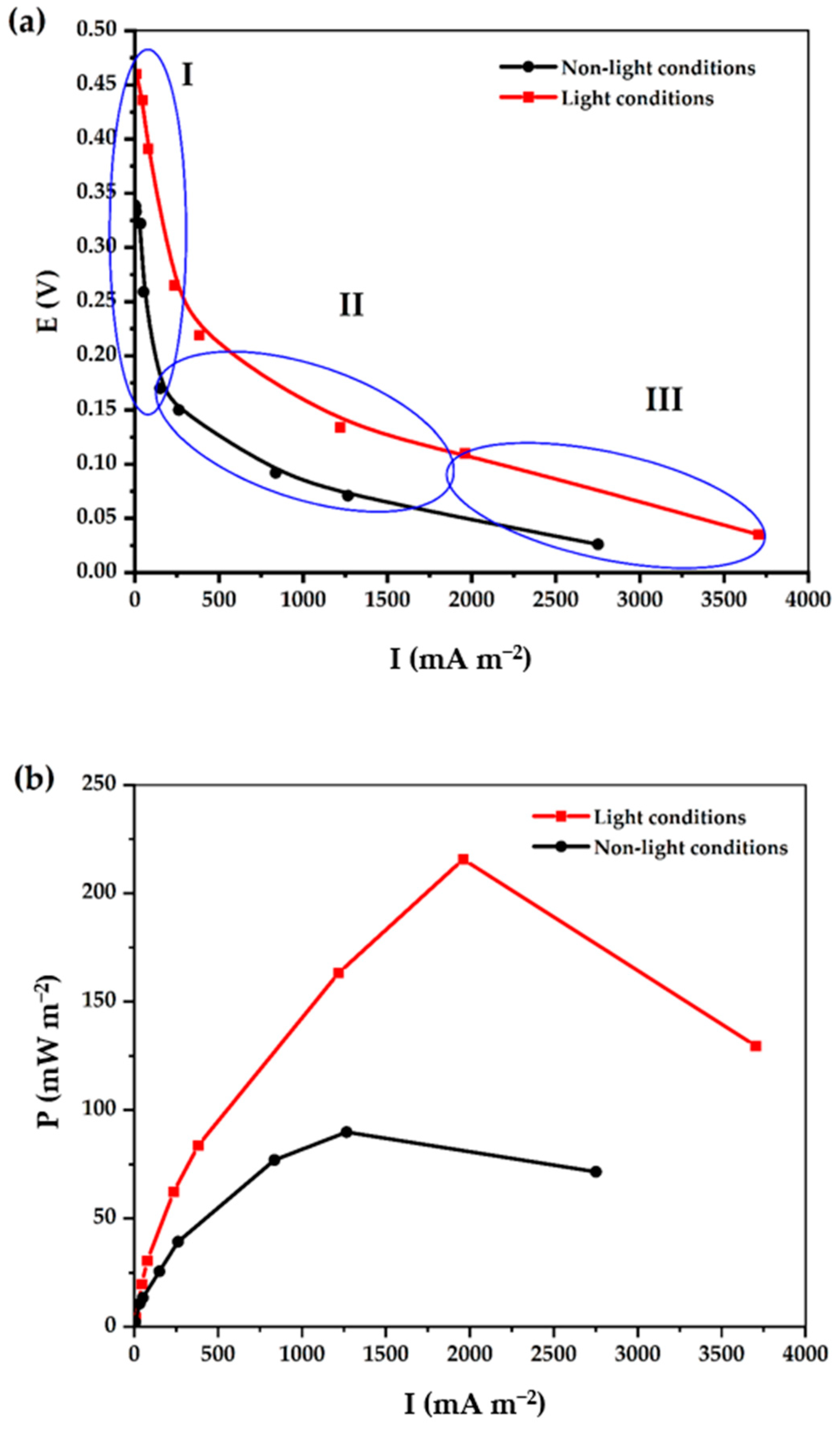
2.3. Power Performance in Air-Cathode MFCs
2.4. Wastewater Treatment by Air-Cathode MFCs
3. Materials and Methods
3.1. Catalyst Preparation and Crystalline Structure Characterization
3.2. UV-Visible Absorption Spectroscopy
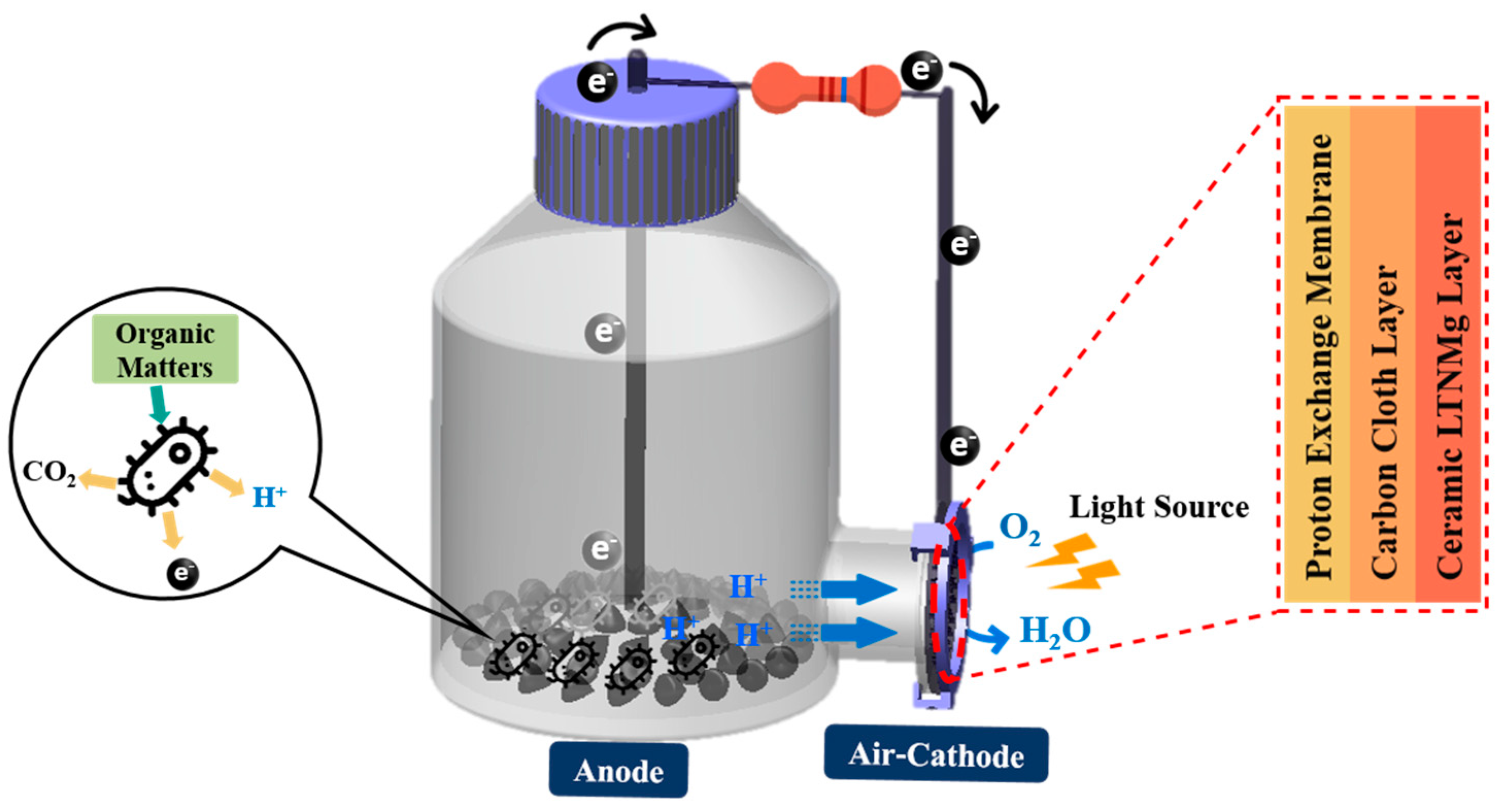
Air-Cathode MFC Setup and Operation
3.3. Electrochemical Characterization and Analysis
3.4. COD Removal Efficiency and Heavy Metals’ Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the Role of Microbial Fuel Cells in Remediation of Environmental Pollutants with Electricity Generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Yang, S.; Jia, B.; Liu, H. Effects of the Pt loading side and cathode-biofilm on the performance of a membrane-less and single-chamber microbial fuel cell. Bioresour. Technol. 2009, 100, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Wang, R.; Yang, Y.; Liu, Y. Design and research progress of nano materials in cathode catalysts of microbial fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 18098–18108. [Google Scholar] [CrossRef]
- Qiu, S.; Guo, Z.; Naz, F.; Yang, Z.; Yu, C. An overview in the development of cathode materials for the improvement in power generation of microbial fuel cells. Bioelectrochemistry 2021, 141, 107834. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Shen, J. Iron-gelatin aerogel derivative as high-performance oxygen reduction reaction electrocatalysts in microbial fuel cells. Int. J. Hydrogen Energy 2022, 47, 17982–17991. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Jin, S.; Ding, H.; Zeng, C.; Wang, X.; Wang, C. Microbial Fuel Cell Equipped with a Photocatalytic Rutile-Coated Cathode. Energy Fuels 2010, 24, 1184–1190. [Google Scholar] [CrossRef]
- Qian, F.; Wang, G.; Li, Y. Solar-Driven Microbial Photoelectrochemical Cells with a Nanowire Photocathode. Nano Lett. 2010, 10, 4686–4691. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, X.; Liu, J.; Wang, L.; Lu, Z.; Li, L.; Sun, L. Visible Light Driven Water Splitting in a Molecular Device with Unprecedentedly High Photocurrent Density. J. Am. Chem. Soc. 2013, 135, 4219–4222. [Google Scholar] [CrossRef]
- Ma, J.; Chen, D.; Zhang, W.; An, Z.; Zeng, K.; Yuan, M.; Shen, J. Enhanced performance and degradation of wastewater in microbial fuel cells using titanium dioxide nanowire photocathodes. RSC Adv. 2021, 11, 2242–2252. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Min, B.-K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light-induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaham-Waldmann, N.; Paz, Y. Away from TiO2: A critical minireview on the developing of new photocatalysts for degradation of contaminants in water. Mater. Sci. Semicond. Process. 2016, 42, 72–80. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced design and synthesis of composite photocatalysts for the remediation of wastewater: A review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.K.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Dunn, S. Effect of Ferroelectricity on Solar-Light-Driven Photocatalytic Activity of BaTiO3—Influence on the Carrier Separation and Stern Layer Formation. Chem. Mater. 2013, 25, 4215–4223. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Park, S.-J. Recent Advances in Carbonaceous Photocatalysts with Enhanced Photocatalytic Performances: A Mini Review. Materials 2019, 12, 1916. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Sharma, M.; Bowen, C.; Vaish, R. 12—Ferroelectric ceramics and glass ceramics for photocatalysis. In Ceramic Science and Engineering; Misra, K.P., Misra, R.D.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–322. [Google Scholar]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A review of advances in multifunctional XTiO3 perovskite-type oxides as piezo-photocatalysts for environmental remediation and energy production. J. Hazard. Mater. 2022, 421, 126792. [Google Scholar] [CrossRef]
- Miller, R.C.; Savage, A. Temperature dependence of the optical properties of ferroelectric LiNbO3 and LiTaO3. Appl. Phys. Lett. 1966, 9, 169–171. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Kurtz, S.K.; Jamieson, P.B. Atomic Displacement Relationship to Curie Temperature and Spontaneous Polarization in Displacive Ferroelectrics. Phys. Rev. 1968, 172, 551–553. [Google Scholar] [CrossRef]
- Benzaouak, A.; Ellouzi, I.; Ouanji, F.; Touach, N.; Kacimi, M.; Ziyad, M.; El Mahi, M.; Lotfi, E.M. Photocatalytic degradation of Methylene Blue (MB) dye in aqueous solution by ferroelectric Li1-xTa1-xWxO3 materials. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 586–592. [Google Scholar] [CrossRef]
- Touach, N.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Benzaouak, A.; Hernández-Fernández, F.P.; de Ríos, A.; El Mahi, M.; Lotfi, E.M. On the use of ferroelectric material LiNbO3 as novel photocatalyst in wastewater-fed microbial fuel cells. Particuology 2017, 34, 147–155. [Google Scholar] [CrossRef]
- Khan, M.A.; Nadeem, M.A.; Idriss, H. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surf. Sci. Rep. 2016, 71, 1–31. [Google Scholar] [CrossRef]
- Stock, M.; Dunn, S. Influence of the Ferroelectric Nature of Lithium Niobate to Drive Photocatalytic Dye Decolorization under Artificial Solar Light. J. Phys. Chem. C 2012, 116, 20854–20859. [Google Scholar] [CrossRef]
- Louki, S.; Touach, N.E.; Benzaouak, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; El Mahi, M.; Lotfi, E.M. Preparation of new ferroelectric Li0.95Ta0.57Nb0.38Cu0.15O3 materials as photocatalysts in microbial fuel cells. Can. J. Chem. Eng. 2018, 96, 1656–1662. [Google Scholar] [CrossRef]
- Reichenbach, P.; Kämpfe, T.; Thiessen, A.; Schröder, M.; Haußmann, A.; Woike, T.; Eng, L. Multiphoton-induced luminescence contrast between antiparallel ferroelectric domains in Mg-doped LiNbO3. J. Appl. Phys. 2014, 115, 213509. [Google Scholar] [CrossRef]
- Reichenbach, P.; Kämpfe, T.; Thiessen, A.; Haußmann, A.; Woike, T.; Eng, L. Multiphoton photoluminescence contrast in switched Mg: LiNbO3 and Mg: LiTaO3 single crystals. Appl. Phys. Lett. 2014, 105, 122906. [Google Scholar] [CrossRef]
- Benzaouak, A.; Touach, N.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Hernández-Fernández, F.J.; Perez de los Rios, A.; Mahi, M.E.; Lotfi, E.M. Ferroelectric LiTaO3 as novel photo-electrocatalyst in microbial fuel cells. Environ. Prog. Sustain. Energy 2017, 36, 1568–1574. [Google Scholar] [CrossRef]
- Kamali, A.R.; Fray, D.J. Preparation of lithium niobate particles via reactive molten salt synthesis method. Ceram. Int. 2014, 40, 1835–1841. [Google Scholar] [CrossRef]
- Louki, S.; Touach, N.; Benzaouak, A.; Ortiz-Martínez, V.; Salar-García, M.; Hernández-Fernández, F.; de los Ríos, A.; El Mahi, M.; Lotfi, E. Characterization of New Nonstoichiometric Ferroelectric (Li0.95Cu0.15) Ta0.76Nb0.19O3 and Comparative Study With (Li0.95Cu0.15) Ta0.57Nb0.38O3 as Photocatalysts in Microbial Fuel Cells. J. Electrochem. Energy Convers. Storage 2019, 16, 021009. [Google Scholar] [CrossRef]
- Choudhury, P.; Bhunia, B.; Mahata, N.; Bandyopadhyay, T.K. Optimization for the improvement of power in equal volume of single chamber microbial fuel cell using dairy wastewater. J. Indian Chem. Soc. 2022, 99, 100489. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Dominguez-Benetton, X.; Pant, D. Internal resistance of microfluidic microbial fuel cell: Challenges and potential opportunities. Bioresour. Technol. 2013, 142, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Radeef, A.Y.; Ismail, Z.Z. Polarization model of microbial fuel cell for treatment of actual potato chips processing wastewater associated with power generation. J. Electroanal. Chem. 2019, 836, 176–181. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.; Zhou, X.; Hu, S.; Hong, Y.; Wang, B.; Yao, A.; Li, W.; Chu, B.; He, Q.; Cheng, J.; et al. Ferroelectric K0.5Na0.5NbO3 catalysts for dye wastewater degradation. Ceram. Int. 2021, 47, 28797–28805. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, S.; Guo, S.; Wan, D.; Xu, H.; Yan, W.; Jin, X.; Feng, J. New insights in light-assisted microbial fuel cells for wastewater treatment and power generation: A win-win cooperation. J. Power Sources 2021, 501, 230000. [Google Scholar] [CrossRef]
- Benzaouak, A.; Touach, N.-E.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Hernández-Fernández, F.; de los Ríos, A.P.; Mahi, M.E.; Lotfi, E.M. Ferroelectric solid solution Li1−xTa1−xWxO3 as potential photocatalysts in microbial fuel cells: Effect of the W content. Chin. J. Chem. Eng. 2018, 26, 1985–1991. [Google Scholar] [CrossRef]
- Wang, X.; Yan, W.; Zhang, Y.; Zhang, L.; Shi, L.; Huang, Y.; Wu, M.; Wang, X.; Chen, H. Threshold effect in the Mg-doping dependence of the photocatalytic ability of LiNbO3 nanoparticles. J. Am. Ceram. Soc. 2017, 100, 739–745. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, T.; Chen, Y.; Ma, H.; Wang, Y.; Liu, W.; Wang, Y.; Zhou, G.; Qing, R.; Zhao, Y.; et al. Integrated chamber-free microbial fuel cell for wastewater purification and bioenergy generation. Chem. Eng. J. 2022, 442, 136091. [Google Scholar] [CrossRef]
- Noori, M.T.; Thatikayala, D.; Pant, D.; Min, B. A critical review on microbe-electrode interactions towards heavy metal ion detection using microbial fuel cell technology. Bioresour. Technol. 2022, 347, 126589. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, W.; Huang, S.; Wang, H.; Cao, X.; Li, X.; Sakamaki, T. Application of microbial fuel cell technology to the remediation of compound heavy metal contamination in soil. J. Environ. Manag. 2022, 320, 115670. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, X.; Zha, Z.; Cai, F.; Zhou, Y.; Zhou, S.; Yao, J.; Zhang, Z. Long-time enrofloxacin processing with microbial fuel cells and the influence of coexisting heavy metals (Cu and Zn). J. Environ. Chem. Eng. 2022, 10, 107965. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. Biomass-derived composite anode electrode: Synthesis, characterizations, and application in microbial fuel cells (MFCs). J. Environ. Chem. Eng. 2021, 9, 106111. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Mateo-Ramírez, F.; Godínez, C.; Lozano-Blanco, L.J.; Moreno, J.I.; Tomás-Alonso, F. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 2015, 279, 115–119. [Google Scholar] [CrossRef]
- Larrosa-Guerrero, A.; Scott, K.; Head, I.M.; Mateo, F.; Ginesta, A.; Godinez, C. Effect of temperature on the performance of microbial fuel cells. Fuel 2010, 89, 3985–3994. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]



| Light Source | CODr after 168 h (%) | Power Density, Pmax (mW m−2) | OCV (v) | Rint (Ω) |
|---|---|---|---|---|
| Without | 83.0 | 109 | 340 | 311 |
| With | 95.74 | 228 | 470 | 411 |
| λmax at Maximum Absorption (nm) | Eg (eV) | CODr (mg L−1) | P (mW m−2) | OCV (mV) | Rint (Ω) | Specific Surface Area, m2 g−1 | References | |
|---|---|---|---|---|---|---|---|---|
| LTNMg | 330 | 4.03 | 95.7 | 228 | 460 | 228 | 4.06 | This work |
| LTNCu | 311 | 3.46 | 93 | 200 | 383 | 202 | - | [30] |
| LTW | 270 | 3.85 | 79 | 107 | 316 | 877 | 5.57 | [37] |
| Metal Species | Initial Concentrations in Wastewater (ppb) | Removal Efficiencies of Heavy Metals (%) | |
|---|---|---|---|
| Light Conditions | Non-Light Conditions | ||
| 63Cu | 9.250 | 96.92 | 48.00 |
| 111Cd | 0.390 | 17.29 | 11.10 |
| 56Fe | 35.153 | 89.12 | 31.23 |
| 52Cr | 0.530 | 55.54 | 9.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touach, N.; Benzaouak, A.; Toyir, J.; El Hamidi, A.; El Mahi, M.; Lotfi, E.M.; Kacimi, M.; Liotta, L.F. Bioenergy Generation and Wastewater Purification with Li0.95Ta0.76Nb0.19Mg0.15O3 as New Air-Photocathode for MFCs. Catalysts 2022, 12, 1424. https://doi.org/10.3390/catal12111424
Touach N, Benzaouak A, Toyir J, El Hamidi A, El Mahi M, Lotfi EM, Kacimi M, Liotta LF. Bioenergy Generation and Wastewater Purification with Li0.95Ta0.76Nb0.19Mg0.15O3 as New Air-Photocathode for MFCs. Catalysts. 2022; 12(11):1424. https://doi.org/10.3390/catal12111424
Chicago/Turabian StyleTouach, Noureddine, Abdellah Benzaouak, Jamil Toyir, Adnane El Hamidi, Mohammed El Mahi, El Mostapha Lotfi, Mohamed Kacimi, and Leonarda Francesca Liotta. 2022. "Bioenergy Generation and Wastewater Purification with Li0.95Ta0.76Nb0.19Mg0.15O3 as New Air-Photocathode for MFCs" Catalysts 12, no. 11: 1424. https://doi.org/10.3390/catal12111424