Cu-Doped SrTiO3 Nanostructured Catalysts for CO2 Conversion into Solar Fuels Using Localised Surface Plasmon Resonance
Abstract
:1. Introduction
2. Results and Discussion
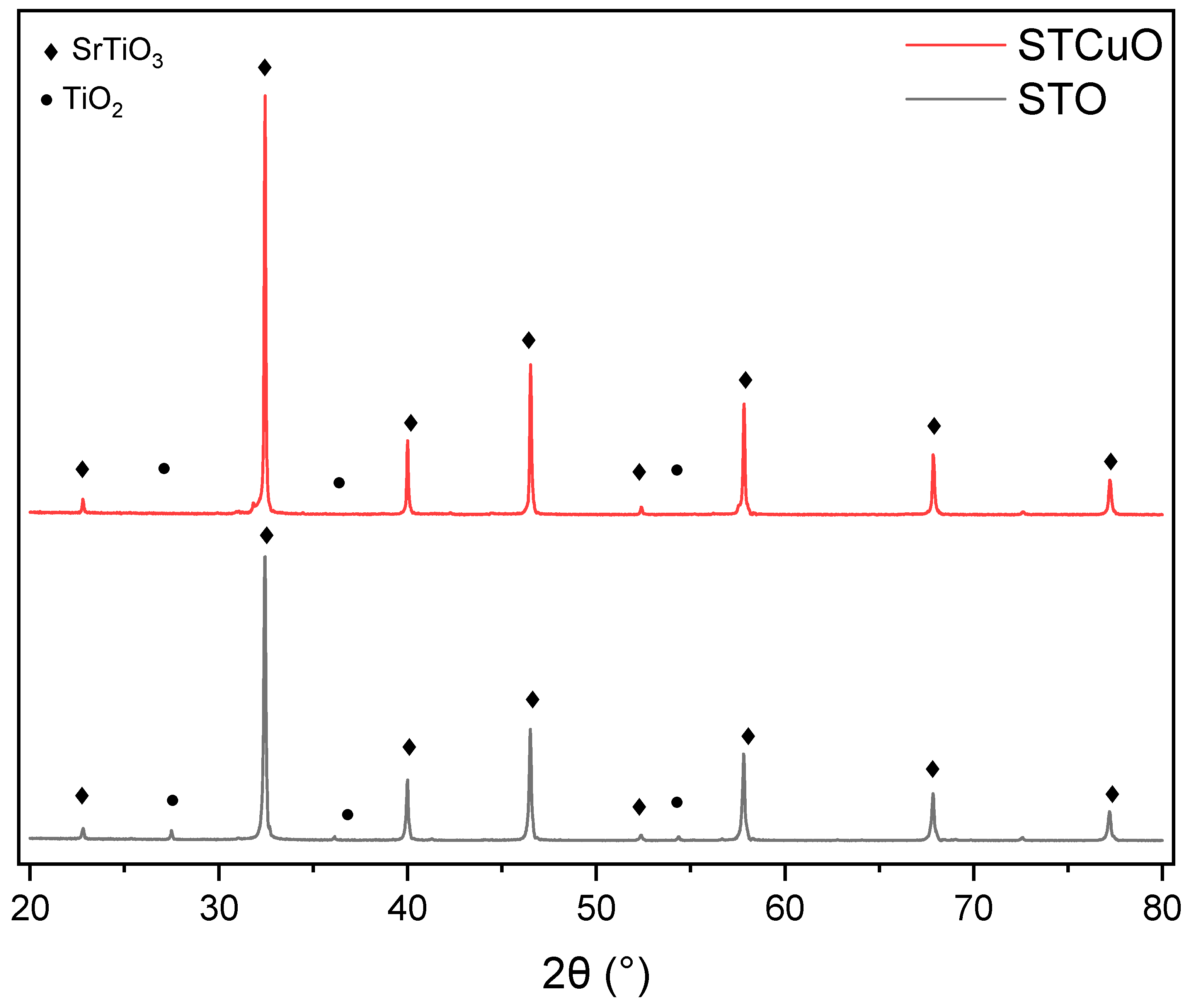
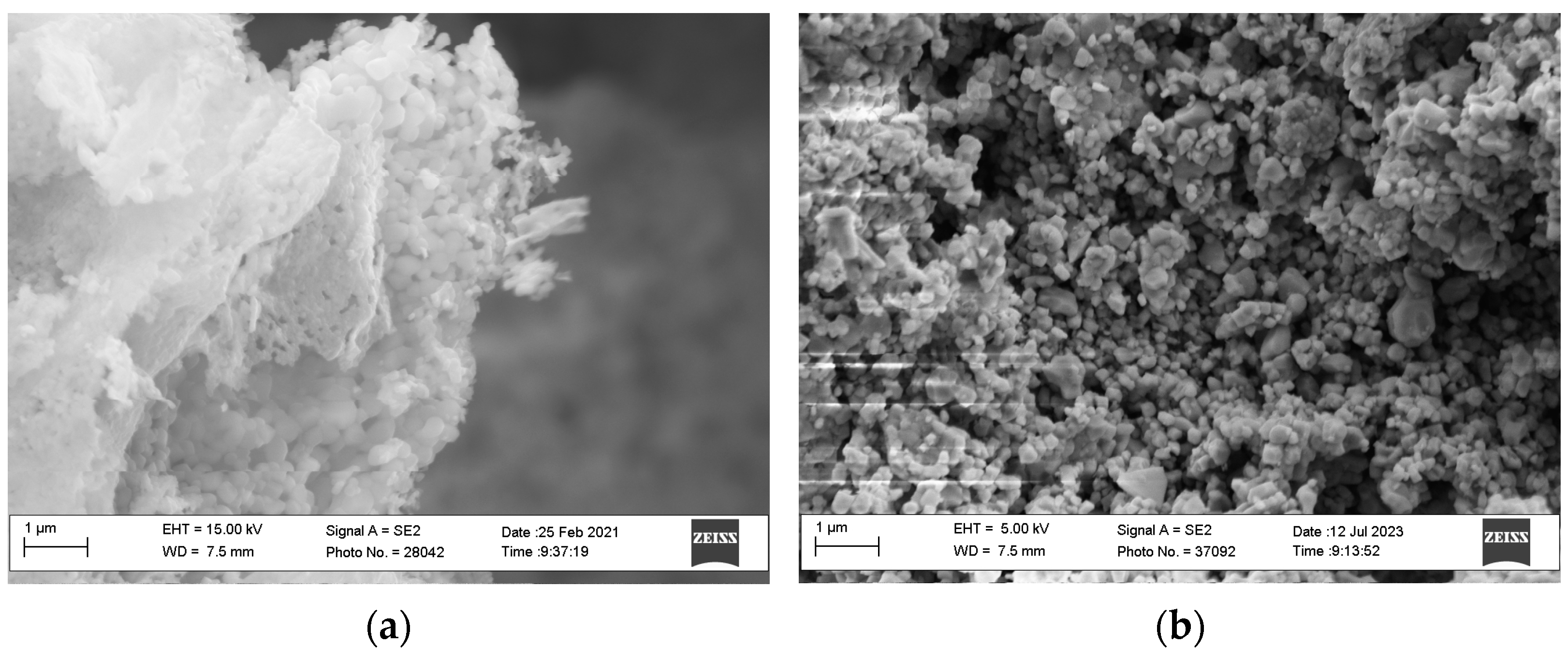
2.1. Structural and Morphological Properties
2.2. Functional Properties
2.2.1. Bulk Reducibility
2.2.2. Electronic Properties
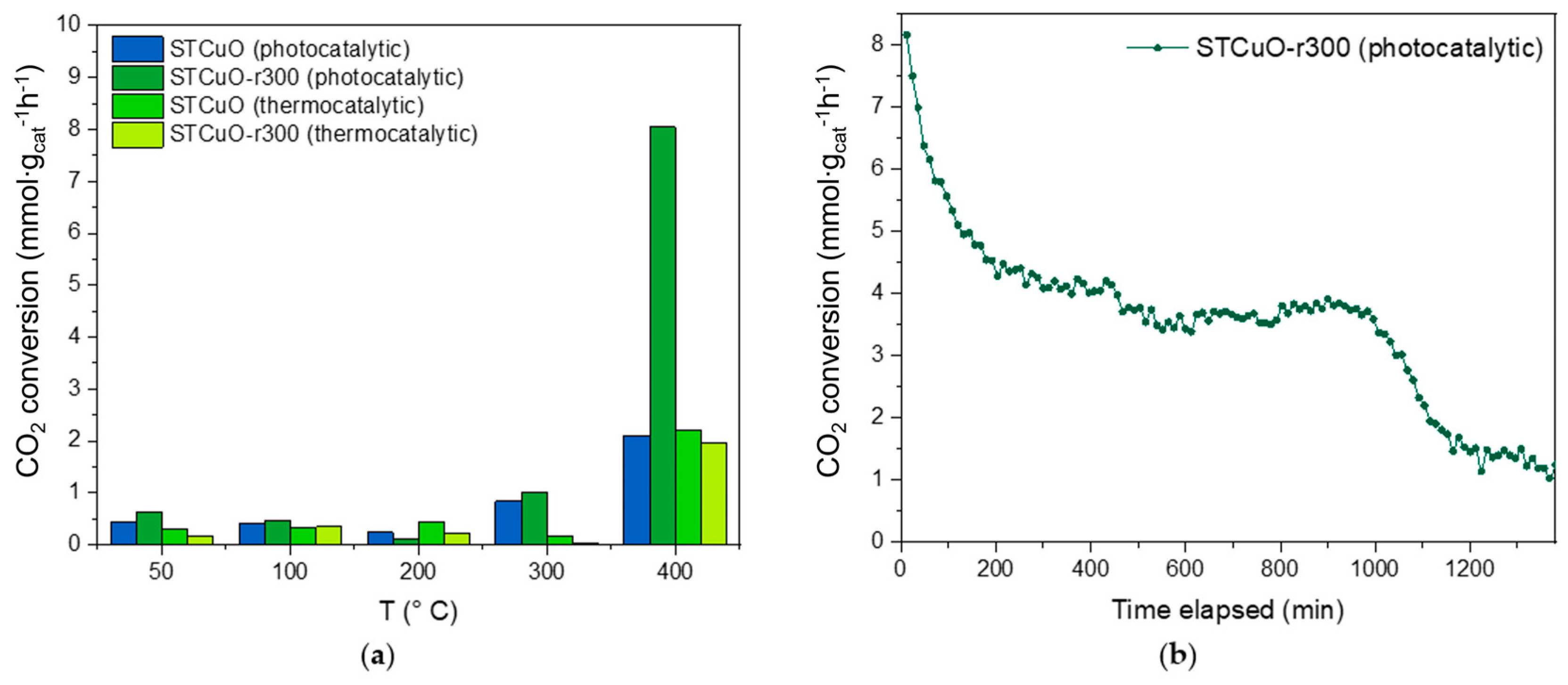
2.3. Catalytic Activity
3. Materials and Methods
3.1. Synthetic Procedure
3.2. Materials’ Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, J.M.; Morgan, V.I. Natural and Anthropogenic Changes in Atmospheric CO2 over the Last 1000 Years from Air in Antarctic Ice and Firn. J. Geophys. Res. Atmos. 1996, 101, 4115–4128. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. Adv. Carbon Capture Methods Technol. Appl. 2020, 3–28. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of Global Warming Due to Carbon-Cycle Feedbacks in a Coupled Climate Model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.T.D.; Nik, V.M.; Chen, D.; Scartezzini, J.L.; Hong, T. Quantifying the Impacts of Climate Change and Extreme Climate Events on Energy Systems. Nat. Energy 2020, 5, 150–159. [Google Scholar] [CrossRef]
- Taylor, J.H.; Benedict, W.S.; Strong, J. Infrared Spectra of H2O and CO2 at 500 °C. J. Chem. Phys. 1952, 20, 1884–1898. [Google Scholar] [CrossRef]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the Greenhouse Effect and Global Warming: From the Pioneering Work of Arrhenius and Callendar to Today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. IPCC, 2019: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and Kinetics of CO2, CO, and H+ Binding to the Metal Centre of CO2 reductioncatalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy Related CO2 Conversion and Utilization: Advanced Materials/Nanomaterials, Reaction Mechanisms and Technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. [Google Scholar] [CrossRef]
- Resasco, J.; Bell, A.T. Electrocatalytic CO2 Reduction to Fuels: Progress and Opportunities. Trends Chem. 2020, 2, 825–836. [Google Scholar] [CrossRef]
- Alam, M.I.; Cheula, R.; Moroni, G.; Nardi, L.; Maestri, M. Mechanistic and Multiscale Aspects of Thermo-Catalytic CO2 Conversion to C 1 Products. Catal. Sci. Technol. 2021, 11, 6601–6629. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Tang, Z.; He, W.; Wang, Y.; Wang, X.; Zhao, Z.; Wei, Y. Recent Progress in Metal Oxide-Based Photocatalysts for CO2 Reduction to Solar Fuels: A Review. Molecules 2023, 28, 1653. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.Q.; Jiang, H.L. Photocatalytic CO2 Reduction over Metal-Organic Framework-Based Materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Zeng, S.; Kar, P.; Thakur, U.K.; Shankar, K. A Review on Photocatalytic CO2 Reduction Using Perovskite Oxide Nanomaterials. Nanotechnology 2018, 29, 052001. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q. Van MXenes: Applications in Electrocatalytic, Photocatalytic Hydrogen Evolution Reaction and CO2 Reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge Carrier Trapping, Recombination and Transfer during TiO2 Photocatalysis: An Overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Vu, N.-N.; Kaliaguine, S.; Do, T.-O.; Vu, N.; Kaliaguine, S.; Do, T. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Garcia, H. Solar Light Photocatalytic CO2 Reduction: General Considerations and Selected Bench-Mark Photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Chen, K.; Leong, E.S.P.; Rukavina, M.; Nagao, T.; Liu, Y.J.; Zheng, Y. Active Molecular Plasmonics: Tuning Surface Plasmon Resonances by Exploiting Molecular Dimensions. Nanophotonics 2015, 4, 186–197. [Google Scholar] [CrossRef]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S.; Yoshimura, K. Wavelength Tuning of Surface Plasmon Resonance Using Dielectric Layers on Silver Island Films. Appl. Phys. Lett. 2003, 82, 3811–3813. [Google Scholar] [CrossRef]
- Klein, N.; Senkovska, I.; Gedrich, K.; Stoeck, U.; Henschel, A.; Mueller, U.; Kaskel, S. A Mesoporous Metal-Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 9954–9957. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Tanaka, T.; Funabiki, T.; Yoshida, S. Photoreduction of CO2 with H2 over ZrO2. A Study on Interaction of Hydrogen with Photoexcited CO2. Phys. Chem. Chem. Phys. 2000, 2, 2635–2639. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic Reduction of Carbon Dioxide in Aqueous Suspensions of Semiconductor Powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic Perovskite Photocatalysts for Solar Energy Utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef]
- Schanze, K.S.; Kamat, P.V.; Yang, P.; Bisquert, J. Progress in Perovskite Photocatalysis. ACS Energy Lett. 2020, 5, 2602–2604. [Google Scholar] [CrossRef]
- Tanaka, H.; Misono, M. Advances in Designing Perovskite Catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Li, Y.; Zhao, W.; Zeng, Y.; Wang, C. Photocatalytic CO2 Reduction over SrTiO3: Correlation between Surface Structure and Activity. Appl. Surf. Sci. 2018, 447, 627–635. [Google Scholar] [CrossRef]
- Cheng, C.; Long, R. Charge-Compensated Doping Extends Carrier Lifetimes in SrTiO3 by Passivating Oxygen Vacancy Defects. J. Phys. Chem. Lett. 2021, 12, 12040–12047. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of Photothermal Gas-Phase Heterogeneous CO2 Catalysis. Energy Env. Sci 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; He, Q.; Xu, R.; Chen, D.; Xu, X.; Hu, H. Review of Doping SrTiO3 for Photocatalytic Applications. Bull. Mater. Sci. 2022, 46, 6. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Efficient Degradation of Methylene Blue Dye over Highly Reactive Cu Doped Strontium Titanate (SrTiO3) Nanoparticles Photocatalyst under Visible Light. J. Nanosci. Nanotechnol. 2012, 12, 7181–7186. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In Situ Growth of Nanoparticles through Control of Non-Stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Cavazzani, J.; Squizzato, E.; Brusamarello, E.; Glisenti, A. Exsolution in Ni-Doped Lanthanum Strontium Titanate: A Perovskite-Based Material for Anode Application in Ammonia-Fed Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2022, 47, 13921–13932. [Google Scholar] [CrossRef]
- Yeshodamma, S.; Hareesh, K.; Nagabhushana, H.; Dwivedi, J.; Petwal, V.C.; Sunitha, D.V. Influence of Electron Beam Irradiation on Structural, Thermo and Photoluminescence Properties of SrTiO3:Sm3+ Nanophosphor. Optik 2020, 223, 165483. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Dao, V.D.; Yasin, A.S.; Mousa, H.M.; Mohamed, H.O.; Choi, H.S.; Hassan, M.K.; Barakat, N.A.M. Nitrogen-Doped&SnO2-Incoportaed TiO2 Nanofibers as Novel and Effective Photoanode for Enhanced Efficiency Dye-Sensitized Solar Cells. Chem. Eng. J. 2016, 304, 48–60. [Google Scholar] [CrossRef]
- Osti, A.; Rizzato, L.; Cavazzani, J.; Glisenti, A. Optimizing Citrate Combustion Synthesis of A-Site-Deficient La,Mn-Based Perovskites: Application for Catalytic CH4 Combustion in Stoichiometric Conditions. Catalysts 2023, 13, 1177. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Boyd, I.W.; O’Sullivan, B.J.; Hurley, P.K.; Kelly, P.V.; Sénateur, J.P. Nanocrystalline TiO2 Films Studied by Optical, XRD and FTIR Spectroscopy. J. Non-Cryst. Solids 2002, 303, 134–138. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Fan, Y.; Xing, J.; Gu, H.; Zhou, Z.; Lu, X.; Zhang, Q.; Wang, L.; Jiang, W. The Effect of Reduced Graphene Oxide on Microstructure and Thermoelectric Properties of Nb-Doped A-Site-Deficient SrTiO3 Ceramics. J. Alloy. Compd. 2019, 786, 884–893. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Wang, L.; Chen, X.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Synergistic Effect of Cu2+ and Cu+ in SrTiO3 Nanofibers Promotes the Photocatalytic Reduction of CO2 to Methanol. Appl. Surf. Sci. 2023, 609, 155297. [Google Scholar] [CrossRef]
- Blennow, P.; Hagen, A.; Hansen, K.K.; Wallenberg, L.R.; Mogensen, M. Defect and Electrical Transport Properties of Nb-Doped SrTiO3. Solid State Ion. 2008, 179, 2047–2058. [Google Scholar] [CrossRef]
- Zhang, W.; Du, L.; Bi, F.; He, H. A Novel SrTiO3/HZSM-5 Photocatalyst Prepared by Sol–Gel Method. Mater. Lett. 2015, 157, 103–105. [Google Scholar] [CrossRef]
- Divya, A.; Mathavan, T.; Harish, S.; Archana, J.; Benial, A.M.F.; Hayakawa, Y.; Navaneethan, M. Synthesis and Characterization of Branchlet-like SrCO3 Nanorods Using Triethylamine as a Capping Agent by Wet Chemical Method. Appl. Surf. Sci. 2019, 487, 1271–1278. [Google Scholar] [CrossRef]
- Li, B.; Hong, J.; Ai, Y.; Hu, Y.; Shen, Z.; Li, S.; Zou, Y.; Zhang, S.; Wang, X.; Zhao, G.; et al. Visible-near-Infrared-Light-Driven Selective Oxidation of Alcohols over Nanostructured Cu Doped SrTiO3 in Water under Mild Condition. J. Catal. 2021, 399, 142–149. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS Study of the Cu@Cu2O Core-Shell Nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Gaudin, P.; Fioux, P.; Dorge, S.; Nouali, H.; Vierling, M.; Fiani, E.; Molière, M.; Brilhac, J.F.; Patarin, J. Formation and Role of Cu+ Species on Highly Dispersed CuO/SBA-15 Mesoporous Materials for SOx Removal: An XPS Study. Fuel Process. Technol. 2016, 153, 129–136. [Google Scholar] [CrossRef]
- Bai, L.; Polo-Garzon, F.; Bao, Z.; Luo, S.; Moskowitz, B.M.; Tian, H.; Wu, Z. Impact of Surface Composition of SrTiO3 Catalysts for Oxidative Coupling of Methane. ChemCatChem 2019, 11, 2107–2117. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Parres-Esclapez, S.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Role of Surface and Lattice Copper Species in Copper-Containing (Mg/Sr)TiO3 Perovskite Catalysts for Soot Combustion. Appl. Catal. B 2009, 93, 82–89. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Kim, J.Y.; Hanson, J.C.; Pérez, M.; Frenkel, A.I. Reduction of CuO in H2: In Situ Time-Resolved XRD Studies. Catal. Lett. 2003, 85, 247–254. [Google Scholar] [CrossRef]
- Gwóźdź, P.; Łącz, A.; Mizera, A.; Drożdż, E. Some Aspects of Cu Incorporation into SrTiO3 Structure. J. Therm. Anal. Calorim. 2022, 147, 9949–9958. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, A.; Usman, Z.; Khalid, N.R.; Jin, H.B.; Cao, C.B. Structural, Electronic and Optical Properties of Copper-Doped SrTiO3 Perovskite: A DFT Study. Phys. B Condens. Matter 2019, 552, 52–57. [Google Scholar] [CrossRef]
- Huang, H.; Song, H.; Kou, J.; Lu, C.; Ye, J. Atomic-Level Insights into Surface Engineering of Semiconductors for Photocatalytic CO2 Reduction. J. Energy Chem. 2022, 67, 309–341. [Google Scholar] [CrossRef]
- Huang, H.B.; Yu, K.; Zhang, N.; Xu, J.Y.; Yu, X.T.; Liu, H.X.; Cao, H.L.; Lü, J.; Cao, R. Localized Surface Plasmon Resonance Enhanced Visible-Light-Driven CO2 Photoreduction in Cu Nanoparticle Loaded ZnInS Solid Solutions. Nanoscale 2020, 12, 15169–15174. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Zhang, W.; Zhu, L.; Cao, B.; Gao, J.; Shi, X.; Huang, Y.; Liu, P.; Hojamberdiev, M. Dual-Functional Copper (Cu0/Cu2+)-Modified SrTiO3-δ Nanosheets with Enhanced Photothermal Catalytic Performance for CO2 Reduction and H2 Evolution. Chem. Eng. J. 2023, 452, 139378. [Google Scholar] [CrossRef]
- Shang, D.S.; Sun, J.R.; Shi, L.; Wang, J.; Wang, Z.H.; Shen, B.G. Electronic Transport and Colossal Electroresistance in SrTiO3:Nb -Based Schottky Junctions. Appl. Phys. Lett. 2009, 94, 052105. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au–Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays. Angew. Chem. 2015, 127, 855–859. [Google Scholar] [CrossRef]
- Ma, H.; Yang, W.; Tang, H.; Pan, Y.; Li, W.; Fang, R.; Shen, Y.; Dong, F. Enhance the Stability of Oxygen Vacancies in SrTiO3 via Metallic Ag Modification for Efficient and Durable Photocatalytic NO Abatement. J. Hazard. Mater. 2023, 452, 131269. [Google Scholar] [CrossRef]
- Rusevich, L.L.; Tyunina, M.; Kotomin, E.A.; Nepomniashchaia, N.; Dejneka, A. The Electronic Properties of SrTiO3-δ with Oxygen Vacancies or Substitutions. Sci. Rep. 2021, 11, 23341. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.W.; Chee, M.K.T.; Kong, X.Y.; Yong, S.T.; Chai, S.P. An Insight into Perovskite-Based Photocatalysts for Artificial Photosynthesis. Sustain. Energy Fuels 2020, 4, 973–984. [Google Scholar] [CrossRef]
- Luo, S.; Ren, X.; Lin, H.; Song, H.; Ye, J. Plasmonic Photothermal Catalysis for Solar-to-Fuel Conversion: Current Status and Prospects. Chem. Sci. 2021, 12, 5701–5719. [Google Scholar] [CrossRef] [PubMed]






| Sample | Composition | SSA (m2/g) | Grain Size (nm) 1 | Mean Pore Size (nm) 2 |
|---|---|---|---|---|
| STO | Sr0.9TiO3 | 5.9 | 74.6 | 25.5 |
| STCuO | Sr0.9Ti0.95Cu0.05O3 | 2.5 | 94.6 | 17.4 |
| STCuO-r300 | Sr0.9Ti0.95Cu0.05O3 | - | 100.9 | - |
| STCuO-r500 | Sr0.9Ti0.95Cu0.05O3 | - | 80.5 | - |
| STCuO-r700 | Sr0.9Ti0.95Cu0.05O3 | - | 67.9 | - |
| Element | Sample | Element Concentration | ||
|---|---|---|---|---|
| Nominal | EDS | XPS | ||
| Sr | STO | 0.47 | 0.52 | 0.55 |
| STCuO | 0.47 | 0.42 | 0.48 | |
| STCuO-r300 | 0.47 | 0.41 | 0.47 | |
| STCuO-r500 | 0.47 | 0.42 | 0.48 | |
| STCuO-r700 | 0.47 | 0.47 | 0.47 | |
| Ti | STO | 0.53 | 0.48 | 0.45 |
| STCuO | 0.50 | 0.46 | 0.49 | |
| STCuO-r300 | 0.50 | 0.46 | 0.49 | |
| STCuO-r500 | 0.50 | 0.48 | 0.49 | |
| STCuO-r700 | 0.50 | 0.46 | 0.50 | |
| Cu | STO | - | - | - |
| STCuO | 0.03 | 0.12 | 0.03 | |
| STCuO-r300 | 0.03 | 0.13 | 0.04 | |
| STCuO-r500 | 0.03 | 0.10 | 0.03 | |
| STCuO-r700 | 0.03 | 0.07 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzato, L.; Cavazzani, J.; Osti, A.; Scavini, M.; Glisenti, A. Cu-Doped SrTiO3 Nanostructured Catalysts for CO2 Conversion into Solar Fuels Using Localised Surface Plasmon Resonance. Catalysts 2023, 13, 1377. https://doi.org/10.3390/catal13101377
Rizzato L, Cavazzani J, Osti A, Scavini M, Glisenti A. Cu-Doped SrTiO3 Nanostructured Catalysts for CO2 Conversion into Solar Fuels Using Localised Surface Plasmon Resonance. Catalysts. 2023; 13(10):1377. https://doi.org/10.3390/catal13101377
Chicago/Turabian StyleRizzato, Lorenzo, Jonathan Cavazzani, Andrea Osti, Marco Scavini, and Antonella Glisenti. 2023. "Cu-Doped SrTiO3 Nanostructured Catalysts for CO2 Conversion into Solar Fuels Using Localised Surface Plasmon Resonance" Catalysts 13, no. 10: 1377. https://doi.org/10.3390/catal13101377






