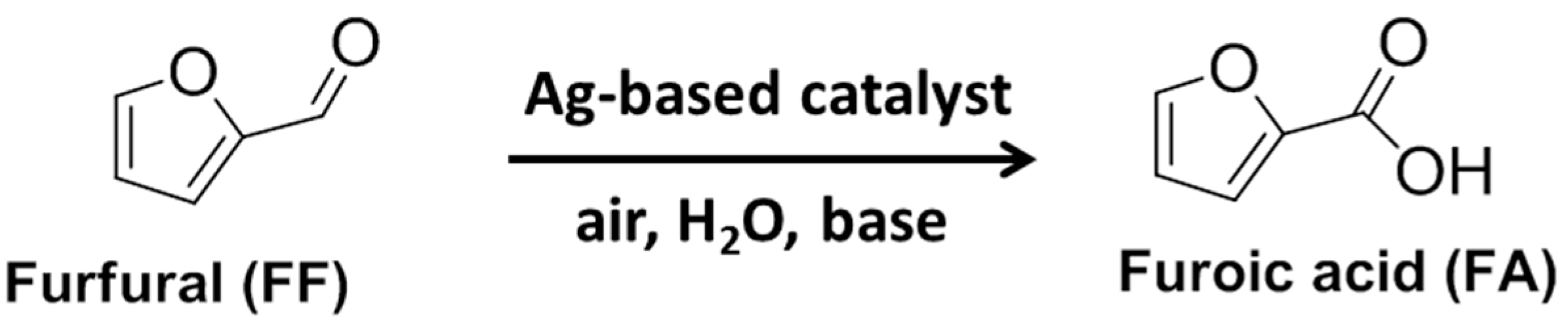
Selective Oxidation of Furfural at Room Temperature on a TiO2-Supported Ag Catalyst
Abstract
:1. Introduction
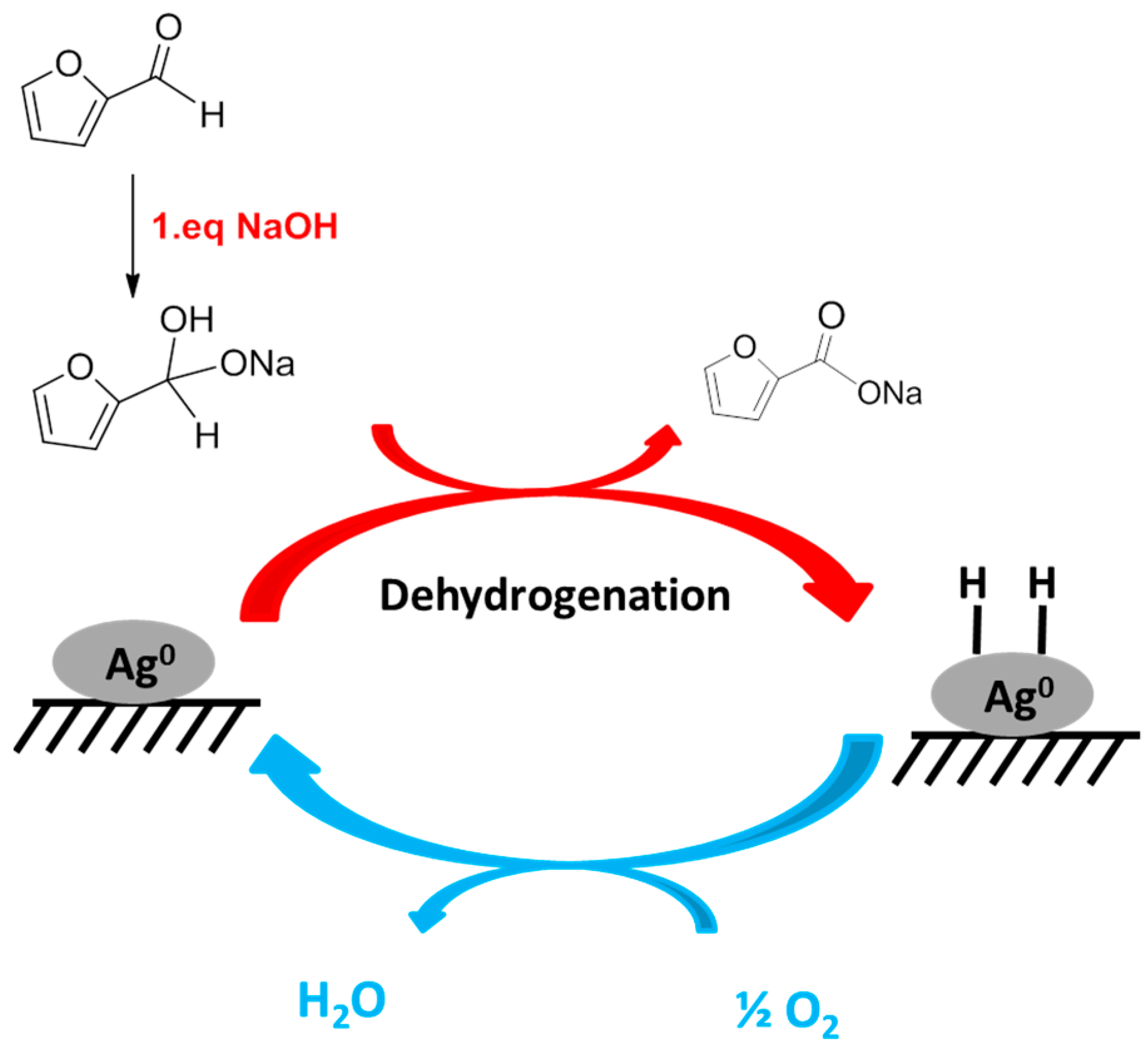
2. Results and Discussion
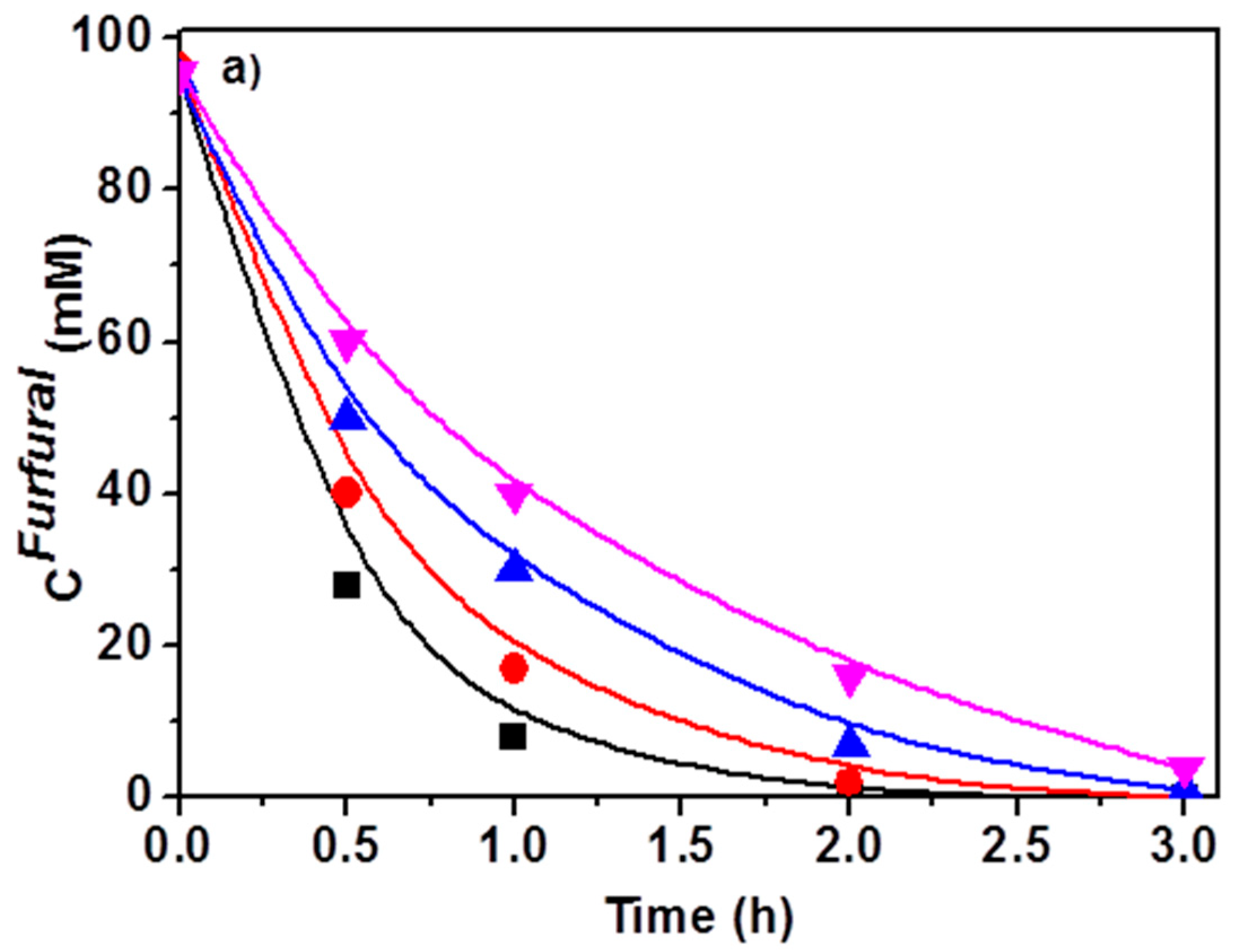
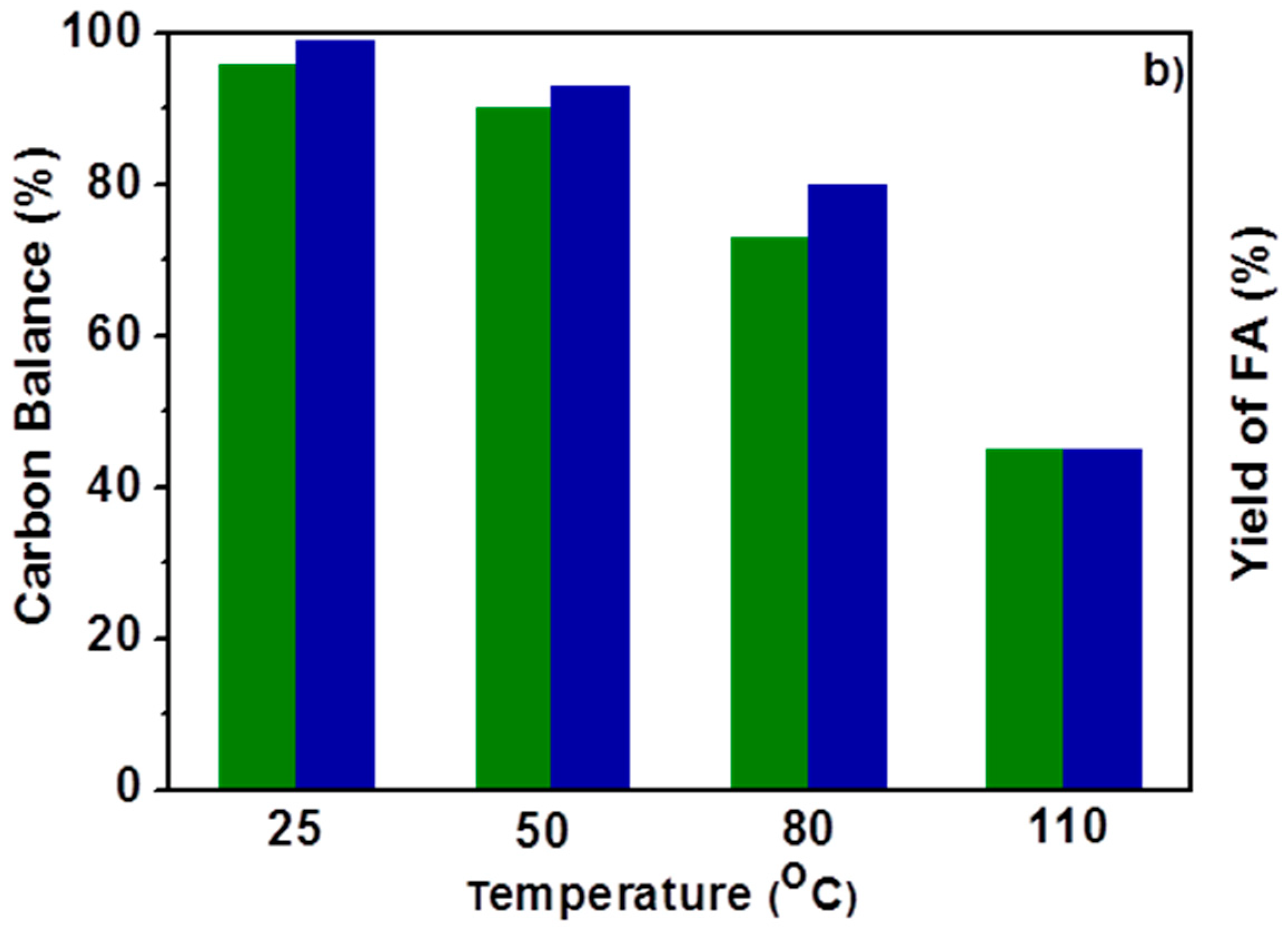
2.1. Effect of the Temperature
2.2. Effect of the Atmosphere
2.3. Effect of Base: Nature and Ratio
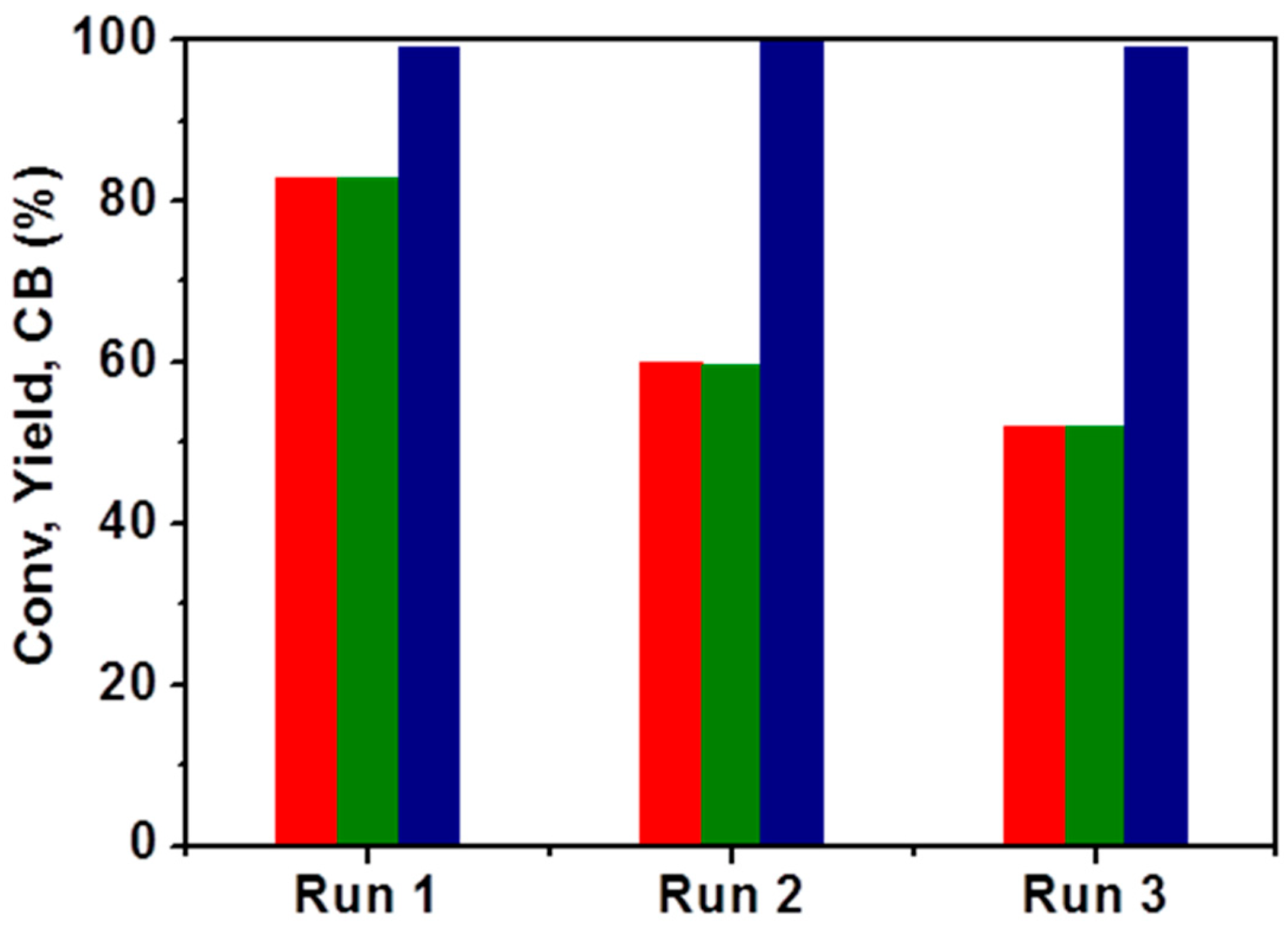
2.4. Recyclability Tests
3. Materials and Methods
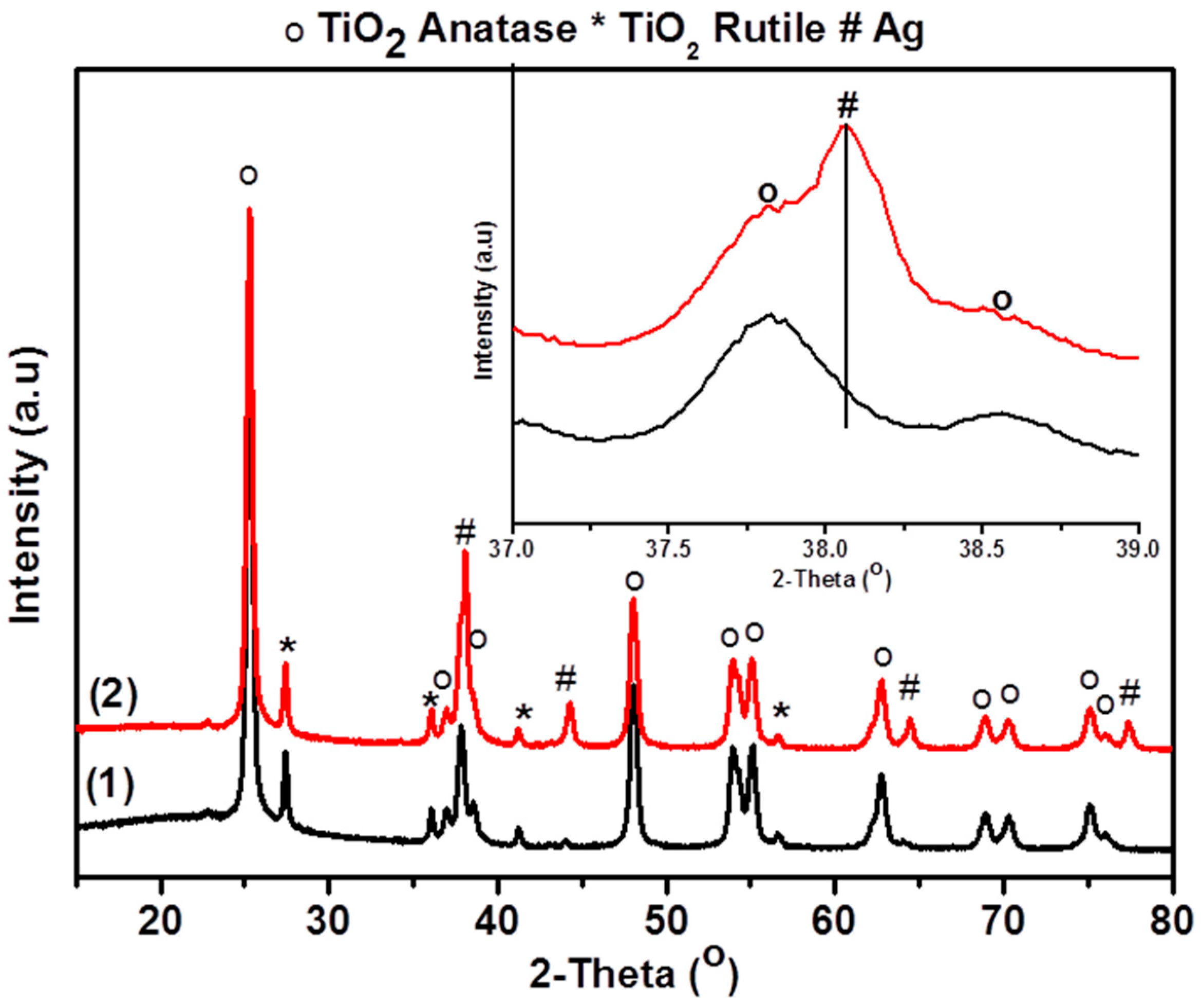
3.1. Catalyst Preparation
3.2. Characterization of Catalysts
3.3. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis Goes Bio: From Carbohydrates and Sugar Alcohols to Platform Chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef]
- Pothu, R.; Gundeboyina, R.; Boddula, R.; Perugopu, V.; Ma, J. Recent advances in biomass-derived platform chemicals to valeric acid synthesis. New J. Chem. 2022, 13, 5907–5921. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—the US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Bertrand, E.; Tolvanen, P.; Devyatkov, S.; Rahkila, J.; Eränen, K.; Wärnå, J.; Salmi, T. Heterogeneous Catalytic Oxidation of Furfural with Hydrogen Peroxide over Sulfated Zirconia. Ind. Eng. Chem. Res. 2020, 59, 13516–13527. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Drault, F.; Snoussi, Y.; Thuriot-Roukos, J.; Itabaiana, I.; Paul, S.; Wojcieszak, R. Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles. Catalysts 2021, 11, 326. [Google Scholar] [CrossRef]
- Drault, F.; Snoussi, Y.; Paul, S.; Itabaiana, I.; Wojcieszak, R. Recent Advances in Carboxylation of Furoic Acid into 2,5-Furandicarboxylic Acid: Pathways towards Bio-Based Polymers. ChemSusChem 2020, 13, 5164–5172. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H. An Overview of the Applications of Furfural and Its Derivatives. Int. J. Architect. Comput. 2015, 3, 42. [Google Scholar] [CrossRef]
- Arias, P.L.; Cecilia, J.A.; Gandarias, I.; Iglesias, J.; López Granados, M.; Mariscal, R.; Morales, G.; Moreno-Tost, R.; Maireles-Torres, P. Oxidation of Lignocellulosic Platform Molecules to Value-Added Chemicals Using Heterogeneous Catalytic Technologies. Catal. Sci. Technol. 2020, 10, 2721–2757. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products. Elsevier B 2000, 13, 159–163. [Google Scholar]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.P.; Costa, N.J.S.; Teixeira-Neto, E.; Teixeira-Neto, Â.A.; Liria, C.W.; Thuriot-Roukos, J.; Machini, M.T.; Froidevaux, R.; Dumeignil, F.; Rossi, L.M.; et al. 5-Hydroxymethylfurfural and Furfural Base-Free Oxidation over AuPd Embedded Bimetallic Nanoparticles. Catalysts 2020, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Taarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from Renewables: Aerobic Oxidation of Furfural and Hydroxymethylfurfural over Gold Catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef]
- Roselli, A.; Carvalho, Y.; Dumeignil, F.; Cavani, F.; Paul, S.; Wojcieszak, R. Liquid Phase Furfural Oxidation under Uncontrolled PH in Batch and Flow Conditions: The Role of In Situ Formed Base. Catalysts 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, F.; Manzoli, M.; di Michele, A.; Ghedini, E.; Signoretto, M. Supported Gold Nanoparticles for Furfural Valorization in the Future Bio-Based Industry. Top. Catal. 2018, 61, 1877–1887. [Google Scholar] [CrossRef]
- Tian, Q.; Shi, D.; Sha, Y. CuO and Ag2O/CuO Catalyzed Oxidation of Aldehydes to the Corresponding Carboxylic Acids by Molecular Oxygen. Molecules 2008, 13, 948–957. [Google Scholar] [CrossRef]
- Schade, O.R.; Gaur, A.; Zimina, A.; Saraçi, E.; Grunwaldt, J.-D. Mechanistic Insights into the Selective Oxidation of 5-(Hydroxymethyl)Furfural over Silver-Based Catalysts. Catal. Sci. Technol. 2020, 10, 5036–5047. [Google Scholar] [CrossRef]
- Farfan-Arribas, E.; Madix, R.J. Characterization of the Acid−Base Properties of the TiO2 (110) Surface by Adsorption of Amines. J. Phys. Chem. B 2003, 107, 3225–3233. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the Mechanism of Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Supported Pt and Au Catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Schade, O.R.; Kalz, K.F.; Neukum, D.; Kleist, W.; Grunwaldt, J.-D. Supported Gold- and Silver-Based Catalysts for the Selective Aerobic Oxidation of 5-(Hydroxymethyl)Furfural to 2,5-Furandicarboxylic Acid and 5-Hydroxymethyl-2-Furancarboxylic Acid. Green Chem. 2018, 20, 3530–3541. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Yin, X.; Subramaniam, B.; Raghunath, V.C. Liquid-Phase Oxidation of Ethylene Glycol on Pt and Pt−Fe Catalysts for the Production of Glycolic Acid: Remarkable Bimetallic Effect and Reaction Mechanism. J. Catal. 2019, 58, 18561–18568. [Google Scholar] [CrossRef]
- Ferraz, C.; Garcia, M.; Teixeira-Neto, E.; Rossi, L. Oxidation of benzyl alcohol catalyzed by gold nanoparticles under alkaline conditions: Weak vs. strong bases. RSC Adv. 2016, 6, 25279–25285. [Google Scholar] [CrossRef]



| OH−:FF Molar Ratio | FF Conversion (%) | FA Yield (%) | Carbon Balance (%) |
|---|---|---|---|
| 0.00 | 0 | 0 | 0 |
| 0.25 | 10 | 10 | 100 |
| 0.50 | 63 | 61 | 98 |
| 1.00 | 96 | 96 | 99 |
| 3.00 | 100 | 70 | 70 |
| Base | FF Conversion (%) | FA Yield (%) | Carbon Balance (%) |
|---|---|---|---|
| No base | 0 | 0 | 0 |
| NaHCO3 | 1 | 1 | 99 |
| Na2CO3 | 7 | 7 | 100 |
| NaOH | 96 | 96 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadier, A.; Paul, S.; Wojcieszak, R. Selective Oxidation of Furfural at Room Temperature on a TiO2-Supported Ag Catalyst. Catalysts 2022, 12, 805. https://doi.org/10.3390/catal12080805
Sadier A, Paul S, Wojcieszak R. Selective Oxidation of Furfural at Room Temperature on a TiO2-Supported Ag Catalyst. Catalysts. 2022; 12(8):805. https://doi.org/10.3390/catal12080805
Chicago/Turabian StyleSadier, Achraf, Sébastien Paul, and Robert Wojcieszak. 2022. "Selective Oxidation of Furfural at Room Temperature on a TiO2-Supported Ag Catalyst" Catalysts 12, no. 8: 805. https://doi.org/10.3390/catal12080805