Adsorption of Methylene Blue on Chestnut Shell-Based Activated Carbon: Calculation of Thermodynamic Parameters for Solid–Liquid Interface Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparative Analysis of the Calculation of Thermodynamic Parameters
2.1.1. Calculation of Thermodynamic Parameters
2.1.2. Calculation of Thermodynamic Parameters Using the Langmuir Adsorption Equilibrium Constant KL
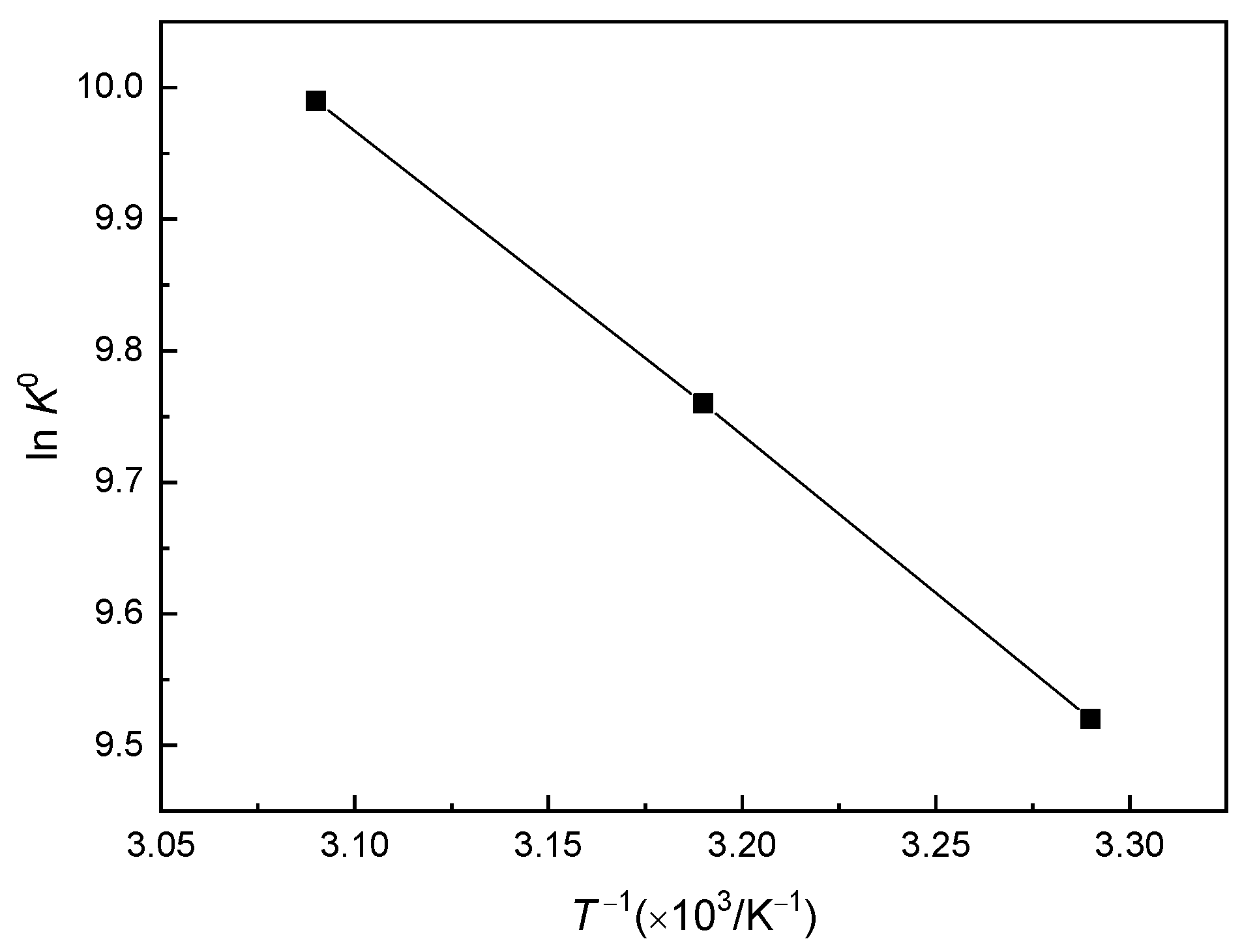
2.1.3. Calculation of Thermodynamic Parameters Using the Adsorption Standard Equilibrium Constant K0
2.2. Adsorption Potential
3. Materials and Methods
3.1. Materials
3.2. Preparation of CnSACs
3.3. Isothermal Adsorption
4. Conclusions
- (1)
- The thermodynamic parameters during the adsorption of MB on chestnut shell-based activated carbon can be calculated more correctly with the standard equilibrium constant K0. The significance of K0 lies in the fact that it enables the equilibrium to be determined by concentration rather than by adsorption rate.
- (2)
- Adsorption enthalpy change ΔH0 = 24.01 KJ mol−1, short of 40 KJ mol−1, indicating that the adsorption mainly involved physical adsorption.
- (3)
- ΔG0 < 0 and ΔS0 > 0, indicating that the adsorption of MB on chestnut shell-based activated carbon was a spontaneous process. The adsorption system was more chaotic after MB entered the activated carbon, and increasing the temperature could facilitate the spontaneous adsorption process.
- (4)
- The adsorption potential was <20 KJ mol−1, further demonstrating that the adsorption consisted mainly of physical adsorption. Increasing the temperature could improve the adsorption potential in the adsorption process, which implies that the adsorption force was affected by temperature during the process of MB molecules entering the activated carbon. The reason is that adsorption first occurred at the site with the largest adsorption force on the adsorbent surface, and the adsorption force of the adsorbent surface decreased as MB covered the surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tatyana, P.; Pablo, C.; Isaac, C. Treatment of textile industrial dyes by simple ozonation with water recirculation. J. Mex. Chem. Soc. 2007, 51, 81–86. [Google Scholar]
- Mittersteiner, M.; Schmitz, F.; Barcellos, I.O. Reuse of dye-colored water post-treated with industrial waste: Its adsorption kinetics and evaluation of method efficiency in cotton fabric dyeing. J. Water Process Eng. 2017, 17, 181–187. [Google Scholar] [CrossRef]
- Kraan, M.V.D.; Cid, M.V.F.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluids 2007, 40, 470–476. [Google Scholar] [CrossRef]
- Aparicio, A.C.C.; Oliveira, L.H.S.D.; Silva, J.S.; Coelho, C.P.; Pinheiro, S.R.; Souza, M.F.; Suffredini, I.B.; Cartwright, S.J.; Bonamin, L.V. Interaction between solvatochromic dyes and water sampled from a natural source treated with high dilutions of phosphorus. Homeopathy 2020, 109, 126–132. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Juang, R.S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- Laghrib, F.; Bakasse, M.; Lahrich, S.; Mhammedi, M.A.E. Advanced oxidation processes: Photo-electro-fenton remediation process for wastewater contaminated by organic azo dyes. Int. J. Environ. Anal. Chem. 2021, 101, 2947–2962. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Aliu, Y.D.; Ofomaja, A.E. Evaluation of snail shell as a coagulant aid in the alum precipitation of aniline blue from aqueous solution. Environ. Technol. 2011, 32, 639–652. [Google Scholar] [CrossRef]
- Nippatla, N.; Philip, L. Performance evaluation of a novel electrolytic reactor with rotating and non rotating bipolar disc electrodes for synthetic textile wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103462. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khand, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Nasar, A.; Mashkoor, F. Application of polyaniline-based adsorbents for dye removal from water and wastewater-a review. Environ. Sci. Pollut. Res. 2019, 26, 5333–5356. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Gao, Y.Y.; Zhu, L.; Gu, X.M.; Dou, H.S.; Pei, L.J. Dyeing property and adsorption kinetics of reactive dyes for cotton textiles in salt-free non-aqueous dyeing systems. Polymers 2018, 10, 1030. [Google Scholar] [CrossRef] [Green Version]
- Filipkowska, U.; Rodziewicz, J.; Moczkowska, I. Basic violet 10 dye removal from water solutions onto zeolite. Rocz. Ochr. Sr. 2010, 12, 747–760. [Google Scholar]
- Zhang, H.; Shi, B.L.; Jia, L.N. Preparation of adsorption material through calcining waste diatomite for treatment of dye water. Desalin. Water Treat. 2018, 113, 281–287. [Google Scholar] [CrossRef]
- Chan, L.S.; Cheung, W.H.; Mckay, G. Adsorption of acid dyes by bamboo derived activated carbon. Desalination 2008, 218, 304–312. [Google Scholar] [CrossRef]
- Mezohegyi, G.; Zee, F.P.V.D.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Asadullah, M.; Kabir, M.S.; Ahmed, M.B.; Razak, N.A.; Rasid, N.; Aezzira, A. Role of microporosity and surface functionality of activated carbon in methylene blue dye removal from water. Korean J. Chem. Eng. 2013, 30, 2228–2234. [Google Scholar] [CrossRef]
- Daoud, M.; Benturki, O.; Kecira, Z.; Girods, P.; Donnot, A. Removal of reactive dye (BEZAKTIV Red S-MAX) from aqueous solution by adsorption onto activated carbons prepared from date palm rachis and jujube stones. J. Mol. Liq. 2017, 243, 799–809. [Google Scholar] [CrossRef]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, L.X.; Cui, J.G.; Cui, J.L.; Wang, F.; Zhang, F. High-efficiency adsorption and regeneration of methylene blue and aniline onto activated carbon from waste edible fungus residue and its possible mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Y.; Liu, X.Z.; Li, W.D.; Tan, Z.W.; Wang, Q.; Zhang, L.H. Removal of toxic dyes from aqueous solutions by adsorption onto a novel activated carbon prepared from chestnut shell. Desalin. Water Treat. 2021, 222, 246–258. [Google Scholar] [CrossRef]
- Gerstner, J.A.; Bell, J.A.; Cramer, S.M. Gibbs free energy of adsorption for biomolecules in ion-exchange systems. Biophys. Chem. 1994, 52, 97–106. [Google Scholar] [CrossRef]
- Na, L.Y.; Zhang, L.Y.; Zhang, F.J.; Hua, R.N. Calculation of Adsorption Thermodynamic Parameters at Solid-liquid Interfaces. Mater. Rep. 2020, 34, 22030–22035. [Google Scholar]
- Zhang, M.Y.; Liu, X.Z.; Li, W.D.; Guo, L.L.; Tan, Z.W.; Wang, Q.; Zhang, L.H. Application of response surface methodology for optimization of methylene blue adsorption onto activated carbons prepared from chestnut shell. Desalin. Water Treat. 2021, 226, 441–451. [Google Scholar] [CrossRef]
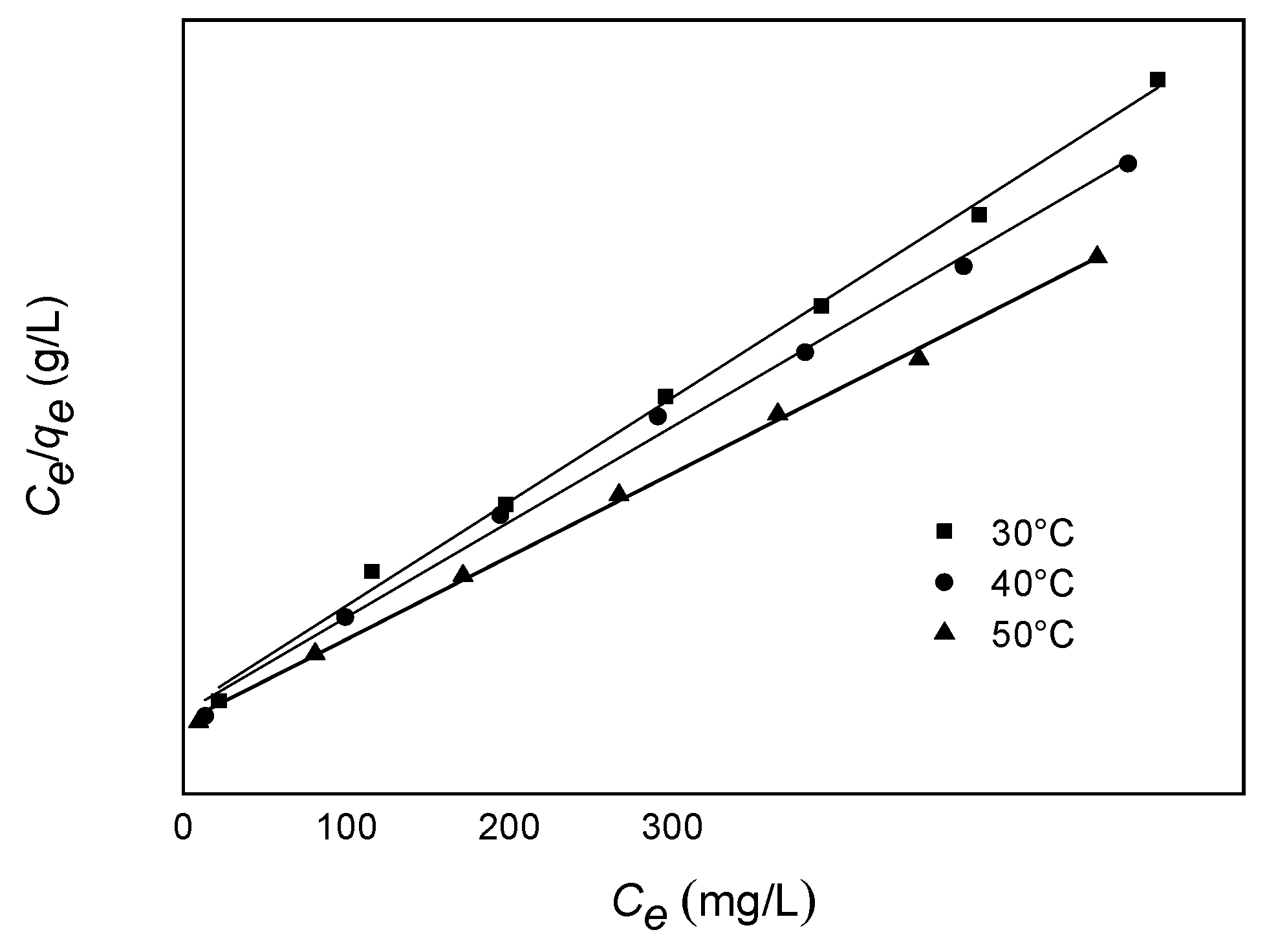


| Langmuir Parameter | 303.15 K | 313.15 K | 323.15 K |
|---|---|---|---|
| qe (mg·g−1) | 1119.12 | 1226.64 | 1429.88 |
| qm (mg·g−1) | 1143.22 | 1248.33 | 1435.87 |
| ce (mg·L−1) | 488.09 | 478.34 | 451.01 |
| θ | 0.979 | 0.982 | 0.996 |
| KL (L·mg−1) | 0.0426 | 0.0442 | 0.0683 |
| Temperature (K) | Δ H0 (kJ·mol−1) | Δ G0 (kJ·mol−1) | Δ S0 (kJ·mol−1·K−1) |
|---|---|---|---|
| 303.15 | 19.62 | 95.99 | 381.37 |
| 313.15 | 19.62 | 95.76 | 381.37 |
| 323.15 | 19.62 | 94.86 | 381.37 |
| Temperature (K) | KL (L·mg−1) | K0 | Δ H0 (kJ·mol−1) | Δ G0 (kJ·mol−1) | Δ S0 (kJ·mol−1·K−1) |
|---|---|---|---|---|---|
| 303.15 | 0.0426 | 13,632 | 24.01 | 23.99 | 76.58 |
| 313.15 | 0.0442 | 14,144 | 24.01 | 24.88 | 76.58 |
| 323.15 | 0.0683 | 21,853 | 24.01 | 26.85 | 76.58 |
| Adsorption Temperature, K | 303.15 | 313.15 | 323.15 |
|---|---|---|---|
| Initial concentration, (mg·L−1) | Adsorption potential ε, (KJ·mol−1) | Adsorption potential ε, (KJ·mol−1) | Adsorption potential ε, (KJ·mol−1) |
| 20 | 3.68 | 5.23 | 6.84 |
| 50 | 3.61 | 5.19 | 6.76 |
| 100 | 3.52 | 5.07 | 6.68 |
| 200 | 3.47 | 5.03 | 6.65 |
| 300 | 3.44 | 4.99 | 6.59 |
| 400 | 3.42 | 4.89 | 6.52 |
| 500 | 3.41 | 4.88 | 6.49 |
| 600 | 3.41 | 4.87 | 6.47 |
| 700 | 3.42 | 4.89 | 6.48 |
| Initial Concentration (mg·L−1) | 20 | 50 | 100 | 200 | 300 | 400 | 500 | 600 | 700 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 303.15 K | m (mg) | 1.7 | 2.1 | 6.4 | 7.1 | 8.0 | 10.2 | 11.9 | 14.0 | 16.3 |
| q (mg·g−1) | 352.9 | 714.3 | 781.3 | 844.5 | 1023.4 | 1040.3 | 1087.4 | 1119.1 | 1086.8 | |
| R | 0.99 | 0.99 | 0.99 | 0.99 | 0.91 | 0.88 | 0.86 | 0.88 | 0.83 | |
| 313.15 K | m (mg) | 1.6 | 1.9 | 6.8 | 7.4 | 8.0 | 9.7 | 11.1 | 12.9 | 13.8 |
| q (mg·g−1) | 375.0 | 789.5 | 865.8 | 1007.8 | 1055.6 | 1090.4 | 1187.1 | 1226.6 | 1208.3 | |
| R | 0.99 | 0.99 | 0.98 | 0.93 | 0.93 | 0.88 | 0.88 | 0.87 | 0.82 | |
| 323.15 K | m (mg) | 1.5 | 1.7 | 5.5 | 7.9 | 6.6 | 8.1 | 9.5 | 10.4 | 12.8 |
| q (mg·g−1) | 400.0 | 882.4 | 904.9 | 1192.1 | 1281.6 | 1328.6 | 1355.3 | 1429.9 | 1397.7 | |
| R | 0.99 | 0.99 | 0.99 | 0.94 | 0.94 | 0.90 | 0.86 | 0.86 | 0.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Zhang, M. Adsorption of Methylene Blue on Chestnut Shell-Based Activated Carbon: Calculation of Thermodynamic Parameters for Solid–Liquid Interface Adsorption. Catalysts 2022, 12, 813. https://doi.org/10.3390/catal12080813
Kong L, Zhang M. Adsorption of Methylene Blue on Chestnut Shell-Based Activated Carbon: Calculation of Thermodynamic Parameters for Solid–Liquid Interface Adsorption. Catalysts. 2022; 12(8):813. https://doi.org/10.3390/catal12080813
Chicago/Turabian StyleKong, Lingjian, and Mingyang Zhang. 2022. "Adsorption of Methylene Blue on Chestnut Shell-Based Activated Carbon: Calculation of Thermodynamic Parameters for Solid–Liquid Interface Adsorption" Catalysts 12, no. 8: 813. https://doi.org/10.3390/catal12080813





