Investigation of Phosphorus Loaded V2O5/ZrO2 Catalysts for the Oxidative Dehydrogenation of Propane (ODH)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Butanol Dehydrogenation over V2O5/ZrO2
2.2. Materials Characterization of the 3.6 wt%V2O5/ZrO2 and Phosphate Loaded Catalysts at Different P/V Ratios
2.3. Catalytic Activity
2.3.1. Acid-Basic and Redox Properties of Phosphorus-Modified V2O5/ZrO2
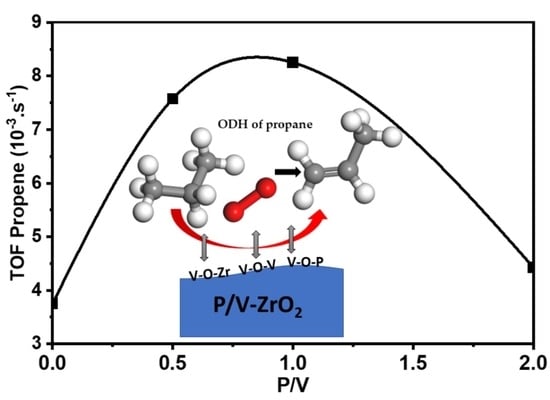
2.3.2. Oxidative Dehydrogenation (ODH) of Propane
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization Methods
3.3. Catalytic Activity
4. Conclusions
- −
- The introduction of vanadium and phosphate promote the tetragonal allotropic phase of ZrO2.
- −
- The most active catalyst corresponds to 3.6 wt% of V2O5/ZrO2.
- −
- The presence of phosphate improves the acid character of the 3.6 wt%V2O5/ZrO2 system as found by dehydration of 2-butanol.
- −
- The addition of phosphorus to V2O5/ZrO2 increased selectivity.
- −
- Good correlation between introduction phosphorus acidity and oxidative dehydrogenation of propane was found.
- −
- The dehydration and dehydrogenation reactions activity of P loaded V2O5/ZrO2 catalysts shows that it is a bi-functional system. V5+ ions appear to play a common role in both dehydration and dehydrogenation reactions.
- −
- The insertion of P modifies the acidity of the system, coupled to the fact that P stabilizes the V4+, leading to the formation of a redox couple responsible for dehydrogenation.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Farsad, A.; Lawson, S.; Rezaei, F.; Rownaghi, A.A. Oxidative dehydrogenation of propane over 3D printed mixed metal oxides/H-ZSM-5 monolithic catalysts using CO2 as an oxidant. Catal. Today 2021, 374, 173–184. [Google Scholar] [CrossRef]
- Gambo, Y.; Adamu, S.; Abdulrasheed, A.A.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. Catalyst design and tuning for oxidative dehydrogenation of propane—A review. Appl. Catal. A: Gen. 2021, 609, 117914. [Google Scholar] [CrossRef]
- Grabowski, R. Kinetics of Oxidative Dehydrogenation of C2-C3 Alkanes on Oxide Catalysts. Catal. Rev. 2006, 48, 199–268. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Carter, J.H.; Bere, T.; Pitchers, J.R.; Hewes, D.G.; Vandegehuchte, B.D.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Direct and oxidative dehydrogenation of propane: From catalyst design to industrial application. Green Chem. 2021, 23, 9747–9799. [Google Scholar] [CrossRef]
- Rase, H.F. Handbook of Commercial Catalysts: Heterogeneous Catalysts; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Khodakov, A.; Olthof, B.; Bell, A.T.; Iglesia, E. Structure and Catalytic Properties of Supported Vanadium Oxides: Support Effects on Oxidative Dehydrogenation Reactions. J. Catal. 1999, 181, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Olthof, B.; Khodakov, A.; Bell, A.T.; Iglesia, E. Effects of Support Composition and Pretreatment Conditions on the Structure of Vanadia Dispersed on SiO2, Al2O3, TiO2, ZrO2, and HfO2. J. Phys. Chem. B 2000, 104, 1516–1528. [Google Scholar] [CrossRef]
- Briand, L.E.; Tkachenko, O.P.; Guraya, M.; Gao, X.; Wachs, I.E.; Grünert, W. Surface-Analytical Studies of Supported Vanadium Oxide Monolayer Catalysts. J. Phys. Chem. B 2004, 108, 4823–4830. [Google Scholar] [CrossRef]
- Bañares, M.A.; Martínez-Huerta, M.V.; Gao, X.; Fierro, J.L.G.; Wachs, I.E. Dynamic behavior of supported vanadia catalysts in the selective oxidation of ethane: In situ Raman, UV–Vis DRS and reactivity studies. Catal. Today 2000, 61, 295–301. [Google Scholar] [CrossRef]
- Bañares, M.A.; Martínez-Huerta, M.; Gao, X.; Wachs, I.E.; Fierro, J.L.G. Identification and roles of the different active sites in supported vanadia catalysts by in situ techniques. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 3125–3130. [Google Scholar]
- Burcham, L.J.; Deo, G.; Gao, X.; Wachs, I.E. In situ IR, Raman, and UV-Vis DRS spectroscopy of supported vanadium oxide catalysts during methanol oxidation. Top. Catal. 2000, 11, 85–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Wang, G.; Dong, M.; Huang, L.; Wu, Z.; Fan, W.; Wang, J. Catalytic performance of V2O5/ZrO2–Al2O3 for methanol oxidation. Fuel 2013, 104, 22–27. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Bedia, J.; Rosas, J.M.; Márquez, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation and characterization of carbon based acid catalysts for the dehydration of 2-propanol. Carbon 2009, 47, 286–294. [Google Scholar] [CrossRef]
- Soto, J.; Rosas, J.M.; Otero, J.C.; Rodríguez-Mirasol, J.; Cordero, T. Reaction Mechanisms of 2-Butanol Dehydration over a Phosphorus-Containing Activated Carbon Acid Catalyst. J. Phys. Chem. C 2018, 122, 16772–16778. [Google Scholar] [CrossRef]
- Baertsch, C.D.; Komala, K.T.; Chua, Y.-H.; Iglesia, E. Genesis of Brønsted Acid Sites during Dehydration of 2-Butanol on Tungsten Oxide Catalysts. J. Catal. 2002, 205, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Rouimi, M.; Ziyad, M.; Leglise, J.J.P.R.B. Characterization and activity of CoMo/AlPO4 catalysts. Phosphorus Res. Bull. 1999, 10, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Kacimi, M.; Ziyad, M.J.J.C.P. Active sites in butan-2-ol conversion over magnésium and zinc phosphates. J. Chim. Phys. 1997, 94, 2007–2015. [Google Scholar] [CrossRef]
- Brik, Y.; Kacimi, M.; Bozon-Verduraz, F.; Ziyad, M. Characterization of active sites on AgHf2(PO4)3 in butan-2-ol conversion. Microporous Mesoporous Mater. 2001, 43, 103–112. [Google Scholar] [CrossRef]
- El Kabouss, K.; Kacimi, M.; Ziyad, M.; Ammar, S.; Ensuque, A.; Piquemal, J.-Y.; Bozon-Verduraz, F. Cobalt speciation in cobalt oxide-apatite materials: Structure–properties relationship in catalytic oxidative dehydrogenation of ethane and butan-2-ol conversion. J. Mater. Chem. 2006, 16, 2453–2463. [Google Scholar] [CrossRef]
- Rajadhyaksha, R.A.; Knözinger, H. Ammonia adsorption on vanadia supported on titania—silica catalyst: An infrared spectroscopic investigation. Appl. Catal. 1989, 51, 81–92. [Google Scholar] [CrossRef]
- Volpe, M.a.A. Partial oxidation of methane over VOx/α-Al2O3 catalysts. Appl. Catal. A Gen. 2001, 210, 355–361. [Google Scholar] [CrossRef]
- Taghavinezhad, P.; Haghighi, M.; Alizadeh, R. Influence of aging temperature and Mg/Zr molar ratio on transformation of C2H6 to C2H4 over VOx catalyst supported on Mg–Zr nanocomposite. Res. Chem. Intermed. 2019, 45, 1907–1927. [Google Scholar] [CrossRef]
- Čičmanec, P.; Ganjkhanlou, Y.; Kotera, J.; Hidalgo, J.M.; Tišler, Z.; Bulánek, R. The effect of vanadium content and speciation on the activity of VOx/ZrO2 catalysts in the conversion of ethanol to acetaldehyde. Appl. Catal. A Gen. 2018, 564, 208–217. [Google Scholar] [CrossRef]
- El-Drissi, J.; Kacimi, M.; Loukah, M.; Ziyad, M.J.J.C.P. Activité0 de V2O5/TiO2 modifié par le phosphore dans la réaction de déshydrogénation oxydante de l’éthane en éthylène. J. Chim. Phys. 1997, 94, 1984–1992. [Google Scholar] [CrossRef]
- Khan, I.; Zada, N.; Khan, I.; Sadiq, M.; Saeed, K. Enhancement of photocatalytic potential and recoverability of Fe3O4 nanoparticles by decorating over monoclinic zirconia. J. Environ. Health Sci. Eng. 2020, 18, 1473–1489. [Google Scholar] [CrossRef]
- Loukah, M.; Coudurier, G.; Vedrine, J.C.; Ziyad, M. Oxidative dehydrogenation of ethane on V- and Cr-based phosphate catalysts. Microporous Mater. 1995, 4, 345–358. [Google Scholar] [CrossRef]
- Sadiq, M.; Bensitel, M.; Nohair, K.; Leglise, J.; Lamonier, C. Effect of calcination temperature on the structure of vanadium phosphorus oxide materials and their catalytic activity in the decomposition of 2-propanol. J. Saudi Chem. Soc. 2012, 16, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Wachs, I.E.; Weckhuysen, B.M. Structure and reactivity of surface vanadium oxide species on oxide supports. Appl. Catal. A Gen. 1997, 157, 67–90. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Jehng, J.-M.; Wachs, I.E. In Situ UV–vis–NIR Diffuse Reflectance and Raman Spectroscopic Studies of Propane Oxidation over ZrO2-Supported Vanadium Oxide Catalysts. J. Catal. 2002, 209, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Védrine, J.C. Partial oxidation reactions on phosphate-based catalysts. Top. Catal. 2000, 11, 147–152. [Google Scholar] [CrossRef]
- Vedrine, J.C. Acid and partial oxidation reactions on phosphorous-based catalysts. Phosphorus Res. Bull. 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Eon, J.G.; Olier, R.; Volta, J.C. Oxidative Dehydrogenation of Propane on γ-Al2O3 Supported Vanadium Oxides. J. Catal. 1994, 145, 318–326. [Google Scholar] [CrossRef]
- Lewandowska, A.E.; Calatayud, M.; Lozano-Diz, E.; Minot, C.; Bañares, M.A. Combining theoretical description with experimental in situ studies on the effect of alkali additives on the structure and reactivity of vanadium oxide supported catalysts. Catal. Today 2008, 139, 209–213. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Guerrero-Pérez, M.O.; Rodríguez-Mirasol, J.; Cordero, T.; Bañares, M.A.; Portela, R.; Bazin, P.; Clet, G.; Daturi, M. Operando Reactor-Cell with Simultaneous Transmission FTIR and Raman Characterization (IRRaman) for the Study of Gas-Phase Reactions with Solid Catalysts. Anal. Chem. 2020, 92, 5100–5106. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Daturi, M.; Clet, G.; Bazin, P.; Bañares, M.A.; Portela, R.; Guerrero-Pérez, M.O.; Rodríguez-Mirasol, J.; Cordero, T. A simultaneous operando FTIR & Raman study of propane ODH mechanism over V-Zr-O catalysts. Catal. Today 2022, 387, 197–206. [Google Scholar] [CrossRef]
- Elkabouss, K.; Kacimi, M.; Ziyad, M.; Ammar, S.; Bozon-Verduraz, F. Cobalt-exchanged hydroxyapatite catalysts: Magnetic studies, spectroscopic investigations, performance in 2-butanol and ethane oxidative dehydrogenations. J. Catal. 2004, 226, 16–24. [Google Scholar] [CrossRef]

















| Samples | SSA (m2/g) | Mean Pore Radius (nm) | Pore Volume (cm³/g) |
|---|---|---|---|
| ZrO2 | 5.6 | 8.81 | 0.047 |
| 3.6 wt%V2O5/ZrO2 | 16.1 | 3.96 | 0.030 |
| P/V = 0.5 | 43.0 | 2.15 | 0.050 |
| P/V = 1 | 33.6 | 2.17 | 0.034 |
| P/V = 2 | 14.7 | 3.37 | 0.020 |
| Operating Conditions | Global Conversion% | Propene Yield% | Propene Selectivity% | |||
|---|---|---|---|---|---|---|
| F °C3H8/F°O2/F°total (cm3/min) | 3.6 wt%V2O5/ZrO2 | P/V = 1 | 3.6 wt%V2O5/ZrO2 | P/V = 1 | 3.6 wt%V2O5/ZrO2 | P/V = 1 |
| 3.6/0.9/60 | 8.92 | 17.54 | 5.91 | 5.31 | 66.25 | 30.27 |
| 3.6/1.8/60 | 12.42 | 20 | 6.49 | 12.67 | 53.82 | 63.35 |
| 3.6/3.6/60 | 13.41 | 31.95 | 6.74 | 11.99 | 50.24 | 37.52 |
| 3.6/7.2/60 | 9.25 | 53.66 | 4.81 | 9.12 | 51.95 | 16.99 |
| 3.6/1.8/30 | 17.95 | 17.56 | 8.72 | 12.19 | 48.58 | 69.38 |
| 3.6/1.8/90 | 9.34 | 12.82 | 4.7 | 7.55 | 50.29 | 58.89 |
| 3.6/1.8/120 | 10.11 | 14.36 | 3.45 | 6.64 | 34.14 | 46.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzaouak, A.; Mahir, H.; El Hamidi, A.; Kacimi, M.; Liotta, L.F. Investigation of Phosphorus Loaded V2O5/ZrO2 Catalysts for the Oxidative Dehydrogenation of Propane (ODH). Catalysts 2022, 12, 811. https://doi.org/10.3390/catal12080811
Benzaouak A, Mahir H, El Hamidi A, Kacimi M, Liotta LF. Investigation of Phosphorus Loaded V2O5/ZrO2 Catalysts for the Oxidative Dehydrogenation of Propane (ODH). Catalysts. 2022; 12(8):811. https://doi.org/10.3390/catal12080811
Chicago/Turabian StyleBenzaouak, Abdellah, Hanane Mahir, Adnane El Hamidi, Mohamed Kacimi, and Leonarda Francesca Liotta. 2022. "Investigation of Phosphorus Loaded V2O5/ZrO2 Catalysts for the Oxidative Dehydrogenation of Propane (ODH)" Catalysts 12, no. 8: 811. https://doi.org/10.3390/catal12080811