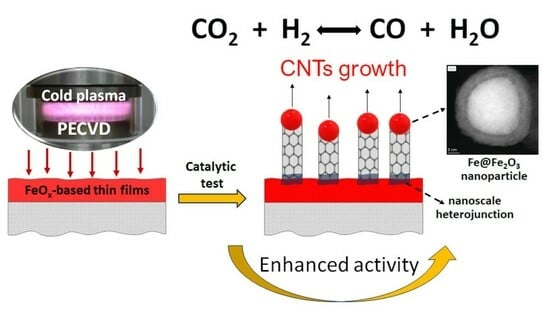
The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Characteristics of Catalytic Films
2.2. Catalytic Activity
2.3. HRTEM Analysis
2.4. What Is the Role of Carbon Nanotubes in the Catalytic Activity?—A Proposed Explanation
3. Materials and Methods
3.1. Thin-Film Nanocatalyst Preparation
3.2. Characteristics of the Film Structure
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tyczkowski, J. Cold Plasma produced catalytic materials. In Plasma Science and Technology—Progress in Physical States and Chemical Reactions; Mieno, T., Ed.; InTech Open: Vienna, Austria, 2016; pp. 53–93. [Google Scholar]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Kołodziej, A.; Łojewska, J.; Tyczkowski, J.; Jodłowski, P.; Redzynia, W.; Iwaniszyn, M.; Zapotoczny, S.; Kustrowski, P. Coupled engineering and chemical approach to the design of a catalytic structured reactor for combustion of VOCs: Cobalt oxide catalyst on knitted wire gauzes. Chem. Eng. J. 2012, 200–202, 329–337. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Kruszczak, E.; Tyczkowski, J. Catalytic activity of plasma-deposited Co3O4-based thin films for CO2 hydration—A new approach to carbon capture applications. Appl. Catal. B Environ. 2022, 304, 120961. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tracz, P.; Redzynia, W.; Tyczkowski, J. Plasma deposited novel nanocatalysts for CO2 hydrogenation to methane. J. CO2 Util. 2017, 17, 312–319. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Ryba, M.; Fronczak, M.; Kapica, R.; Sielski, J.; Sitarz, M.; Zając, P.; Łyszczarz, K.; Tyczkowski, J. Enhancing CO2 conversion to CO over plasma-deposited composites based on mixed Co and Fe oxides. Catalysts 2021, 11, 883. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 2021, 6, 954. [Google Scholar] [CrossRef]
- Mota, F.M.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Wismann, S.Y.; Larsen, K.E.; Mortensen, P.M. Electrical reverse shift: Sustainable CO2 valorization for industrial scale. Angew. Chem. Int. Ed. 2022, 61, e202109696. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Song, C.; Ji, P.; Wang, N.; Wang, W.; Cui, L. Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction. Front. Chem. 2020, 8, 709. [Google Scholar] [CrossRef]
- Chou, C.Y.; Loiland, J.A.; Lobo, R.F. Reverse water-gas shift iron catalyst derived from magnetite. Catalysts 2019, 9, 773. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Wu, B.; Wang, T.; Ma, L.; Xiang, H.; Li, Y. Transformation of carbonaceous species and its influence on catalytic performance for iron-based Fischer–Tropsch synthesis catalyst. J. Mol. Catal. A Chem. 2011, 351, 165–173. [Google Scholar] [CrossRef]
- Otun, K.O.; Yao, Y.; Liu, X.; Hildenbrandt, D. Synthesis, structure, and performance of carbide phases in Fischer-Tropsch synthesis: A critical review. Fuel 2021, 296, 120689. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-based catalysts for the high-temperature water–gas shift (HTWGS) reaction: A review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Landau, M.V.; Meiri, N.; Utsis, N.; Nehemya, R.V.; Herskowitz, M. Conversion of CO2, CO, and H2 in CO2 hydrogenation to fungible liquid fuels on Fe-based catalysts. Ind. Eng. Chem. Res. 2017, 56, 13334–13355. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Zhang, C.; Zhang, Z.; Liu, X.; Yang, Z.; Zhu, M.; Meng, B.; Xu, J.; Han, Y.F. The study of structure-performance relationship of iron catalyst during a full life cycle for CO2 hydrogenation. J. Catal. 2019, 378, 51–62. [Google Scholar] [CrossRef]
- Puga, A.F. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.W.; Yoon, H.S.; Kim, Y.D. Reverse water gas shift reaction catalyzed by Fe nanoparticles with high catalytic activity and stability. J. Ind. Eng. Chem. 2015, 25, 67–71. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- le Sache, E.; Reina, T.R. Analysis of dry reforming as direct route for gas phase CO2 conversion. The past, the present and future of catalytic DRM technologies. Prog. Energy Combust. Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Bost, N.; Ammar, M.R.; Bouchetou, M.L.; Poirier, J. The catalytic effect of iron oxides on the formation of nano-carbon by the Boudouard reaction in refractories. J. Eur. Ceram. Soc. 2016, 36, 2133–2142. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Fu, D.; Weckhuysen, B.M. Carbon deposit analysis in catalyst deactivation, regeneration, and rejuvenation. Angew. Chem. Int. Ed. 2023, 62, e202300319. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Matralis, H.; Verykios, X. Deactivation due to coking. In Catalysis for Alternative Energy Generation; Guczi, L., Erdőhelyi, A., Eds.; Springer: New York, NY, USA, 2012; pp. 80–84. [Google Scholar]
- Lu, W.; Li, N.; Chen, W.; Yao, Y. The role of multiwalled carbon nanotubes in enhancing the catalytic activity of cobalt tetraaminophthalocyanine for oxidation of conjugated dyes. Carbon 2009, 47, 3337–3345. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Bradley, J.P.; Brownlee, D.E. Carbon compounds in interplanetary dust: Evidence for formation by heterogeneous catalysis. Science 1984, 223, 56–57. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Pan, X.; Bao, X. Effect of confinement in carbon nanotubes on the activity of Fischer−Tropsch iron catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef]
- Bai, Z.Y.; Yang, Q.; Wang, J.I. Fe3O4/multi-walled carbon nanotubes as an efficient catalyst for catalytic ozonation of p-hydroxybenzoic acid. Int. J. Environ. Sci. Technol. 2016, 13, 483–492. [Google Scholar] [CrossRef]
- Ismail, A.A.; Ali, A.M.; Harraz, F.A.; Faisal, M.; Shoukry, H.; Al-Salami, A.E. A facile synthesis of α-Fe2O3/carbon nanotubes and their photocatalytic and electrochemical sensing performances. Int. J. Electrochem. Sci. 2019, 14, 15–32. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer–Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.E.; Ramakrishna, S.; Yongzhi, W. Carbon nanotubes characterization by X-ray powder diffraction—A review. Curr. Nanosci. 2015, 11, 23–35. [Google Scholar] [CrossRef]
- Wirth, C.T.; Bayer, B.C.; Gamalski, A.D.; Esconjauregui, S.; Weatherup, R.S.; Ducati, C.; Baehtz, C.; Robertson, J.; Hofmann, S. The phase of iron catalyst nanoparticles during carbon nanotube growth. Chem. Mater. 2012, 24, 4633–4640. [Google Scholar] [CrossRef]
- McCafferty, L.; Stolojan, V.; King, S.G.; Zhang, W.; Haq, S.; Silva, S.R.P. Decoration of multiwalled carbon nanotubes with protected iron nanoparticles. Carbon 2015, 84, 47–55. [Google Scholar] [CrossRef]
- Ou, X. Molecular dynamics simulations of fcc-to-bcc transformation in pure iron: A review. Mater. Sci. Technol. 2017, 33, 822–835. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Liao, P.; Toroker, M.C.; Carter, E.A. Electron transport in pure and doped hematite. Nano Lett. 2011, 11, 1775–1781. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Z.; Wang, Y.; Wang, J.; Wang, H.; Wu, Z.; Lu, G.; Zhang, H.L.; Yu, C. Double doping approach for unusually stable and large n-type thermoelectric voltage from p-type multi-walled carbon nanotube mats. J. Mater. Chem. A 2020, 8, 13095–13105. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 80–90. [Google Scholar]
- Wang, L.; Chen, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef]
- Gao, X.; Cai, P.; Wang, Z.; Lv, X.; Kawi, S. Surface acidity/basicity and oxygen defects of metal oxide: Impacts on catalytic performances of CO2 reforming and hydrogenation reactions. Top. Catal. 2023, 66, 299–325. [Google Scholar] [CrossRef]
- Pour, A.N.; Housaindokht, M.R.; Babakhani, E.G.; Irani, M.; Shahri, S.M.K. Size dependence on reduction kinetic of iron based Fischer–Tropsch catalyst. J. Ind. Eng. Chem. 2011, 17, 596–602. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Kapica, R.; Balcerzak, J.; Sielski, J. Cold plasma − a promising tool for the production of thin-film nanocatalysts. Catal. Today 2019, 337, 44–54. [Google Scholar] [CrossRef]









| Catalyst → | 0.1FeOx Fresh | 0.1FeOx Spent | 0.3FeOx Fresh | 0.3FeOx Spent | 0.3FeOx Spent (CNTs Partially Removed) |
|---|---|---|---|---|---|
| Element ↓ | Content [at%] | ||||
| Fe | 23 ± 2 | 24.0 ± 0.5 | 25.0 ±0.4 | 5.0 ± 0.3 | 10 ± 1 |
| O | 45 ± 2 | 49.7 ±0.4 | 48 ± 1 | 11 ± 1 | 23 ± 1 |
| C | 32.0 ± 0.1 | 26.3 ± 0.8 | 27 ± 2 | 84 ± 1 | 67 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panek, B.; Kierzkowska-Pawlak, H.; Uznański, P.; Nagy, S.; Nagy-Trembošová, V.; Tyczkowski, J. The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction. Catalysts 2023, 13, 1302. https://doi.org/10.3390/catal13091302
Panek B, Kierzkowska-Pawlak H, Uznański P, Nagy S, Nagy-Trembošová V, Tyczkowski J. The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction. Catalysts. 2023; 13(9):1302. https://doi.org/10.3390/catal13091302
Chicago/Turabian StylePanek, Bartosz, Hanna Kierzkowska-Pawlak, Paweł Uznański, Stefan Nagy, Veronika Nagy-Trembošová, and Jacek Tyczkowski. 2023. "The Role of Carbon Nanotube Deposit in Catalytic Activity of FeOX-Based PECVD Thin Films Tested in RWGS Reaction" Catalysts 13, no. 9: 1302. https://doi.org/10.3390/catal13091302