Microreactor Based on Trimetallic Nano-Oxides Obtained by In Situ Growth from German Silver
Abstract
:1. Introduction
2. Results and Discussion
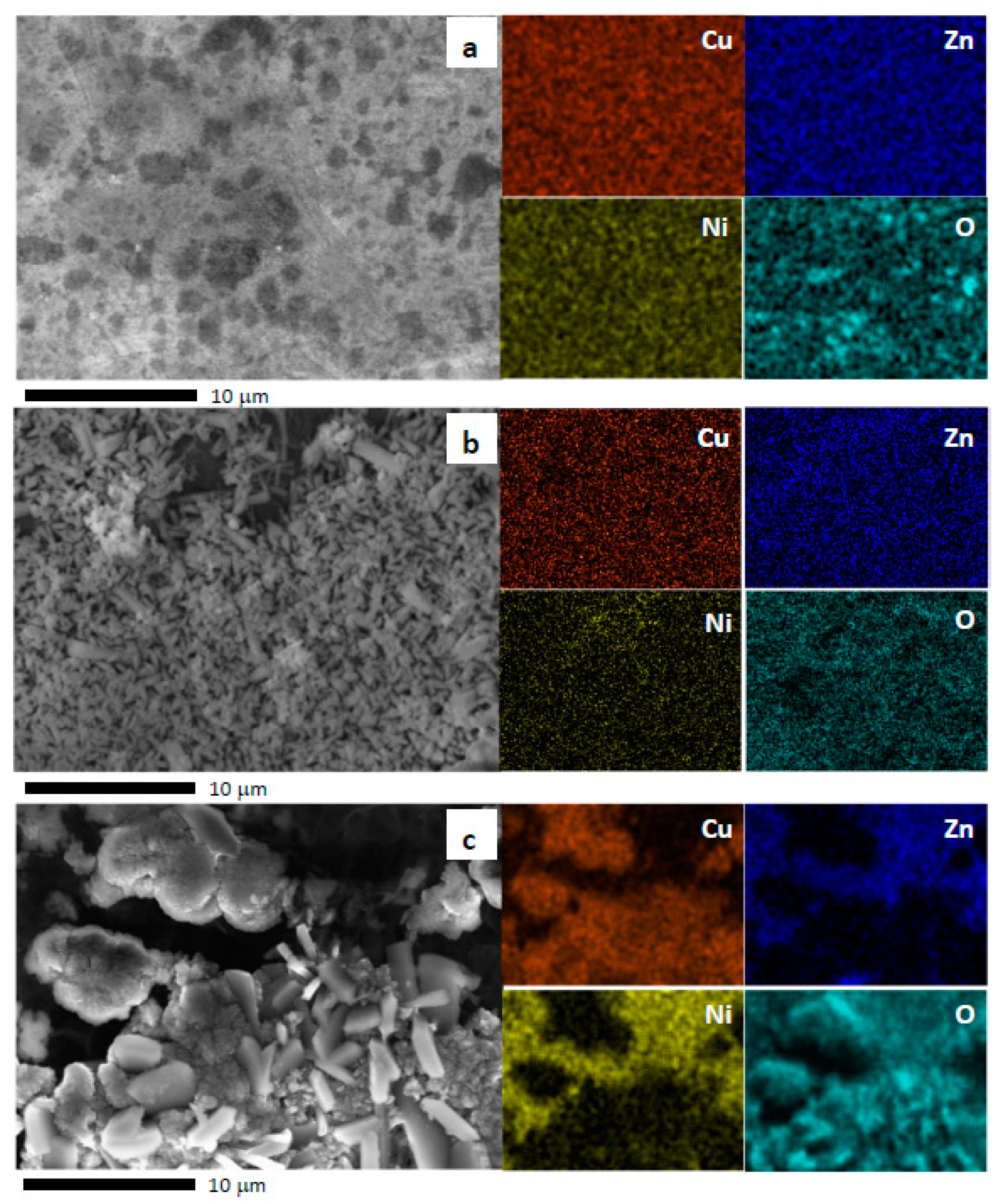
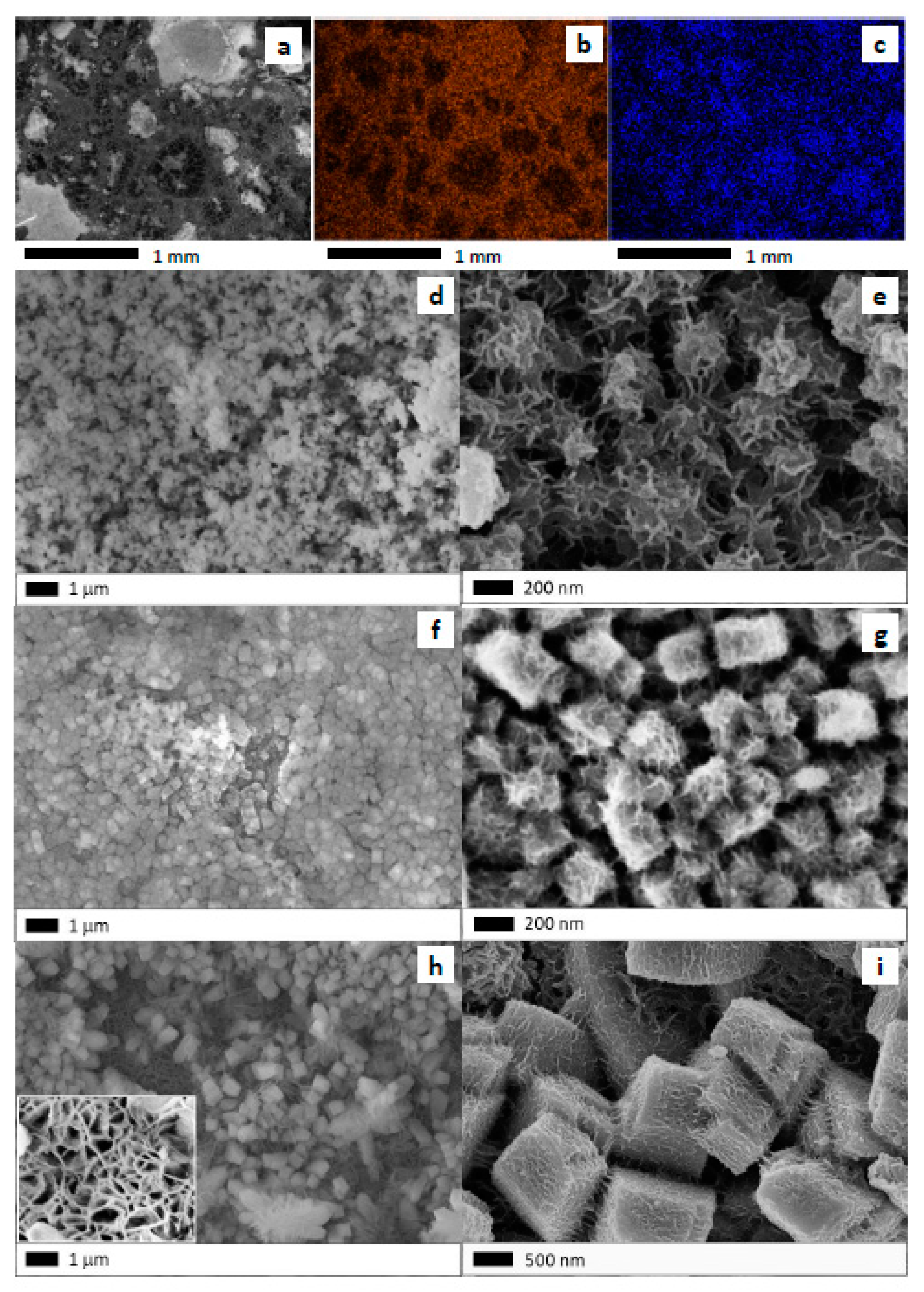
2.1. Physicochemical Characteristics of the Nano-Oxide Film
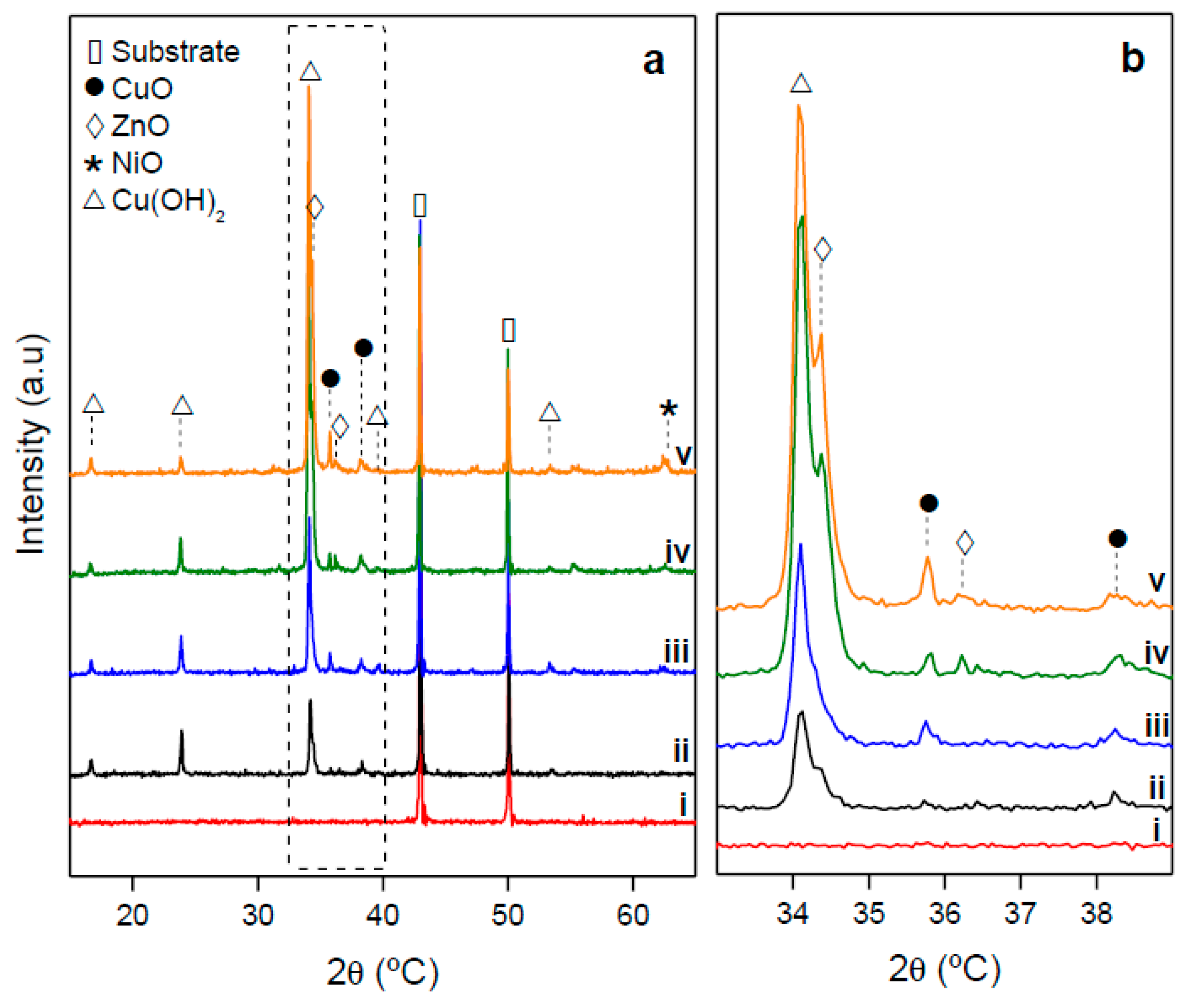
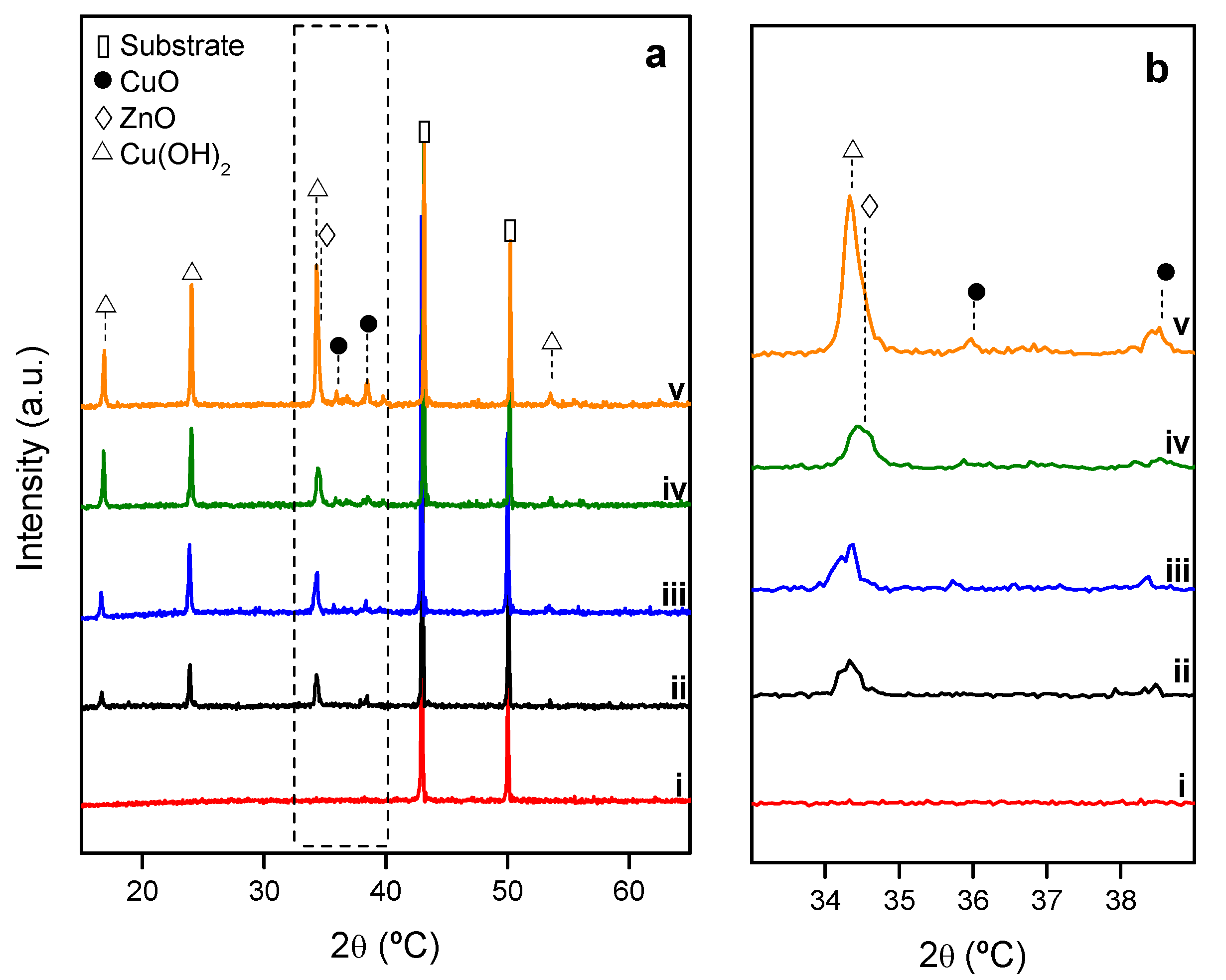
2.1.1. Evolution of Growth with Time
2.1.2. Growth of Nanooxides at Room Temperature
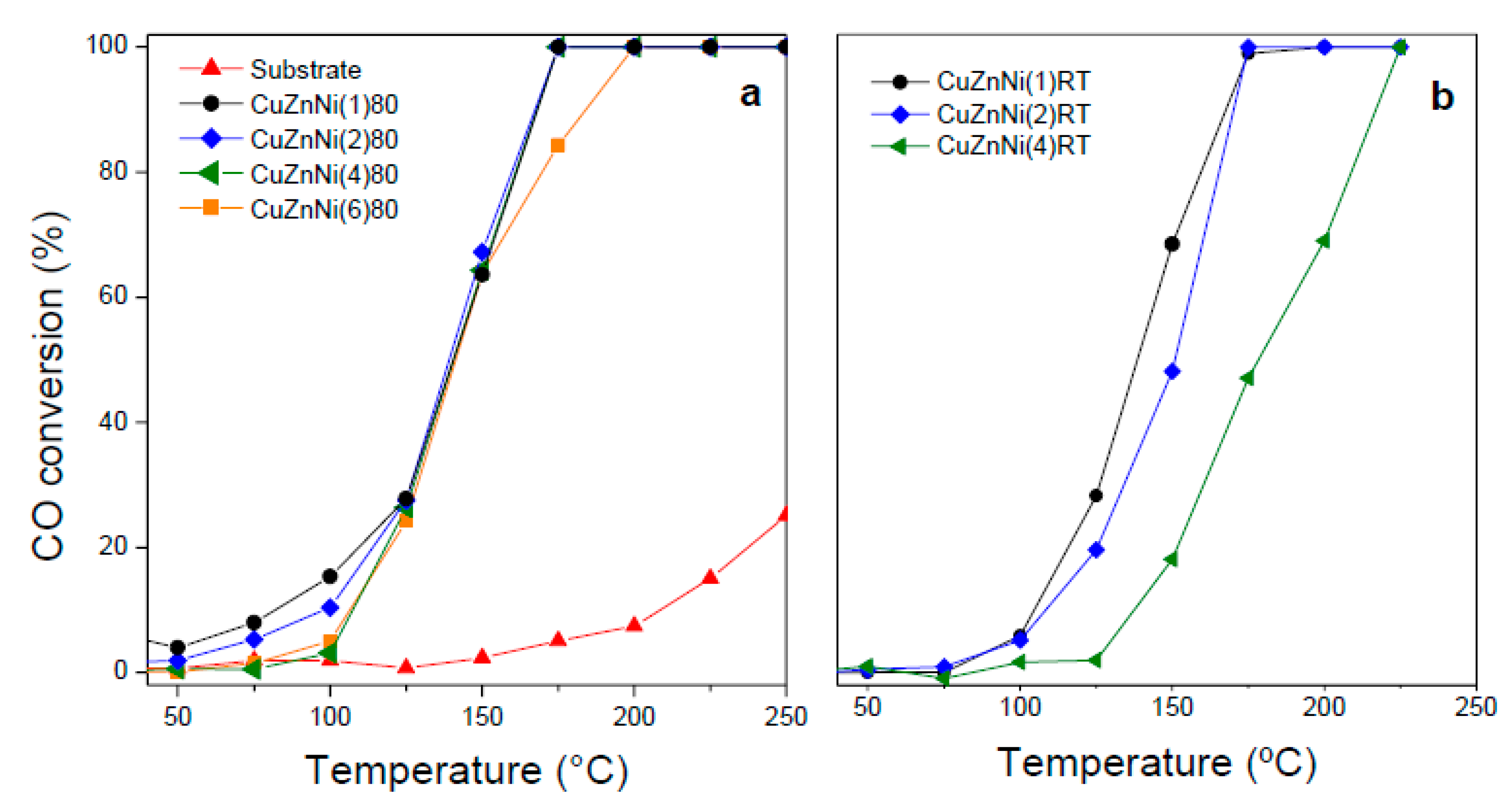
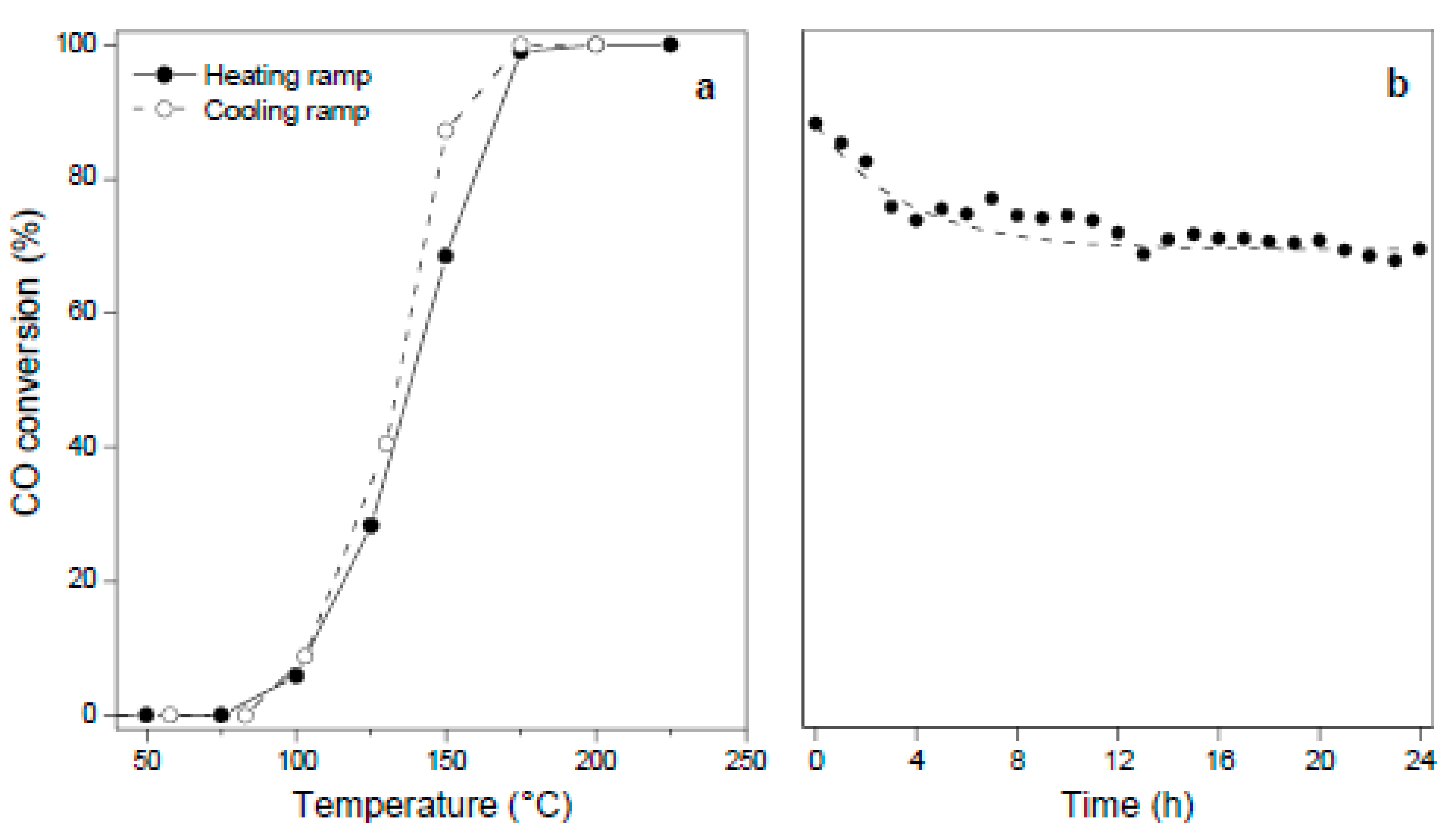
2.2. Catalytic Performance of CuZnNi Oxides Film
3. Materials and Methods
3.1. Synthesis of Nanostructured Oxide Films
3.2. Sample Characterization
3.3. Catalytic Performance in a Microreactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bullock, R.M. Reaction: Earth-Abundant Metal Catalysts for Energy Conversions. Chem 2017, 2, 444–446. [Google Scholar] [CrossRef]
- Bion, N.; Can, F.; Courtois, X.; Duprez, D. Transition Metal Oxides for Combustion and Depollution Processes. In Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 287–353. [Google Scholar]
- Chenoweth, J.A.; Albertson, T.E.; Greer, M.R. Carbon Monoxide Poisoning. Crit. Care Clin. 2021, 37, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J.L. Carbon Monoxide Poisoning and Mitigation Strategies for Polymer Electrolyte Membrane Fuel Cells—A Review. Prog. Energy Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Boix, A.V.; Martínez-Hernández, A. CO Oxidation over Au Supported on Mn–ZSM5 and Mn–MOR. Catal. Commun. 2015, 69, 212–216. [Google Scholar] [CrossRef]
- Abedi, A.; Luo, J.Y.; Epling, W.S. Improved CO, Hydrocarbon and NO Oxidation Performance Using Zone-Coated Pt-Based Catalysts. Catal. Today 2013, 207, 220–226. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO Oxidation over Pd-Based Catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Camposeco, R.; Maturano-Rojas, V.; Zanella, R. Highly Efficient Au/ZnO–ZrO2 Catalysts for CO Oxidation at Low Temperature. Catalysts 2023, 13, 590. [Google Scholar] [CrossRef]
- Saoud, K.M.; El-Shall, M.S. Physical and Chemical Synthesis of Au/CeO2 Nanoparticle Catalysts for Room Temperature CO Oxidation: A Comparative Study. Catalysts 2020, 10, 1351. [Google Scholar] [CrossRef]
- Duan, D.; Hao, C.; He, G.; Wang, H.; Shi, W.; Gao, L.; Sun, Z. Co3O4 Nanosheet/Au Nanoparticle/CeO2 Nanorod Composites as Catalysts for CO Oxidation at Room Temperature. ACS Appl. Nano Mater. 2020, 3, 12416–12426. [Google Scholar] [CrossRef]
- Lu, S.; Song, H.; Xiao, Y.; Qadir, K.; Li, Y.; Li, Y.; He, G. Promoted Catalytic Activity of CO Oxidation at Low Temperatures by Tuning ZnO Morphology for Optimized CuO/ZnO Catalysts. Colloid Interface Sci. Commun. 2023, 52, 100698. [Google Scholar] [CrossRef]
- Du, L.; Wang, W.; Yan, H.; Wang, X.; Jin, Z.; Song, Q.; Si, R.; Jia, C. Copper-Ceria Sheets Catalysts: Effect of Copper Species on Catalytic Activity in CO Oxidation Reaction. J. Rare Earths 2017, 35, 1186–1196. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Song, H.; Cui, Y.; Xue, Y.; Wu, C.; Pan, C.; Xu, J.; Qiu, J.; Xu, L. Transition Metal (Fe2O3, Co3O4 and NiO)-Promoted CuO-Based α-MnO2 Nanowire Catalysts for Low-Temperature CO Oxidation. Catalysts 2023, 13, 588. [Google Scholar] [CrossRef]
- Hu, X.; Mao, D.; Yu, J.; Xue, Z. Low-Temperature CO Oxidation on CuO-CeO2-ZrO2 Catalysts Prepared by a Facile Surfactant-Assisted Grinding Method. Fuel 2023, 340, 127529. [Google Scholar] [CrossRef]
- Stoian, M.; Maurer, T.; Lamri, S.; Fechete, I. Techniques of Preparation of Thin Films: Catalytic Combustion. Catalysts 2021, 11, 1530. [Google Scholar] [CrossRef]
- Wiame, F.; Maurice, V.; Marcus, P. Initial Stages of Oxidation of Cu0.7Zn0.3(1 1 1). Surf. Sci. 2007, 601, 4402–4406. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, T.; Lin, C.; Lee, W.-J.; Kim, S.-J.; Gorte, R.J.; Jung, W. The Effects of Iron Oxide Overlayers on Pt for CO Oxidation. Catal. Commun. 2022, 172, 106549. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Li, H.; Xu, R.; Sun, Z. Highly Active and Water-Resistant Lanthanum-Doped Platinum-Cobalt Oxide Catalysts for CO Oxidation. Appl. Catal. B Environ. 2023, 331, 122678. [Google Scholar] [CrossRef]
- Taylor, S.H.; Hutchings, G.J.; Mirzaei, A.A. The Preparation and Activity of Copper Zinc Oxide Catalysts for Ambient Temperature Carbon Monoxide Oxidation. Catal. Today 2003, 84, 113–119. [Google Scholar] [CrossRef]
- Wang, D.; Xu, R.; Wang, X.; Li, Y. NiO Nanorings and Their Unexpected Catalytic Property for CO Oxidation. Nanotechnology 2006, 17, 979–983. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Kim, I.H.; Han, S.W.; Kim, D.H.; Kim, Y.D. Room Temperature CO Oxidation Catalyzed by NiO Particles on Mesoporous SiO2 Prepared via Atomic Layer Deposition: Influence of Pre-Annealing Temperature on Catalytic Activity. J. Mol. Catal. A Chem. 2016, 414, 87–93. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.L.; Qiu, J.X.; Chen, Z.H. Charaterization and Catalytic Activity of CuO and NiO Supported on ZrO2 for CO Low Temperature Oxidation. Adv. Mater. Res. 2013, 842, 237–241. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Yehia, N.S.; Hassan, H.M.A.; Badawy, A.R.A.A. Catalytic Oxidation of CO by O2 over Nanosized CuO–ZnO System Prepared under Various Conditions. Can. J. Chem. Eng. 2009, 87, 792–800. [Google Scholar] [CrossRef]
- Soliman, N.K. Factors Affecting CO Oxidation Reaction over Nanosized Materials: A Review. J. Mater. Res. Technol. 2019, 8, 2395–2407. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Miró, E.E. Copper Alloy Microstructures for Application in Microreactors. In Copper Alloys: Preparation, Properties and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 127–139. [Google Scholar]
- Groppi, G.; Airoldi, G.; Cristiani, C.; Tronconi, E. Characteristics of Metallic Structured Catalysts with High Thermal Conductivity. Catal. Today 2000, 60, 57–62. [Google Scholar] [CrossRef]
- Neyertz, C.A.; Gallo, A.D.; Ulla, M.A.; Zamaro, J.M. Nanostructured CuOx Coatings onto Cu Foils: Surface Growth by the Combination of Gas-Phase Treatments. Surf. Coat. Technol. 2016, 285, 262–269. [Google Scholar] [CrossRef]
- Papurello, R.L.; Cabello, A.P.; Ulla, M.A.; Neyertz, C.A.; Zamaro, J.M. Microreactor with Copper Oxide Nanostructured Films for Catalytic Gas Phase Oxidations. Surf. Coat. Technol. 2017, 328, 231–239. [Google Scholar] [CrossRef]
- Cabello, A.P.; Ulla, M.A.; Zamaro, J.M. In Situ Growth of Nanostructured Copper and Zinc Mixed Oxides on Brass Supports as Efficient Microreactors for the Catalytic CO Oxidation. J. Mater. Sci. 2022, 57, 12797–12809. [Google Scholar] [CrossRef]
- Neikov, O.D.; Naboychenko, S.S.; Murashova, I.B. Production of Copper and Copper Alloy Powders. In Handbook of Non-Ferrous Metal Powders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 571–614. [Google Scholar]
- Amin, A.; El-dissouky, A. One-Step Synthesis of Novel Cu2ZnNiO3 Complex Oxide Nanowires with Tuned Band Gap for Photoelectrochemical Water Splitting. J. Appl. Crystallogr. 2020, 53, 1425–1433. [Google Scholar] [CrossRef]
- Bahnasawy, N.; Elbanna, A.M.; Ramadan, M.; Allam, N.K. Fabrication of Polyhedral Cu–Zn Oxide Nanoparticles by Dealloying and Anodic Oxidation of German Silver Alloy for Photoelectrochemical Water Splitting. Sci. Rep. 2022, 12, 16785. [Google Scholar] [CrossRef]
- Babikier, M.; Wang, D.; Wang, J.; Li, Q.; Sun, J.; Yan, Y.; Yu, Q.; Jiao, S. Cu-Doped ZnO Nanorod Arrays: The Effects of Copper Precursor and Concentration. Nanoscale Res. Lett. 2014, 9, 199. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, Y.; Li, Z.; Liu, X.; Tian, Z.; Liu, C.; Li, J.; Wang, L. Electronic Metal-Support Interactions Between CuxO and ZnO for CuxO/ZnO Catalysts with Enhanced CO Oxidation Activity. Front. Chem. 2022, 10, 912550. [Google Scholar] [CrossRef] [PubMed]
- Cabello, A.P.; Ulla, M.A.; Zamaro, J.M. CeO2/CuOx Nanostructured Films for CO Oxidation and CO Oxidation in Hydrogen-Rich Streams Using a Micro-Structured Reactor. Top. Catal. 2019, 62, 931–940. [Google Scholar] [CrossRef]
- Pérez, N.C.; Miró, E.E.; Zamaro, J.M. Microreactors Based on CuO–CeO2/Zeolite Films Synthesized onto Brass Microgrids for the Oxidation of CO. Appl. Catal. B Environ. 2013, 129, 416–425. [Google Scholar] [CrossRef]
- Papurello, R.L.; Fernández, J.L.; Miró, E.E.; Zamaro, J.M. Microreactor with Silver-Loaded Metal-Organic Framework Films for Gas-Phase Reactions. Chem. Eng. J. 2017, 313, 1468–1476. [Google Scholar] [CrossRef]

| Sample | Cu/Zn | Cu/Ni | Zn/Ni |
|---|---|---|---|
| Substrate | 3.2 | 4.6 | 1.4 |
| CuZnNi(1)80 | 3.2 | 4.3 | 1.4 |
| CuZnNi(2)80 | 3.9 | 8.0 | 2.1 |
| CuZnNi(6)80 | 3.4 | 4.1 | 1.2 |
| CuZnNi(1)RT | 3.2 | 4.8 | 1.5 |
| CuZnNi(2)RT | 4.6 | 6.1 | 1.3 |
| Sample | mO a | T50 | X150 b | Ra c |
|---|---|---|---|---|
| CuZnNi(1)80 | 5.9 | 142 | 63.6 | 10.8 |
| CuZnNi(2)80 | 10.0 | 140 | 67.2 | 6.7 |
| CuZnNi(4)80 | 13.7 | 142 | 64.3 | 4.7 |
| CuZnNi(6)80 | 17.5 | 141 | 63.9 | 3.7 |
| CuZnNi(1)RT | 0.5 | 138 | 65.9 | 146.4 |
| CuZnNi(2)RT | 2.3 | 151 | 48.2 | 20.5 |
| CuZnNi(4)RT | 3.4 | 182 | 18.6 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello, A.P.; Franco Murcia, M.A.; Ulla, M.A.; Zamaro, J.M. Microreactor Based on Trimetallic Nano-Oxides Obtained by In Situ Growth from German Silver. Catalysts 2023, 13, 932. https://doi.org/10.3390/catal13060932
Cabello AP, Franco Murcia MA, Ulla MA, Zamaro JM. Microreactor Based on Trimetallic Nano-Oxides Obtained by In Situ Growth from German Silver. Catalysts. 2023; 13(6):932. https://doi.org/10.3390/catal13060932
Chicago/Turabian StyleCabello, Ana P., Mayra A. Franco Murcia, María A. Ulla, and Juan M. Zamaro. 2023. "Microreactor Based on Trimetallic Nano-Oxides Obtained by In Situ Growth from German Silver" Catalysts 13, no. 6: 932. https://doi.org/10.3390/catal13060932






