Comparison of Industrial and Lab-Scale Ion Exchange for the DeNOx-SCR Performance of Cu Chabazites: A Case Study
Abstract
:1. Introduction
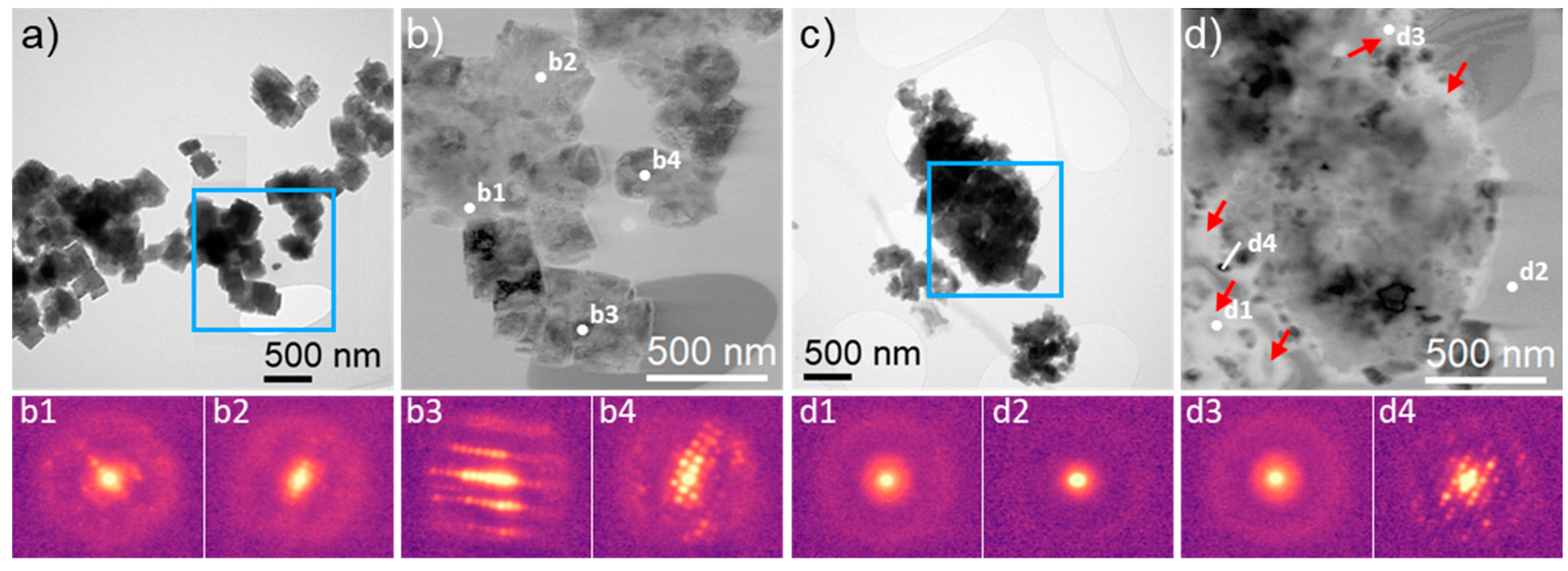
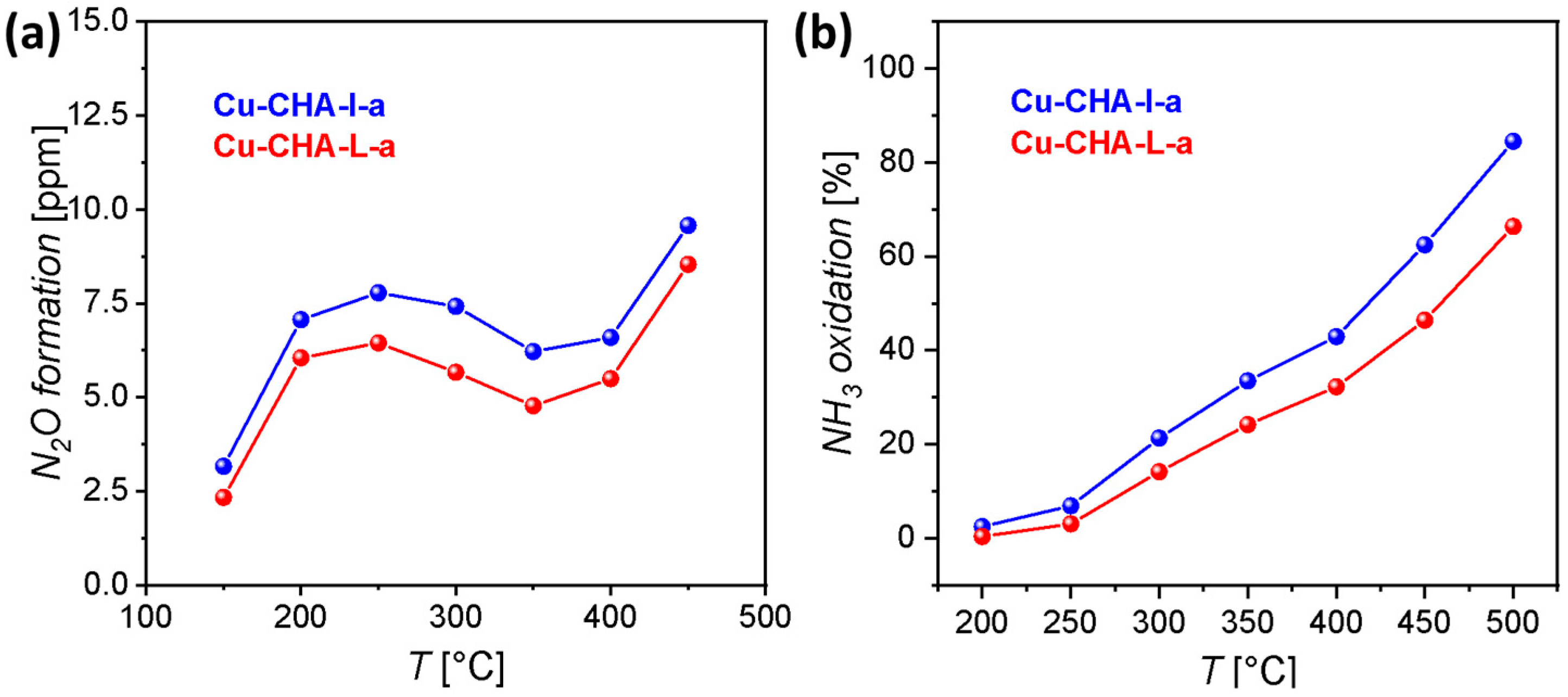
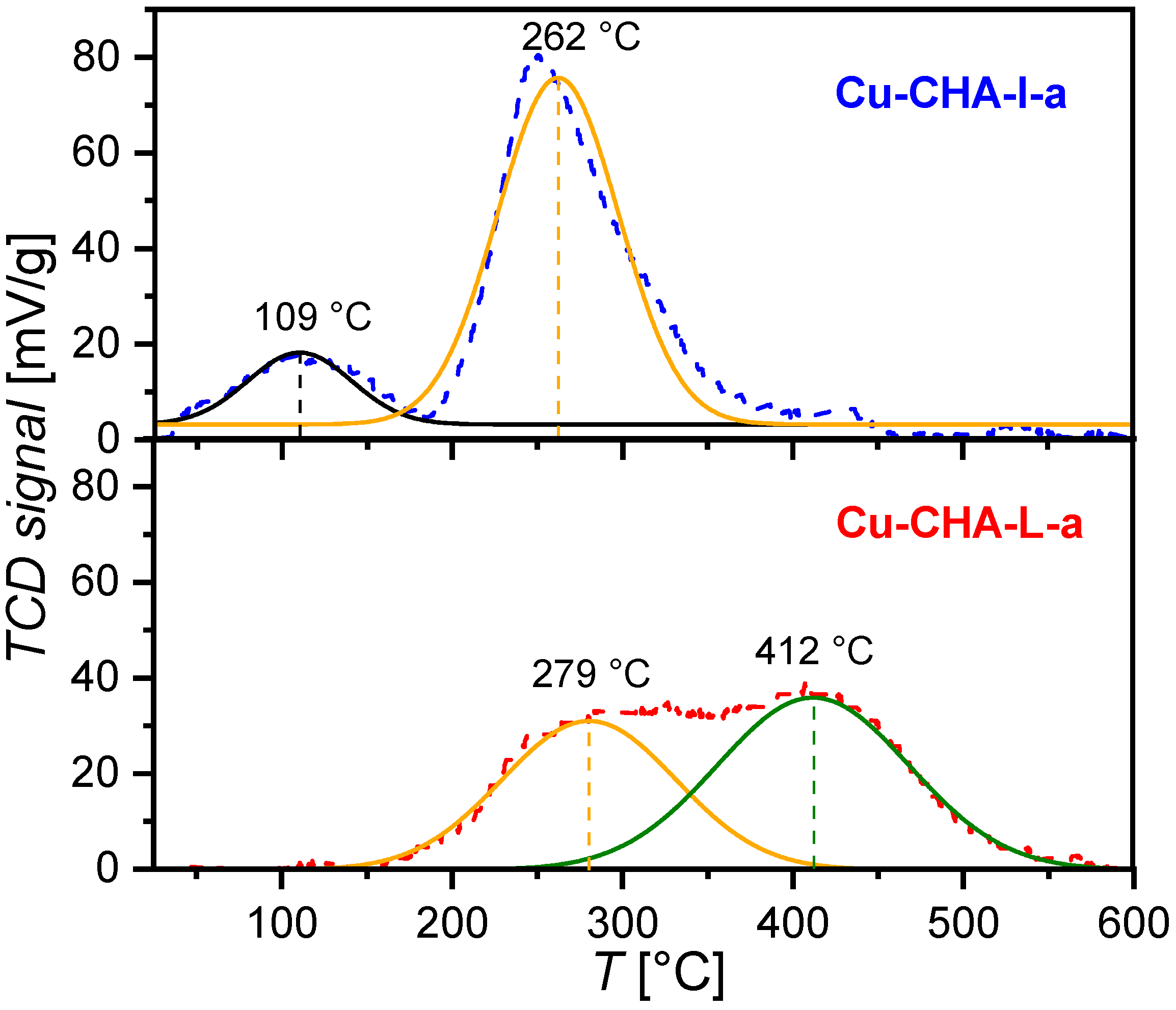
2. Results
3. Materials and Methods
3.1. Experimental Section
3.1.1. Synthesis via Liquid Ion-Exchange (LIE)
3.1.2. Hydrothermal Aging
3.2. Physicochemical Characterization
3.3. Temperature-Programmed Desorption with NH3 (NH3-TPD)
3.4. SCR Catalytic Tests
3.5. NH3 Oxidation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Zhang, Y.; Shan, W.; Shi, X.; Yu, Y.; Zhang, R.; Meng, X.; Xiao, F.-S.; He, H. Selective catalytic reduction of NOx with NH3: Opportunities and challenges of Cu-based small-pore zeolites. Natl. Sci. Rev. 2021, 8, nwab010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A-Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Cheng, S.; Cao, L.; Liu, L.; Xu, Y.; Liu, J.; Ran, R.; Si, Z.; Weng, D. Relationships between copper speciation and Brønsted acidity evolution over Cu-SSZ-13 during hydrothermal aging. Appl. Catal. A-Gen. 2020, 602, 117650. [Google Scholar] [CrossRef]
- Gao, F.; Toops, T.J. Selective Catalytic Reduction: From Basic Science to deNOx Applications. Catalysts 2021, 11, 250. [Google Scholar] [CrossRef]
- Newton, M.A.; Knorpp, A.J.; Sushkevich, V.L.; Palagin, D.; Van Bokhoven, J.A. Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: A critical examination. Chem. Soc. Rev. 2020, 49, 1449–1486. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Rizzotto, V.; Guo, A.; Ye, D.; Simon, U.; Chen, P. Recent understanding of low-temperature copper dynamics in Cu-chabazite NH3-SCR catalysts. Catalysts 2021, 11, 52. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H. Recent progress in atomic-level understanding of Cu/SSZ-13 selective catalytic reduction catalysts. Catalysts 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B-Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Şanlı, D.; Özener, B.; Hisar, G.; Erkey, C. An aging model of NH3 storage sites for predicting kinetics of NH3 adsorption, desorption and oxidation over hydrothermally aged Cu-Chabazite. Catalysts 2020, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B-Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Q.; Yang, X.; Yu, J. A dual-template method for the synthesis of bimetallic CuNi/SSZ-13 zeolite catalysts for NH3-SCR reaction. Inorg. Chem. Commun. 2019, 105, 203–207. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, L.; Chen, Z.; Ming, S.; Dong, Y.; Liu, Q.; Liu, P.; Cai, W.; Li, T. Cu/SSZ-13 and Cu/SAPO-34 catalysts for deNOx in diesel exhaust: Current status, challenges, and future perspectives. Appl. Catal. A-Gen. 2020, 607, 117855. [Google Scholar] [CrossRef]
- Li, S.; Gounder, R.; Debellis, A.; Müller, I.B.; Prasad, S.; Moini, A.; Schneider, W.F. Influence of the N, N, N-Trimethyl-1-adamantyl Ammonium Structure-Directing Agent on Al Substitution in SSZ-13 Zeolite. J. Phys. Chem. C 2019, 123, 17454–17458. [Google Scholar] [CrossRef]
- Nishitoba, T.; Yoshida, N.; Kondo, J.N.; Yokoi, T. Control of Al distribution in the CHA-type aluminosilicate zeolites and its impact on the hydrothermal stability and catalytic properties. Ind. Eng. Chem. Res. 2018, 57, 3914–3922. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, B.; Qin, P.; Lv, N.; Wang, T.; Bi, X.; Zhu, H.; Yuan, P.; Bai, Z.; Cui, Q. One-pot synthesis of FeCu-SSZ-13 zeolite with superior performance in selective catalytic reduction of NO by NH3 from natural aluminosilicates. Chem. Eng. J. 2020, 398, 125515. [Google Scholar] [CrossRef]
- Zhang, N.; Xin, Y.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts 2019, 9, 882. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhu, J.; Wakihara, T.; Okubo, T. Ultrafast synthesis of zeolites: Breakthrough, progress and perspective. Inorg. Chem. Front. 2019, 6, 14–31. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.-Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Microporous Mesoporous Mater. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Peng, X.; Zhan, R.; Lin, H.; Huang, Z. Influence of synthesis method on catalytic properties and hydrothermal stability of Cu/SSZ-13 for NH3-SCR reaction. Chem. Eng. J. 2020, 379, 122358. [Google Scholar] [CrossRef]
- Rizzotto, V.; Chen, D.; Tabak, B.M.; Yang, J.-Y.; Ye, D.; Simon, U.; Chen, P. Spectroscopic identification and catalytic relevance of NH4+ intermediates in selective NOx reduction over Cu-SSZ-13 zeolites. Chemosphere 2020, 250, 126272. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.V.L.; Nicolle, A.; Berthout, D. Hydrothermal aging effects on Cu-zeolite NH3-SCR catalyst. Catal. Today 2015, 258, 424–431. [Google Scholar] [CrossRef]
- Rauch, E.; Véron, M.; Portillo, J.; Bultreys, D.; Maniette, Y.; Nicolopoulos, S. Automatic crystal orientation and phase mapping in TEM by precession diffraction. Microsc. Anal.-UK 2008, 128, S5–S8. [Google Scholar]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Song, C.; Zhang, L.; Li, Z.; Lu, Y.; Li, K. Co-exchange of Mn: A simple method to improve both the hydrothermal stability and activity of Cu–SSZ-13 NH3–SCR catalysts. Catalysts 2019, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Yashnik, S.; Ismagilov, Z. Cu-substituted ZSM-5 catalyst: Controlling of DeNOx reactivity via ion-exchange mode with copper–ammonia solution. Appl. Catal. B-Environ. 2015, 170, 241–254. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Liu, B.; Wu, F.; Li, Y.; Hu, X.; Jin, W.; Wang, X. N2O Formation Mechanism During Low-Temperature NH3-SCR over Cu-SSZ-13 Catalysts with Different Cu Loadings. Ind. Eng. Chem. Res. 2021, 60, 10083–10093. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H. Selective catalytic reduction over Cu/SSZ-13: Linking homo-and heterogeneous catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a Cage: Condition-Dependent Speciation and Dynamics of Exchanged Cu Cations in SSZ-13 Zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A.; Wang, Y.; Washton, N.M.; Walter, E.D.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C.H. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B-Environ. 2017, 201, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Derewinski, M.A.; Kwak, J.H.; Szanyi, J. On the Nature of Extra-Framework Aluminum Species and Improved Catalytic Properties in Steamed Zeolites. Molecules 2022, 27, 2352. [Google Scholar] [CrossRef]
- Gackowski, M.; Podobiński, J.; Broclawik, E.; Datka, J. IR and NMR Studies of the status of Al and acid sites in desilicated zeolite Y. Molecules 2019, 25, 31. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.J.; Kim, D.H. Facile synthesis of KFI-type zeolite and its application to selective catalytic reduction of NOx with NH3. ACS Catal. 2017, 7, 6070–6081. [Google Scholar] [CrossRef]
- Grundner, S.; Markovits, M.A.; Li, G.; Tromp, M.; Pidko, E.A.; Hensen, E.J.; Jentys, A.; Sanchez-Sanchez, M.; Lercher, J.A. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat. Commun. 2015, 6, 7546. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Peng, Y.; Li, J. Correlation of the changes in the framework and active Cu sites for typical Cu/CHA zeolites (SSZ-13 and SAPO-34) during hydrothermal aging. Phys. Chem. Chem. Phys. 2015, 17, 29142–29149. [Google Scholar] [CrossRef]
- Mitchell, D.; Schaffer, B. Scripting-customised microscopy tools for Digital Micrograph™. Ultramicroscopy 2005, 103, 319–332. [Google Scholar] [CrossRef]




| Sample | Si [wt%] | Al [wt%] | Cu [wt%] | Cu/Al Molar Ratio |
|---|---|---|---|---|
| Cu-CHA-I | 38.40 | 5.40 | 2.94 | 0.231 |
| Cu-CHA-L | 22.99 | 4.09 | 2.29 | 0.238 |
| Cu-CHA-I-a | 35.88 | 6.18 | 3.62 | 0.249 |
| Cu-CHA-L-a | 29.18 | 5.04 | 2.86 | 0.241 |
| NH3 Uptake | Cu-CHA-I-a | Cu-CHA-L-a |
|---|---|---|
| Total [mmol/g] | 0.682 | 0.430 |
| LT range [mmol/g] | 0.155 | 0.096 |
| MT range [mmol/g] | 0.371 | 0.181 |
| HT range [mmol/g] | 0.163 | 0.147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzotto, V.; Bajić, S.; Formenti, D.; Wu, X.; Sauerbeck, S.; Werner, J.; Weirich, T.E.; Janke, T.; Mauermann, P.; Pischinger, S.; et al. Comparison of Industrial and Lab-Scale Ion Exchange for the DeNOx-SCR Performance of Cu Chabazites: A Case Study. Catalysts 2022, 12, 880. https://doi.org/10.3390/catal12080880
Rizzotto V, Bajić S, Formenti D, Wu X, Sauerbeck S, Werner J, Weirich TE, Janke T, Mauermann P, Pischinger S, et al. Comparison of Industrial and Lab-Scale Ion Exchange for the DeNOx-SCR Performance of Cu Chabazites: A Case Study. Catalysts. 2022; 12(8):880. https://doi.org/10.3390/catal12080880
Chicago/Turabian StyleRizzotto, Valentina, Stefan Bajić, Dario Formenti, Xiaochao Wu, Silke Sauerbeck, Jonas Werner, Thomas E. Weirich, Tobias Janke, Peter Mauermann, Stefan Pischinger, and et al. 2022. "Comparison of Industrial and Lab-Scale Ion Exchange for the DeNOx-SCR Performance of Cu Chabazites: A Case Study" Catalysts 12, no. 8: 880. https://doi.org/10.3390/catal12080880







