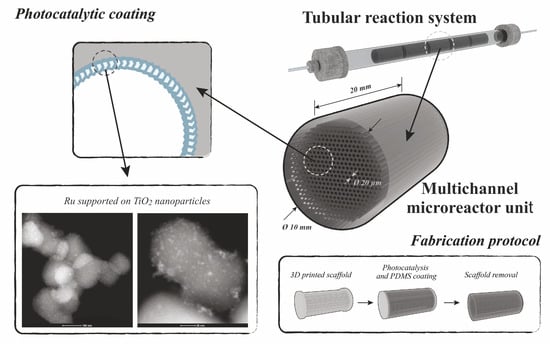
The 3D-Printing Fabrication of Multichannel Silicone Microreactors for Catalytic Applications
Abstract
:1. Introduction
2. Results and Discussion
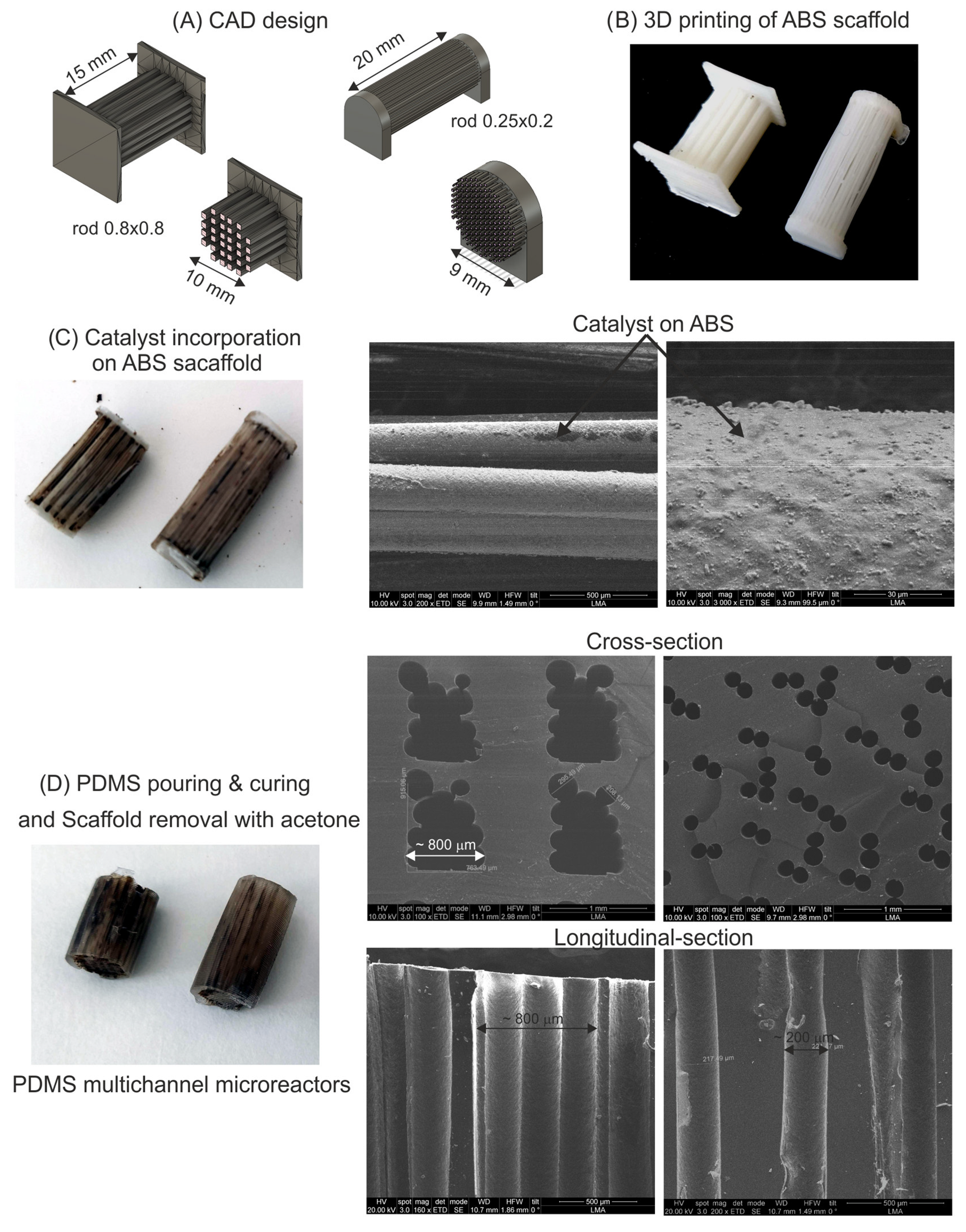
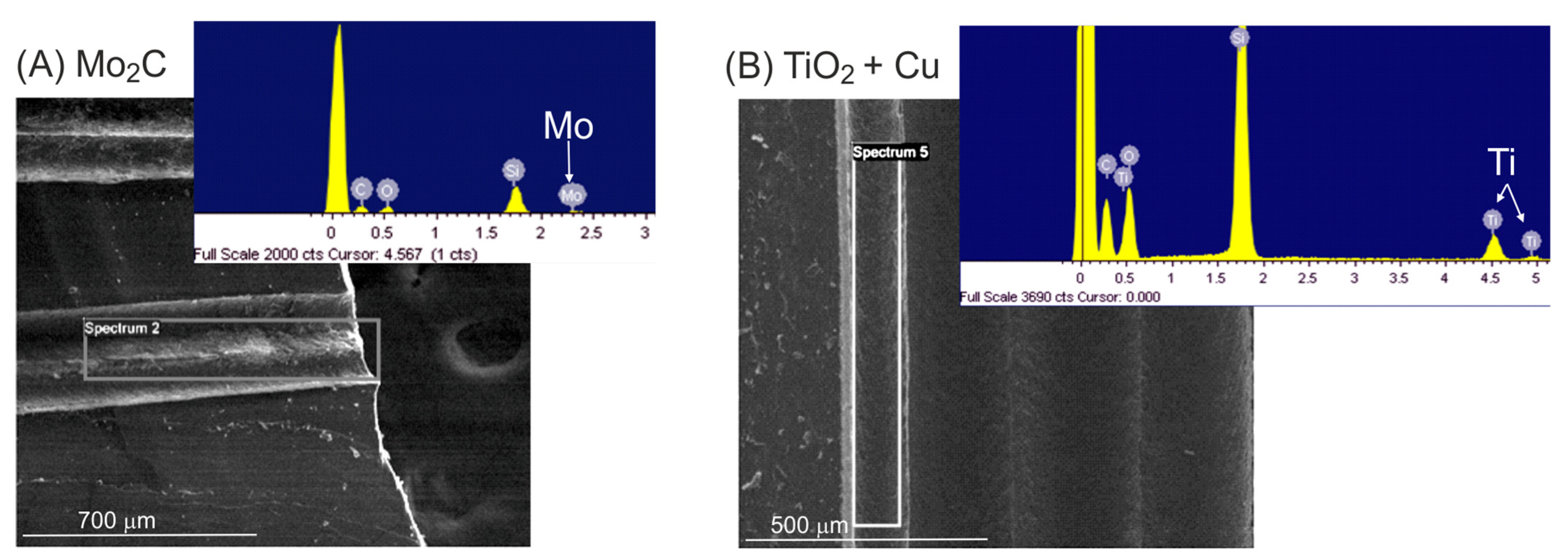
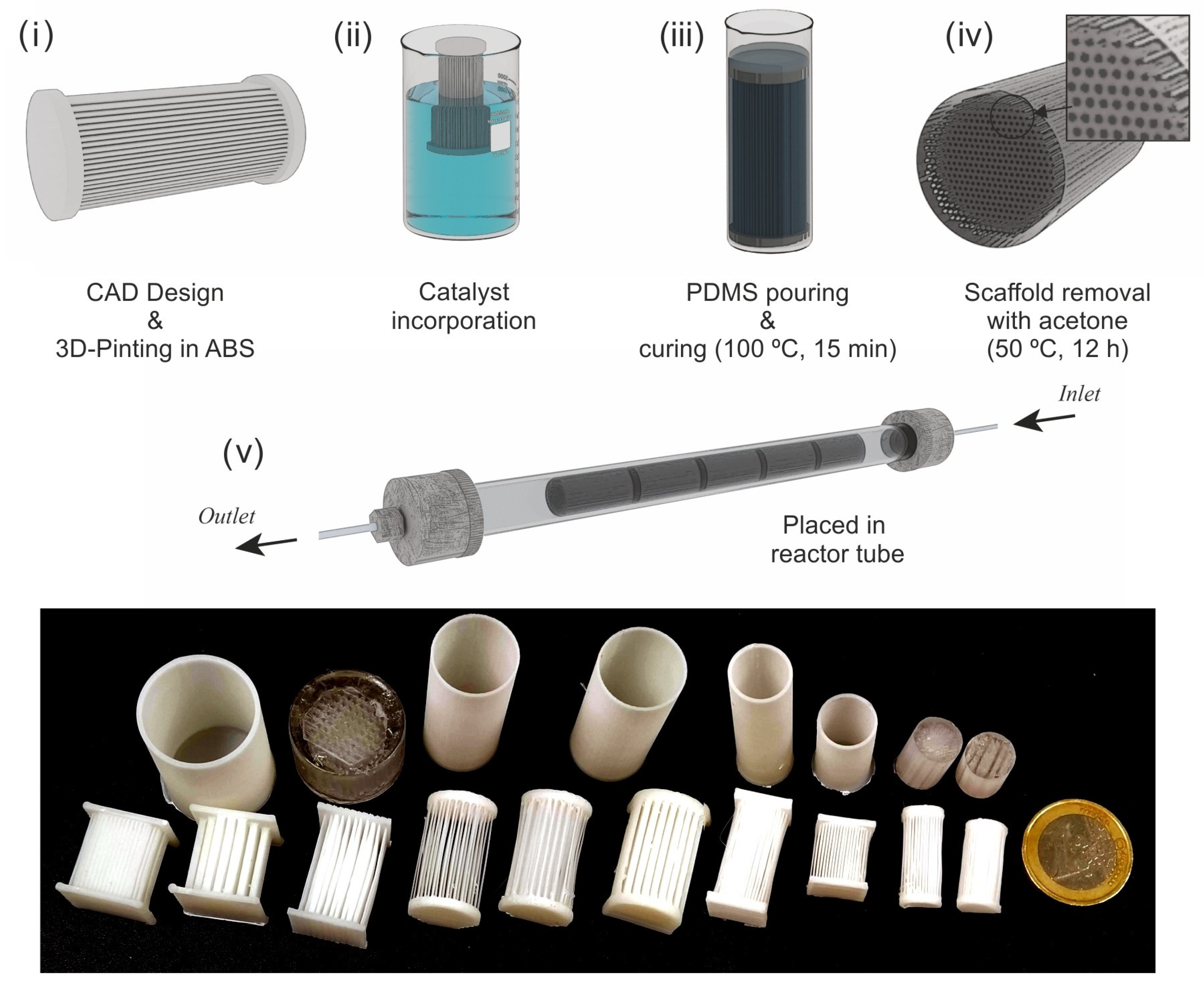
2.1. Multichannel Microreactor Fabrication
2.2. Multichannel Microreactor Performance
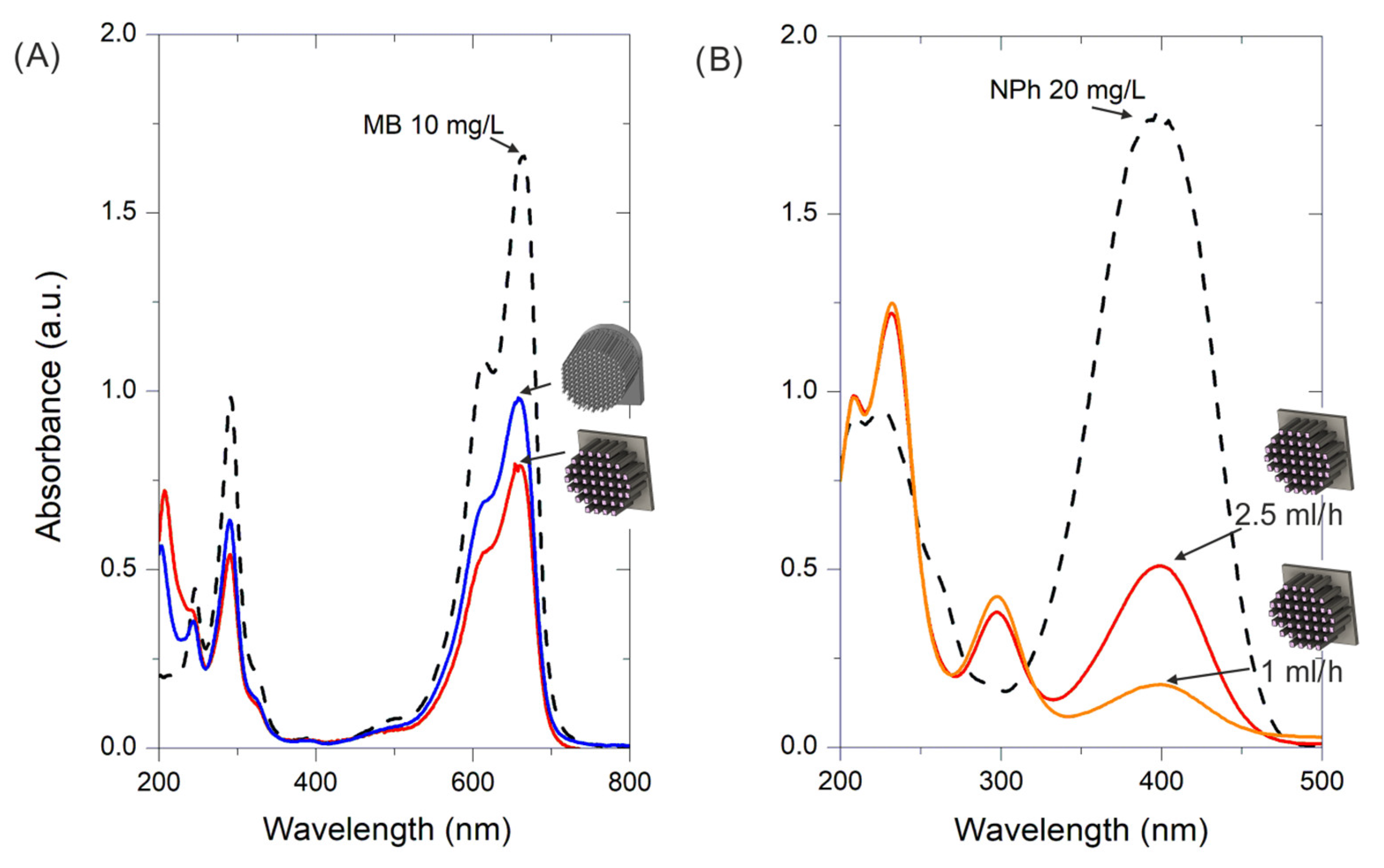
2.2.1. Photocatalytic Degradation of Methylene Blue (MB)
2.2.2. 2-Nitrophenol (2NP) Photohydrogenation to 2-Aminophenol (2AP)
2.2.3. Lignin Depolymerization
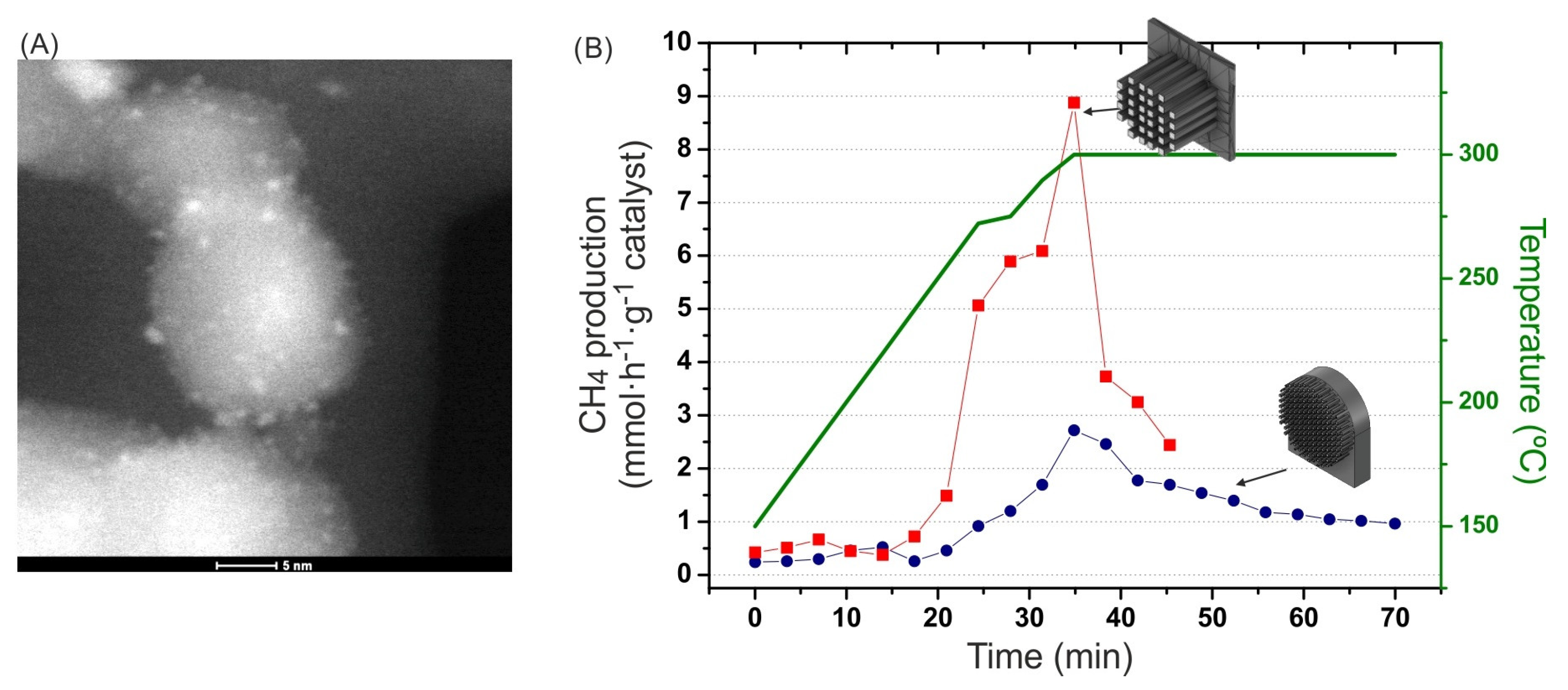
2.2.4. CO2 Hydrogenation to CH4
3. Materials and Methods
3.1. Microreactor Fabrication Protocol
3.2. Catalytic Materials and Experiments
- (i)
- Cu (3 wt. %) on TiO2 was prepared via flame spray pyrolysis following the procedure described in a previous study [27] and was selected for the photocatalytic degradation of methylene blue (MB) in water (10 mg/L). The microreactors containing the catalyst were placed in a quartz reaction tube (10 mm inner diameter), and an aqueous MB dissolution was fed using a syringe pump at flow rates ranging from 0.5 mL/h to 5 mL/h at room temperature. Illumination was performed with two UV LEDs (1200 mW radiant flux, 365 nm, Engin LZ1-10U600) placed on opposite sides with the reactor in the middle at a distance of 1 cm from each LED. The liquid effluent was collected in a cuvette, and the MB degradation reaction was monitored via UV–Vis spectroscopy (Flame Ocean Optics) through the analysis of the characteristic absorption peak at a wavelength (λ) of 665 nm.
- (ii)
- Au (1 wt. %) on the TiO2 (TECNAN, Los Arcos, Spain) catalyst was prepared as described in a previous study, in which the photohydrogenation procedure of 2-nitrophenol (2NP) to 2-aminophenol (2AP) reaction was also reported [26]. An aqueous solution of 2NP (Aldrich, 20 mg/L) was treated with sodium borohydride (1 g/L) as a reducing agent. The experimental setup was the same as the one used for MB degradation, though the reaction evolution was monitored through the analysis of the characteristic absorption band at λ = 400 nm.
- (iii)
- β-Mo2C on activated charcoal (AC) was prepared through the incipient wetness impregnation of the support using aqueous ammonium heptamolybdate tetrahydrate until achieving a nominal Mo content of 35 wt. %, followed by carboreduction as described by Wu et al. [46]. Lignin depolymerization was carried out in a two-bed in-series tubular flow reactor (½ inch o.d. stainless steel tube with 10.0 mm i.d. and 100 mm of length). The first bed contained the solid Poplar sawdust feedstock (ca. 980 mg Populus sp., dp <500 μm), separated by glass wool from the second bed that was charged with β-Mo2C/AC particles (typically 300 mg) or with the microreactor containing the catalyst. The tube was placed inside a reaction oven (JASCO RO-4068), and ethanol was circulated at 9 mL/min using an HPLC pump (JASCO PU-1585). A back-pressure regulator (JASCO BP-1580-81) allowed for controlling the reaction pressure at 3.6 MPa-g. Liquid product collection was conducted at atmospheric pressure and room temperature, and its composition was analyzed via gas chromatography and HPLC as described elsewhere [47]. The reaction temperature was fixed at 195 °C, and the temperature inside the catalyst bed was monitored by means of a K-type thermocouple (1319 A, RS Amidata). In this way, the semicontinuous reductive catalytic fractionation (RCF) of lignin was divided into two separate processes: (1) the extraction of the polymer from the raw material with ethanol, and (2) subsequent lignin depolymerization in ethanol over β-Mo2C/AC catalyst.
- (iv)
- Ru (2 wt. %) on the TiO2 (P25 Evonik) catalyst was prepared following the amino-acid-based method described in [41] using RuCl3 (Aldrich) as the Ru precursor, NaBH4 (Aldrich) as the reducing agent, and aminobutyric acid (ABA) (Aldrich) as the capping agent to control the size of the Ru nanoparticles. The actual Ru content was 1.6 wt. %, as determined by using ICP-MS (inductively coupled plasma mass spectroscopy). High Ru dispersion was confirmed by the finding that CO chemisorption gave a value of 72.9%. Temperature-programmed reduction (TPR) patterns showed two reduction events, with a main peak at 90 °C corresponding to 71% of the H2 consumption and another peak at 140 °C. The overall degree of reduction was 20%. CO2 methanation experiments were carried out in a quartz tubular reactor (10 mm inner diameter) placed in a programmable oven with temperature control through a PT100 probe located in contact with the MS reactor. The experiments were carried out with a feed stream of 5 N-mL/min with a H2/CO2 molar ratio of 4 (1 atm). Prior to the reaction, the catalyst was activated through a reduction process in 150 °C flowing H2 (10 N-mL/min) at 1 atm.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renken, A. Microstructured Reactors for Heterogeneous Catalytic Processes. Springer Ser. Chem. Phys. 2004, 75, 521–542. [Google Scholar]
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chem. Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef] [PubMed]
- Venvik, H.J.; Yang, J. Catalysis in Microstructured Reactors: Short Review on Small-Scale Syngas Production and Further Conversion into Methanol, DME and Fischer-Tropsch Products. Catal. Today 2017, 285, 135–146. [Google Scholar] [CrossRef]
- Kolb, G. Review: Microstructured Reactors for Distributed and Renewable Production of Fuels and Electrical Energy. Chem. Eng. Process. Process Intensif. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Reyero, I.; Moral, A.; Bimbela, F.; Radosevic, J.; Sanz, O.; Montes, M.; Gandía, L.M. Metallic Monolithic Catalysts Based on Calcium and Cerium for the Production of Biodiesel. Fuel 2016, 182, 668–676. [Google Scholar] [CrossRef]
- Almeida, L.C.; Echave, F.J.; Sanz, O.; Centeno, M.A.; Arzamendi, G.; Gandía, L.M.; Sousa-Aguiar, E.F.; Odriozola, J.A.; Montes, M. Fischer–Tropsch Synthesis in Microchannels. Chem. Eng. J. 2011, 167, 536–544. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. Microfluidic-Based Photocatalytic Microreactor for Environmental Application: A Review of Fabrication Substrates and Techniques, and Operating Parameters. Photochem. Photobiol. Sci. 2016, 15, 714–730. [Google Scholar] [CrossRef]
- Yusuf, A.; Garlisi, C.; Palmisano, G. Overview on Microfluidic Reactors in Photocatalysis: Applications of Graphene Derivatives. Catal. Today 2018, 315, 79–92. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A Review on Microreactors: Reactor Fabrication, Design, and Cutting-Edge Applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Liu, B.; Li, C.; Yu, Y.; Tao, S.; Li, T. Fluorescent Fluid in 3D-Printed Microreactors for the Acceleration of Photocatalytic Reactions. Adv. Sci. 2019, 6, 1900583. [Google Scholar] [CrossRef]
- Anciaux, S.K.; Geiger, M.; Bowser, M.T. 3D Printed Micro Free-Flow Electrophoresis Device. Anal. Chem. 2016, 88, 7675–7682. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, J.M.; Madani, A.; Klein, T.; Ziegler, T. Low-Budget 3D-Printed Equipment for Continuous Flow Reactions. Beilstein J. Org. Chem. 2019, 15, 558–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castedo, A.; Mendoza, E.; Angurell, I.; Llorca, J. Silicone Microreactors for the Photocatalytic Generation of Hydrogen. Catal. Today 2016, 273, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Saggiomo, V. 3D Printed Devices for Catalytic Systems. In Catalyst Immobilization; Wiley: Hoboken, NJ, USA, 2020; pp. 369–408. [Google Scholar]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Mehla, S.; Das, J.; Jampaiah, D.; Periasamy, S.; Nafady, A.; Bhargava, S.K. Recent Advances in Preparation Methods for Catalytic Thin Films and Coatings. Catal. Sci. Technol. 2019, 9, 3582–3602. [Google Scholar] [CrossRef]
- Almeida, L.C.; Echave, F.J.; Sanz, O.; Centeno, M.A.; Odriozola, J.A.; Montes, M. Washcoating of Metallic Monoliths and Microchannel Reactors. Stud. Surf. Sci. Catal. 2010, 175, 25–33. [Google Scholar]
- McDonald, J.C.; Whitesides, G.M. Poly(Dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Ren, K.; Zheng, Y.; Dai, W.; Ryan, D.; Fung, C.Y.; Wu, H. Soft-Lithography-Based High Temperature Molding Method to Fabricate Whole Teflon Microfluidic Chips. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 2010, MicroTAS, Groningen, The Netherlands, 3–7 October 2010; p. 554. [Google Scholar]
- Saggiomo, V.; Velders, A.H. Simple 3D Printed Scaffold-Removal Method for the Fabrication of Intricate Microfluidic Devices. Adv. Sci. 2015, 2, 1500125. [Google Scholar] [CrossRef]
- Yamashita, T.; Yasukawa, K.; Yunoki, E. Fabrication of a Polydimethylsiloxane Fluidic Chip Using a Sacrificial Template Made by Fused Deposition Modeling 3D Printing and Application for Flow-Injection Analysis. Anal. Sci. 2019, 35, 769–775. [Google Scholar] [CrossRef] [Green Version]
- Alkayyali, T.; Ahmadi, A. Fabrication of Microfluidic Chips Using Controlled Dissolution of 3D Printed Scaffolds. J. Appl. Polym. Sci. 2020, 137, 49524. [Google Scholar] [CrossRef]
- Montazerian, H.; Mohamed, M.G.A.; Montazeri, M.M.; Kheiri, S.; Milani, A.S.; Kim, K.; Hoorfar, M. Permeability and Mechanical Properties of Gradient Porous PDMS Scaffolds Fabricated by 3D-Printed Sacrificial Templates Designed with Minimal Surfaces. Acta Biomater. 2019, 96, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, H.; Zhu, L.; Shi, J.; Li, Z.; Xiang, N.; Yang, J. Fabrication of Different Microchannels by Adjusting the Extrusion Parameters for Sacrificial Molds. Micromachines 2019, 10, 544. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chou, C.C. Fabrication of a Nonplanar Microfluidics by Using Sonication-Assisted Dissolution Technique. In Proceedings of the NEMS 2018—13th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Singapore, 22–26 April 2018; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018; pp. 421–424. [Google Scholar]
- Pellejero, I.; Clemente, A.; Reinoso, S.; Cornejo, A.; Navajas, A.; Vesperinas, J.J.; Urbiztondo, M.A.; Gandía, L.M. Innovative Catalyst Integration on Transparent Silicone Microreactors for Photocatalytic Applications. Catal. Today 2022, 383, 164–172. [Google Scholar] [CrossRef]
- Cabrera, A.; Pellejero, I.; Oroz-Mateo, T.; Salazar, C.; Navajas, A.; Fernández-Acevedo, C.; Gandía, L.M. Three-Dimensional Printing of Acrylonitrile Butadiene Styrene Microreactors for Photocatalytic Applications. Ind. Eng. Chem. Res. 2020, 59, 20686–20692. [Google Scholar] [CrossRef]
- Mingmongkol, Y.; Trinh, D.T.T.; Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles. Nanomaterials 2022, 12, 1198. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 Phase on the Photocatalytic Degradation of Methylene Blue Dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Phan, D.P.; Sarwar, A.; Tran, M.H.; Lee, O.K.; Lee, E.Y. Valorization of Industrial Lignin to Value-Added Chemicals by Chemical Depolymerization and Biological Conversion. Ind. Crops Prod. 2021, 161, 113219. [Google Scholar] [CrossRef]
- Welker, C.; Balasubramanian, V.; Petti, C.; Rai, K.; DeBolt, S.; Mendu, V. Engineering Plant Biomass Lignin Content and Composition for Biofuels and Bioproducts. Energies 2015, 8, 7654–7676. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(Dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Ramirez, A.; Sarathy, S.M.; Gascon, J. CO2 Derived E-Fuels: Research Trends, Misconceptions, and Future Directions. Trends Chem. 2020, 2, 785–795. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The Renaissance of the Sabatier Reaction and Its Applications on Earth and in Space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Kim, A.; Debecker, D.P.; Devred, F.; Dubois, V.; Sanchez, C.; Sassoye, C. CO2 Methanation on Ru/TiO2 Catalysts: On the Effect of Mixing Anatase and Rutile TiO2 Supports. Appl. Catal. B Environ. 2018, 220, 615–625. [Google Scholar] [CrossRef]
- Mateo, D.; Maity, P.; Shterk, G.; Mohammed, O.F.; Gascon, J. Tunable Selectivity in CO2 Photo-Thermal Reduction by Perovskite-Supported Pd Nanoparticles. ChemSusChem 2021, 14, 5525–5533. [Google Scholar] [CrossRef] [PubMed]
- Bando, K.K.; Arakawa, H.; Ichikuni, N. CO2 Hydrogenation over Micro- and Mesoporous Oxides Supported Ru Catalysts. Catal. Lett. 1999, 60, 125–132. [Google Scholar] [CrossRef]
- Khan, I.S.; Mateo, D.; Shterk, G.; Shoinkhorova, T.; Poloneeva, D.; Garzón-Tovar, L.; Gascon, J. An Efficient Metal–Organic Framework-Derived Nickel Catalyst for the Light Driven Methanation of CO2. Angew. Chem. Int. Ed. 2021, 60, 26476–26482. [Google Scholar] [CrossRef]
- Mateo, D.; Albero, J.; García, H. Graphene Supported NiO/Ni Nanoparticles as Efficient Photocatalyst for Gas Phase CO2 Reduction with Hydrogen. Appl. Catal. B Environ. 2018, 224, 563–571. [Google Scholar] [CrossRef]
- Yu, J.W.; Li, W.Z.; Zhang, T.; Ma, D.; Zhang, Y.W. Ruthenium Nanoclusters Dispersed on Titania Nanorods and Nanoparticles as High-Performance Catalysts for Aqueous-Phase Fischer–Tropsch Synthesis. Catal. Sci. Technol. 2016, 6, 8355–8363. [Google Scholar] [CrossRef]
- Koh, T.; Koo, H.M.; Yu, T.; Lim, B.; Bae, J.W. Roles of Ruthenium-Support Interactions of Size-Controlled Ruthenium Nanoparticles for the Product Distribution of Fischer-Tropsch Synthesis. ACS Catal. 2014, 4, 1054–1060. [Google Scholar] [CrossRef]
- Kim, A.; Sanchez, C.; Patriarche, G.; Ersen, O.; Moldovan, S.; Wisnet, A.; Sassoye, C.; Debecker, D.P. Selective CO2 Methanation on Ru/TiO2 Catalysts: Unravelling the Decisive Role of the TiO2 Support Crystal Structure. Catal. Sci. Technol. 2016, 6, 8117–8128. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Li, J.; You, Z. A Facile TiO2 Containing Oxygen Vacancies and Hydroxyl as a Ru-Loaded Underlay for CO2 Hydrogenation to CH4. Appl. Surf. Sci. 2022, 587, 152856. [Google Scholar] [CrossRef]
- Falbo, L.; Visconti, C.G.; Lietti, L.; Szanyi, J. The Effect of CO on CO2 Methanation over Ru/Al2O3 Catalysts: A Combined Steady-State Reactivity and Transient DRIFT Spectroscopy Study. Appl. Catal. B Environ. 2019, 256, 117791. [Google Scholar] [CrossRef]
- Ewald, S.; Kolbeck, M.; Kratky, T.; Wolf, M.; Hinrichsen, O. On the Deactivation of Ni-Al Catalysts in CO2 Methanation. Appl. Catal. A Gen. 2019, 570, 376–386. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.; Zhu, Y.; Wang, X.; Yang, C.; Liu, Y.; Liu, C.; Lu, H.; Liang, B.; Li, Y. Supported β-Mo2C on Carbon Materials for Kraft Lignin Decomposition into Aromatic Monomers in Ethanol. Ind. Eng. Chem. Res. 2019, 58, 12602–12610. [Google Scholar] [CrossRef]
- Cornejo, A.; Bimbela, F.; Moreira, R.; Hablich, K.; García-Yoldi, Í.; Maisterra, M.; Portugal, A.; Gandía, L.M.; Martínez-Merino, V. Production of Aromatic Compounds by Catalytic Depolymerization of Technical and Downstream Biorefinery Lignins. Biomolecules 2020, 10, 1338. [Google Scholar] [CrossRef]
- Ling, F.W.M.; Abdulbari, H.A.; Chin, S.Y. Heterogeneous Microfluidic Reactors: A Review and an Insight of Enzymatic Reactions. ChemBioEng Rev. 2022, 9, 265–285. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Q.; Shao, L.; Jia, Y.; Zhang, X. Microfluidic Immobilized Enzyme Reactors for Continuous Biocatalysis. React. Chem. Eng. 2020, 5, 9–32. [Google Scholar] [CrossRef]
| Design | Reactor Length-Diameter (mm) | Rod Dimension (mm) | Rod Number (Density %) | Fluidic Volume (mm3) | Nozzle (mm) | Layer Height | Printing T (°C) and Velocity (mm/s) | |
|---|---|---|---|---|---|---|---|---|
 | 10–20 | 9 | 0.2 × 0.25 | 154 (12%) | 77–154 | 0.25 | 0.2 | 255/50 |
 | 20 | 14 | 0.4 × 0.3 | 98 (7.6%) | 235.2 | 0.4 | 0.3 | 255/60 |
 | 20 | 14 | 0.8 × 0.8 | 60 (25%) | 768 | 0.4 | 0.2 | 255/60 |
 | 20 | 14 | 0.8 × 0.3 | 198 (30%) | 950.4 | 0.8 | 0.3 | 250/70 |
 | 10–15 | 10 | 0.8 × 0.8 | 32 (26%) | 204.8–307.2 | 0.4 | 0.2 | 255/60 |
 | 15 | 15 | 1.0 × 1.0 | 44 (24%) | 660 | 0.4 | 0.2 | 255/60 |
| Multichannel Microreactor | Fixed Bed | |||
|---|---|---|---|---|
| Reaction Cycle Number | Phenolic Monomer Yield (%) | Phenolic Monomer Yield (%)/mgcat. | Phenolic Monomer Yield (%) | Phenolic Monomer Yield (%)/mgcat. |
| Fresh catalyst | 26 | 2.83 | 13.3 | 0.044 |
| 2 | 15 | 1.63 | 12.4 | 0.041 |
| 3 | 10 | 1.09 | 19.4 | 0.065 |
| 4 | 10 | 1.09 | 15.5 | 0.052 |
| 5 | 10 | 1.09 | 13.8 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez-de-Garayo, A.; Imizcoz, M.; Maisterra, M.; Almazán, F.; Sanz, D.; Bimbela, F.; Cornejo, A.; Pellejero, I.; Gandía, L.M. The 3D-Printing Fabrication of Multichannel Silicone Microreactors for Catalytic Applications. Catalysts 2023, 13, 157. https://doi.org/10.3390/catal13010157
Ibáñez-de-Garayo A, Imizcoz M, Maisterra M, Almazán F, Sanz D, Bimbela F, Cornejo A, Pellejero I, Gandía LM. The 3D-Printing Fabrication of Multichannel Silicone Microreactors for Catalytic Applications. Catalysts. 2023; 13(1):157. https://doi.org/10.3390/catal13010157
Chicago/Turabian StyleIbáñez-de-Garayo, Alejandro, Mikel Imizcoz, Maitane Maisterra, Fernando Almazán, Diego Sanz, Fernando Bimbela, Alfonso Cornejo, Ismael Pellejero, and Luis M. Gandía. 2023. "The 3D-Printing Fabrication of Multichannel Silicone Microreactors for Catalytic Applications" Catalysts 13, no. 1: 157. https://doi.org/10.3390/catal13010157