Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr(VI) Reduction
Abstract
:1. Introduction
2. Results and Discussion
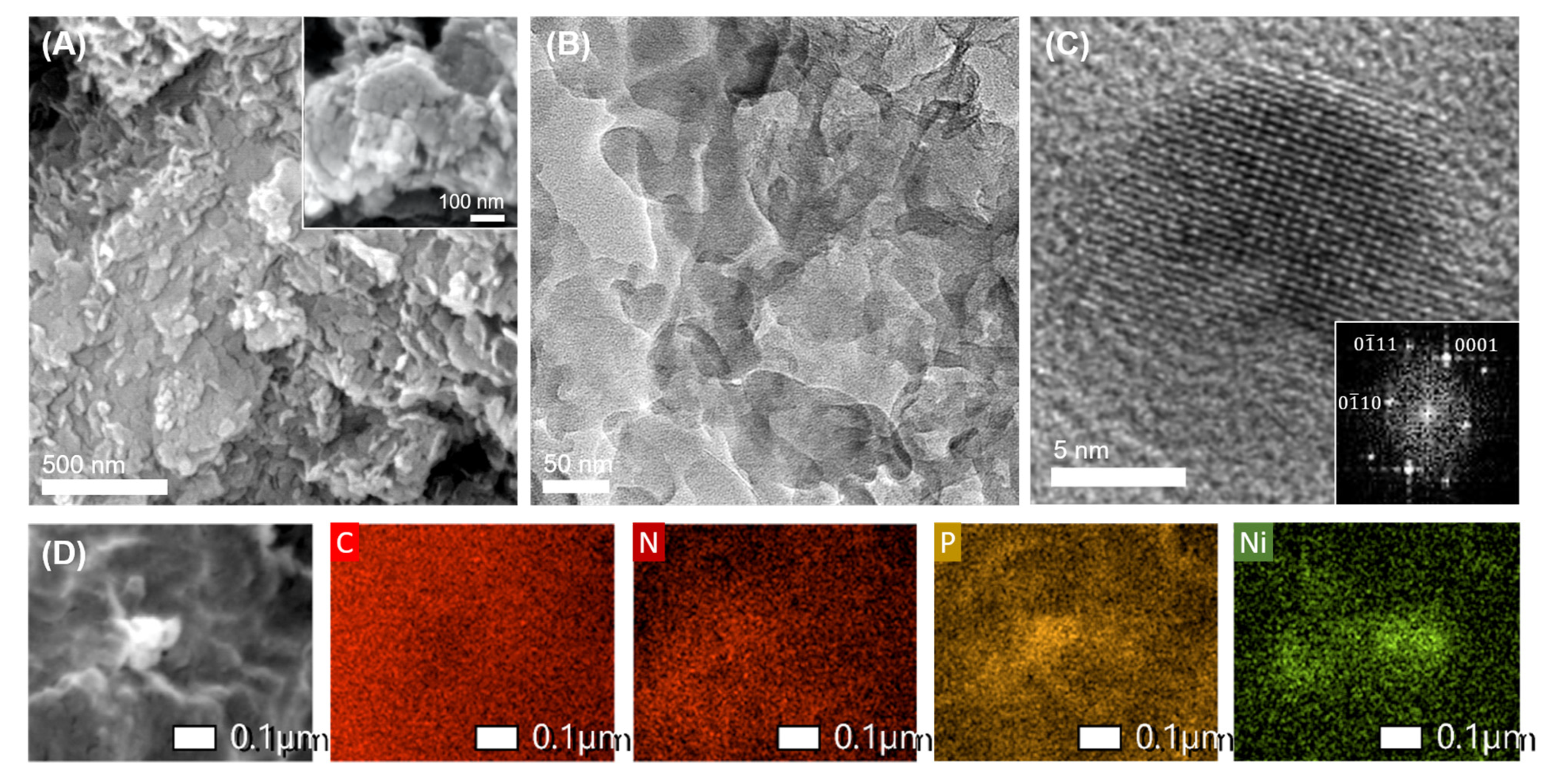
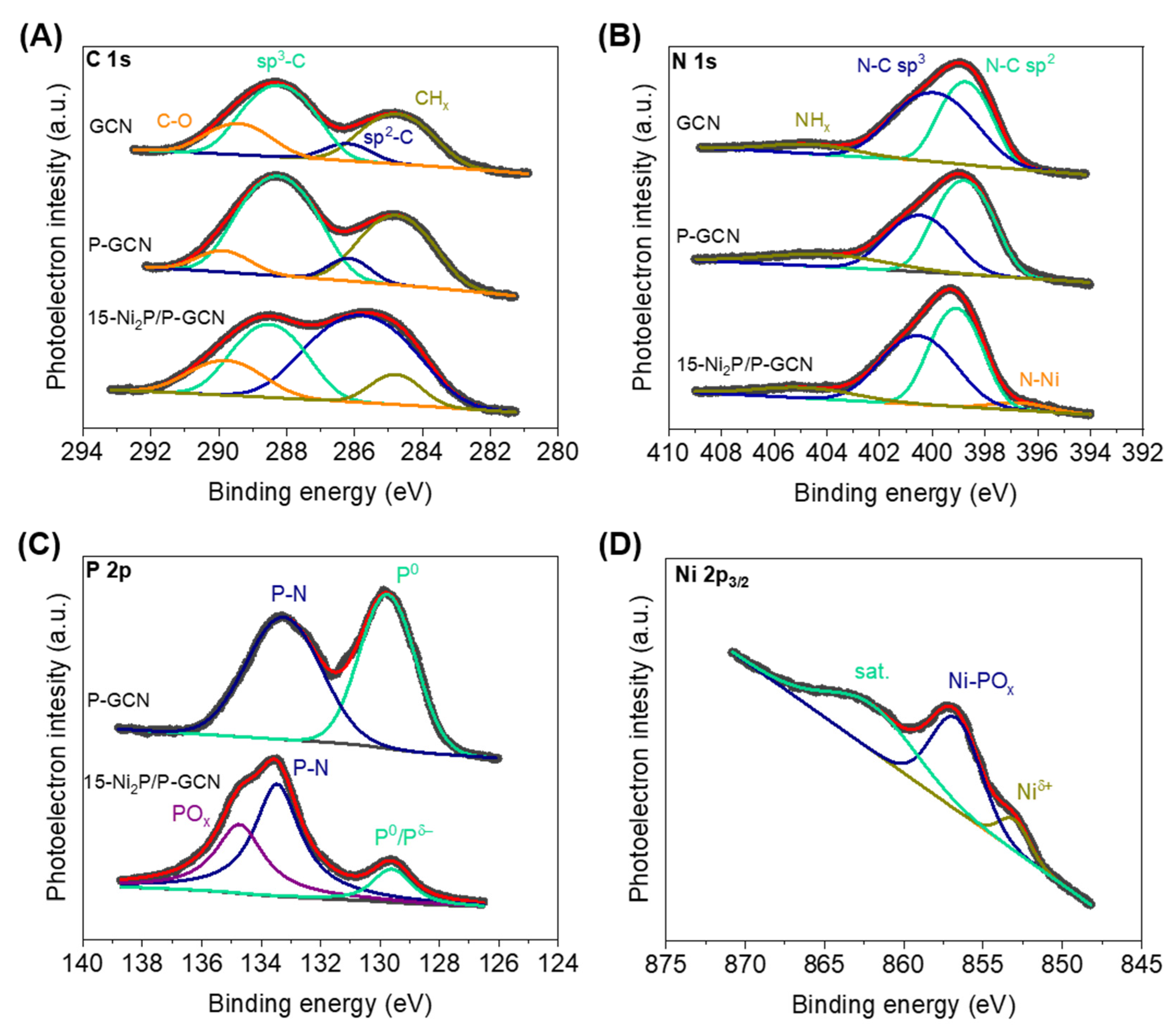
2.1. Synthesis and Structural Characterization
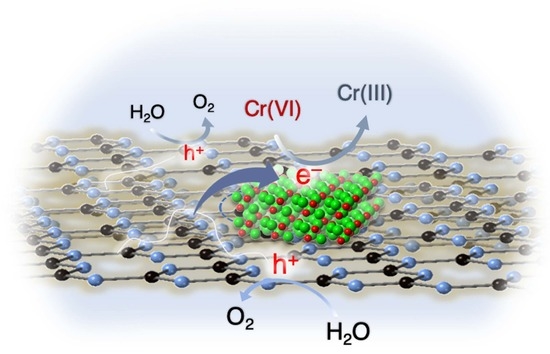
2.2. Photocatalytic Reduction of Cr(VI)
2.3. Effect of Ni2P on the Photocatalytic Activity
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of g-C3N4
3.3. Synthesis of Ni2P/g-C3N4 Heterostructures
3.4. Physicochemical Characterization
3.5. Photocatalytic Reactions
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Efremova Aaron, S.; Aaron, J.J. Toxic Heavy Metals: Impact on the Environment and Human Health, and Treatment with Conducting Organic Polymers, a Review. Environ. Sci. Pollut. Res. Int. 2020, 27, 29927–29942. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; el Nemr, A.; Madkour, F.F. Environmental Assessment of Heavy Metal Pollution and Human Health Risk. Am. J. Water Sci. Eng. 2016, 2, 14–19. [Google Scholar]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and Individuality in Chromium Toxicity in Humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and Carcinogenicity of Chromium Compounds in Humans. Crit. Rev. Toxicol. 2008, 36, 155–163. [Google Scholar] [CrossRef]
- Baral, A.; Engelken, R.; Stephens, W.; Farris, J.; Hannigan, R. Evaluation of Aquatic Toxicities of Chromium and Chromium-Containing Effluents in Reference to Chromium Electroplating Industries. Arch. Environ. Contam. Toxicol. 2006, 50, 496–502. [Google Scholar] [CrossRef]
- Levankumar, L.; Muthukumaran, V.; Gobinath, M.B. Batch Adsorption and Kinetics of Chromium (VI) Removal from Aqueous Solutions by Ocimum Americanum L. Seed Pods. J. Hazard. Mater. 2009, 161, 709–713. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent Advances in Removal Techniques of Cr(VI) Toxic Ion from Aqueous Solution: A Comprehensive Review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective Capture of Hexavalent Chromium from an Anion-Exchange Column of Metal Organic Resin–Alginic Acid Composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Evangelou, D.; Pournara, A.; Tziasiou, C.; Andreou, E.; Armatas, G.S.; Manos, M.J. Robust Al3+ MOF with Selective As(V) Sorption and Efficient Luminescence Sensing Properties toward Cr(VI). Inorg. Chem. 2022, 61, 2017–2030. [Google Scholar] [CrossRef]
- Djellabi, R.; Su, P.; Elimian, E.A.; Poliukhova, V.; Nouacer, S.; Abdelhafeez, I.A.; Abderrahim, N.; Aboagye, D.; Andhalkar, V.V.; Nabgan, W.; et al. Advances in Photocatalytic Reduction of Hexavalent Chromium: From Fundamental Concepts to Materials Design and Technology Challenges. J. Water Process Eng. 2022, 50, 103301. [Google Scholar] [CrossRef]
- Anthony, E.T.; Oladoja, N.A. Process Enhancing Strategies for the Reduction of Cr(VI) to Cr(III) via Photocatalytic Pathway. Environ. Sci. Pollut. Res. Int. 2021, 29, 8026–8053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yi, X.H.; Wang, C.C.; Wang, P.; Zheng, W. Photocatalytic Cr(VI) Reduction over MIL-101(Fe)–NH2 Immobilized on Alumina Substrate: From Batch Test to Continuous Operation. Chem. Eng. J. 2022, 429, 132497. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Loureiro, J.M.; Boaventura, R.A.R.; Vilar, V.J.P. Solar Photocatalytic Reduction of Cr(VI) over Fe(III) in the Presence of Organic Sacrificial Agents. Appl. Catal. B 2016, 192, 208–219. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Chen, Y.; Wu, F.; Deng, N. Simultaneous Photocatalytic Reduction of Cr(VI) and Oxidation of Bisphenol A Induced by Fe(III)–OH Complexes in Water. J. Hazard. Mater. 2007, 139, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Enviromental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 12, 7159–7329. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Kavitha, R.; Nithya, P.M.; Girish Kumar, S. Noble Metal Deposited Graphitic Carbon Nitride Based Heterojunction Photocatalysts. Appl. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Zheng, Y. Graphitic Carbon Nitride Heterojunction Photocatalysts for Solar Hydrogen Production. Int. J. Hydrog. Energy 2021, 46, 37242–37267. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of Graphitic Carbon Nitride for Photocatalysis: A Reveiw. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, W.; Dang, L.; Mao, Y.; Wu, J.; Xu, K. Recent Progress in Doped g-C3N4 Photocatalyst for Solar Water Splitting: A Review. Front. Chem. 2022, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in g-C3N4-Based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Koutsouroubi, E.D.; Vamvasakis, I.; Drivas, C.; Kennou, S.; Armatas, G.S. Photochemical Deposition of SnS2 on Graphitic Carbon Nitride for Photocatalytic Aqueous Cr(VI) Reduction. Chem. Eng. J. Adv. 2022, 9, 100224. [Google Scholar] [CrossRef]
- Koutsouroubi, E.D.; Vamvasakis, I.; Minotaki, M.G.; Papadas, I.T.; Drivas, C.; Choulis, S.A.; Kopidakis, G.; Kennou, S.; Armatas, G.S. Ni-Doped MoS2 Modified Graphitic Carbon Nitride Layered Hetero-Nanostructures as Highly Efficient Photocatalysts for Environmental Remediation. Appl. Catal. B 2021, 297, 120419. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, L.; Wang, P.; Shi, Z.; Zhang, L. Photocatalytic Activity of N-TiO2/O-Doped N Vacancy g-C3N4 and the Intermediates Toxicity Evaluation under Tetracycline Hydrochloride and Cr(VI) Coexistence Environment. Appl. Catal. B 2020, 262, 118308. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Serendipitous Assembly of Mixed Phase BiVO4 on B-Doped g-C3N4: An Appropriate p-n Heterojunction for Photocatalytic O2 Evolution and Cr(VI) Reduction. Inorg. Chem. 2019, 58, 12480–12491. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, M.; Lv, X.; Liu, Y.; Shi, C.; Dai, Y.; Wang, A.; Majima, T. Insight into Iron Group Transition Metal Phosphides (Fe2P, Co2P, Ni2P) for Improving Photocatalytic Hydrogen Generation. Appl. Catal. B 2019, 246, 330–336. [Google Scholar] [CrossRef]
- Hua, S.; Qu, D.; An, L.; Jiang, W.; Wen, Y.; Wang, X.; Sun, Z. Highly Efficient P-Type Cu3P/n-Type g-C3N4 Photocatalyst through Z-Scheme Charge Transfer Route. Appl. Catal. B 2019, 240, 253–261. [Google Scholar] [CrossRef]
- Zeng, D.; Ong, W.J.; Zheng, H.; Wu, M.; Chen, Y.; Peng, D.L.; Han, M.Y. Ni12P5 Nanoparticles Embedded into Porous g-C3N4 Nanosheets as a Noble-Metal-Free Hetero-Structure Photocatalyst for Efficient H2 Production under Visible Light. J. Mater. Chem. A 2017, 5, 16171–16178. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, H.; Li, J.; Du, P. Extraordinarily Efficient Photocatalytic Hydrogen Evolution in Water Using Semiconductor Nanorods Integrated with Crystalline Ni2P Cocatalysts. Energy Environ. Sci. 2015, 8, 2668–2676. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Wang, W.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Song, B.; Xue, W.; Li, X.; et al. Recent Advances in Application of Transition Metal Phosphides for Photocatalytic Hydrogen Production. Chem. Eng. J. 2021, 405, 126547. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zou, J.; Ou-Yang, L.; Zheng, A.; Tang, H. Micro/Nano-Structured Graphitic Carbon Nitride–Ag Nanoparticle Hybrids as Surface-Enhanced Raman Scattering Substrates with Much Improved Long-Term Stability. Carbon 2015, 87, 193–205. [Google Scholar] [CrossRef]
- Shi, J.R.; Xu, Y.J.; Zhang, J. Study on Amorphous Carbon Nitride Film Prepared by Facing Target Sputtering. Thin Solid Films 2005, 483, 169–174. [Google Scholar] [CrossRef]
- Marton, D.; Boyd, K.J.; Al-Bayati, A.H.; Todorov, S.S.; Rabalais, J.W. Carbon Nitride Deposited Using Energetic Species: A Two-Phase System. Phys. Rev. Lett. 1994, 73, 118. [Google Scholar] [CrossRef]
- Hellgren, N.; Haasch, R.T.; Schmidt, S.; Hultman, L.; Petrov, I. Interpretation of X-Ray Photoelectron Spectra of Carbon-Nitride Thin Films: New Insights from in Situ XPS. Carbon 2016, 108, 242–252. [Google Scholar] [CrossRef]
- Wei, H.; Xi, Q.; Chen, X.; Guo, D.; Ding, F.; Yang, Z.; Wang, S.; Li, J.; Huang, S. Molybdenum Carbide Nanoparticles Coated into the Graphene Wrapping N-Doped Porous Carbon Microspheres for Highly Efficient Electrocatalytic Hydrogen Evolution Both in Acidic and Alkaline Media. Adv. Sci. 2018, 5, 1700733. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand New P-Doped g-C3N4: Enhanced Photocatalytic Activity for H2 Evolution and Rhodamine B Degradation under Visible Light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Yao, W.; Chen, K.; Chen, D. One Step Synthesis of P-Doped g-C3N4 with the Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 309–315. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective Photocatalytic Oxidation of Aromatic Alcohols in Water by Using P-Doped g-C3N4. Appl. Catal. B 2018, 220, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-Doped Carbon Nitride Solid: Enhanced Electrical Conductivity and Photocurrent Generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Z.; Fan, X.; Leung, D.Y.C.; Long, J.; Zhang, Z.; Miao, T.; Meng, S.; Chen, S.; Fu, X. Intimately Contacted Ni2P on CdS Nanorods for Highly Efficient Photocatalytic H2 Evolution: New Phosphidation Route and the Interfacial Separation Mechanism of Charge Carriers. Appl. Catal. B 2021, 281, 119443. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhu, J.; Li, Y.; Zhao, J.; Li, F.T. Fabrication of Two-Dimensional Ni2P/ZnIn2S4 Heterostructures for Enhanced Photocatalytic Hydrogen Evolution. Chem. Eng. J. 2018, 353, 15–24. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The Enhancement of CdS Photocatalytic Activity for Water Splitting via Anti-Photocorrosion by Coating Ni2P Shell and Removing Nascent Formed Oxygen with Artificial Gill. Appl. Catal. B 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, D.; Zhou, H.; Pi, M.; Wang, X.; Chen, S. Coupling P Nanostructures with P-Doped g-C3N4 As Efficient Visible Light Photocatalysts for H2 Evolution and RhB Degradation. ACS Sustain. Chem. Eng. 2018, 6, 6342–6349. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Zhang, L.; Yu, J. Review on DFT Calculation of S-Triazine-Based Carbon Nitride. Carbon Energy 2019, 1, 32–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Li, X.L.; Liu, C.; Li, F.T.; Li, Y.P.; Zhao, J.; Liu, R.H.; Li, G.D. Metalloid Ni2P and Its Behavior for Boosting the Photocatalytic Hydrogen Evolution of CaIn2S4. Int. J. Hydrog. Energy 2018, 43, 219–228. [Google Scholar] [CrossRef]
- Li, S.; Hu, Z.; Xie, S.; Liu, H.; Liu, J. Removal of Cr(VI) From Electroplating Industry Effluent via Electrochemical Reduction. Int. J. Electrochem. Sci. 2018, 13, 655–663. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L.; Bellakhal, N.; Belgaied, J.E. Treatment of Electroplating Wastewater Containing Cu2+, Zn2+ and Cr(VI) by Electrocoagulation. J. Hazard. Mater. 2004, 112, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Ghorab, M.F. Photoreduction of Toxic Chromium Using TiO2-Immobilized under Natural Sunlight: Effects of Some Hole Scavengers and Process Parameters. Desalin. Water Treat. 2014, 55, 1900–1907. [Google Scholar] [CrossRef]
- Burns, R.A.; Crittenden, J.C.; Hand, D.W.; Selzer, V.H.; Sutter, L.L.; Salman, S.R. Effect of Inorganic Ions in Heterogeneous Photocatalysis of TCE. J. Environ. Eng. 1999, 125, 77–85. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jiang, C. Principle and Surface Science of Photocatalysis. Interface Sci. Technol. 2020, 31, 1–38. [Google Scholar]
- Dai, D.; Wang, L.; Xiao, N.; Li, S.; Xu, H.; Liu, S.; Xu, B.; Lv, D.; Gao, Y.; Song, W.; et al. In-Situ Synthesis of Ni2P Co-Catalyst Decorated Zn0.5Cd0.5S Nanorods for High-Quantum-Yield Photocatalytic Hydrogen Production under Visible Light Irradiation. Appl. Catal. B 2018, 233, 194–201. [Google Scholar] [CrossRef]
- Koutsouroubi, E.D.; Vamvasakis, I.; Papadas, I.T.; Drivas, C.; Choulis, S.A.; Kennou, S.; Armatas, G.S. Interface Engineering of MoS2-Modified Graphitic Carbon Nitride Nano-Photocatalysts for an Efficient Hydrogen Evolution Reaction. Chempluschem 2020, 85, 1379–1388. [Google Scholar] [CrossRef]
- Patra, P.C.; Mohapatra, Y.N. Dielectric Constant of Thin Film Graphitic Carbon Nitride (g-C3N4) and Double Dielectric Al2O3/g-C3N4. Appl. Phys. Lett. 2021, 118, 103501. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zhu, S.; Luo, S.; Yan, L.; Dai, Y.; Guo, Y.; Yang, Y. Removal of Cr(VI) from Water by a Biochar-Coupled g-C3N4 Nanosheets Composite and Performance of a Recycled Photocatalyst in Single and Combined Pollution Systems. Appl. Catal. B 2019, 243, 386–396. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal Organic Framework g-C3N4/MIL-53(Fe) Heterojunctions with Enhanced Photocatalytic Activity for Cr(VI) Reduction under Visible Light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D Porous Tubular g-C3N4 Capture Black Phosphorus Quantum Dots as 1D/0D Metal-Free Photocatalysts for Oxytetracycline Hydrochloride Degradation and Hexavalent Chromium Reduction. Appl. Catal. B 2020, 273, 119051. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Jia, Z.; Fang, J.; Yang, F.; Tang, Y.; Wu, K.; Liu, Z.; Fang, Z.Q. Efficient Visible-Light-Driven Hydrogen Evolution and Cr(VI) Reduction over Porous P and Mo Co-Doped g-C3N4 with Feeble N Vacancies Photocatalyst. J. Hazard. Mater. 2019, 361, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Park, S.J. Phosphorus-Doped g-C3N4/SnS Nanocomposite for Efficient Photocatalytic Reduction of Aqueous Cr(VI) under Visible Light. Appl. Surf. Sci. 2020, 531, 147325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Shi, Q.; Cai, Z.; Yang, Z. Acid-Treated g-C3N4 with Improved Photocatalytic Performance in the Reduction of Aqueous Cr(VI) under Visible-Light. Sep. Purif. Technol. 2015, 142, 251–257. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, S.; Liu, Y.; Yang, W.; Yu, Y.; Feng, M.; Li, K. Efficient Photocatalytic Reduction of Cr(VI) in Aqueous Solution over CoS2/g-C3N4-RGO Nanocomposites under Visible Light. Appl. Surf. Sci. 2020, 510, 145495. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Zhang, Y.; Yang, Z.; Zhu, A.; Dionysiou, D.D. Enhancement of the Cr(VI) Adsorption and Photocatalytic Reduction Activity of g-C3N4 by Hydrothermal Treatment in HNO3 Aqueous Solution. Appl. Catal. A. 2016, 521, 9–18. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.; Zhang, F.; Zhuang, Z.; Yu, Y. Recyclable Nanoscale Zero Valent Iron Doped g-C3N4/MoS2 for Efficient Photocatalysis of RhB and Cr(VI) Driven by Visible Light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, W.; Li, Y.K.; Qin, R.; Yue, D.; Ge, C.; Liao, J. Constructing Built-in Electric Field in Graphitic Carbon Nitride Hollow Nanospheres by Co-Doping and Modified in-Situ Ni2P for Broad Spectrum Photocatalytic Activity. J. Mater. Sci. Technol. 2021, 90, 143–149. [Google Scholar] [CrossRef]


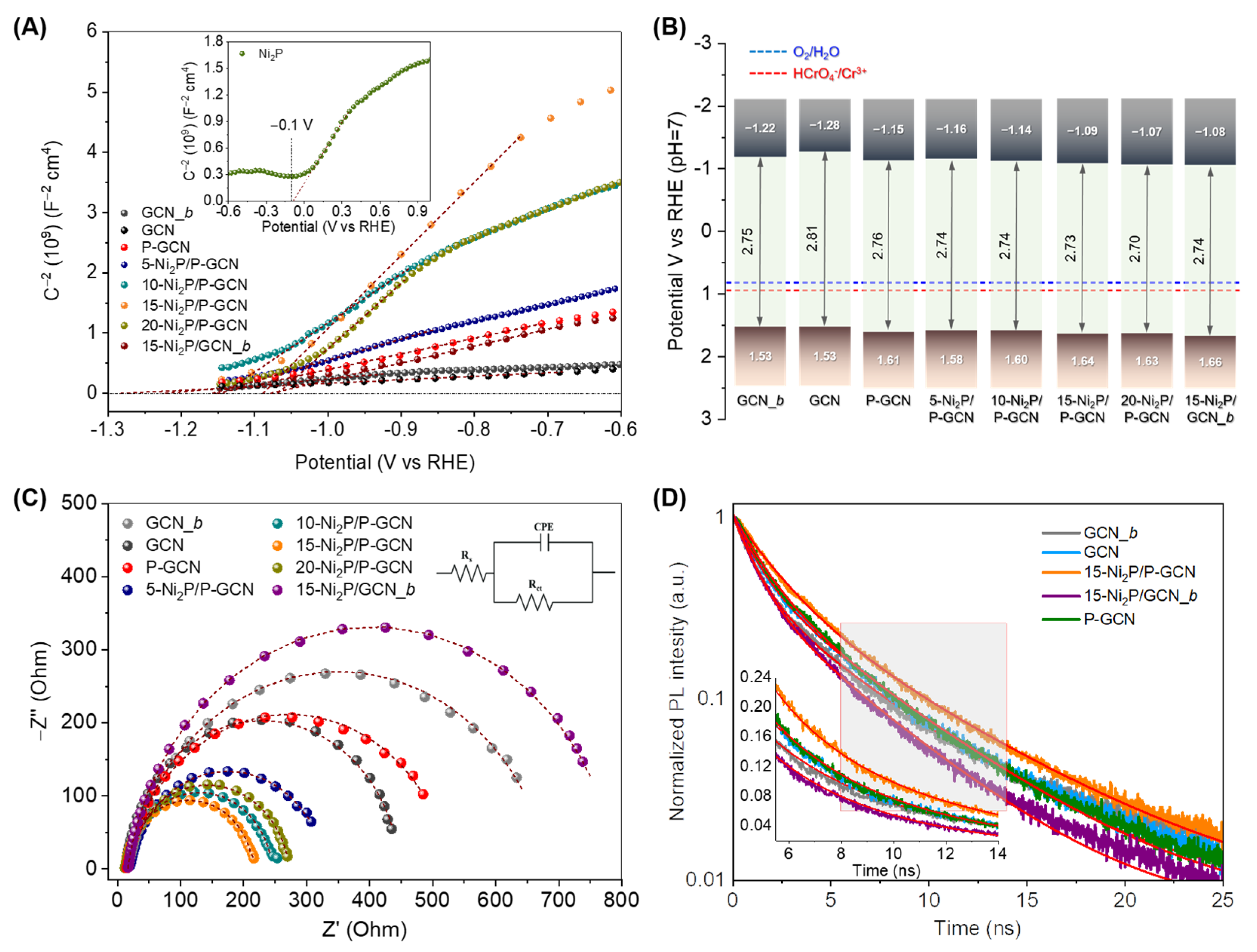

| Sample | EFB (V vs. RHE) | EVB (V vs. RHE) | Donor Density, ND (cm−3) | Rct (Ohm) |
|---|---|---|---|---|
| GCN_b | −1.22 | 1.53 | 1.72 × 1018 | 661 |
| GCN | −1.28 | 1.53 | 3.17 × 1018 | 436 |
| P-GCN | −1.15 | 1.61 | 7.12 × 1017 | 514 |
| 5-Ni2P/P-GCN | −1.16 | 1.58 | 5.30 × 1017 | 311 |
| 10-Ni2P/P-GCN | −1.14 | 1.60 | 2.28 × 1017 | 233 |
| 15-Ni2P/P-GCN | −1.09 | 1.64 | 1.53 × 1017 | 203 |
| 20-Ni2P/P-GCN | −1.07 | 1.63 | 1.71 × 1017 | 261 |
| 15-Ni2P/GCN_b | −1.08 | 1.66 | 6.60 × 1017 | 777 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreou, E.K.; Koutsouroubi, E.D.; Vamvasakis, I.; Armatas, G.S. Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr(VI) Reduction. Catalysts 2023, 13, 437. https://doi.org/10.3390/catal13020437
Andreou EK, Koutsouroubi ED, Vamvasakis I, Armatas GS. Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr(VI) Reduction. Catalysts. 2023; 13(2):437. https://doi.org/10.3390/catal13020437
Chicago/Turabian StyleAndreou, Evangelos K., Eirini D. Koutsouroubi, Ioannis Vamvasakis, and Gerasimos S. Armatas. 2023. "Ni2P-Modified P-Doped Graphitic Carbon Nitride Hetero-Nanostructures for Efficient Photocatalytic Aqueous Cr(VI) Reduction" Catalysts 13, no. 2: 437. https://doi.org/10.3390/catal13020437



_Dionysiou.jpg)