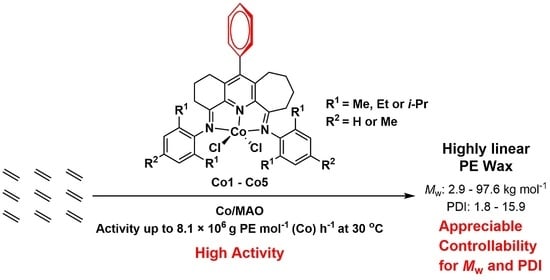
Exploring Long Range para-Phenyl Effects in Unsymmetrically Fused bis(imino)pyridine-Cobalt Ethylene Polymerization Catalysts
Abstract
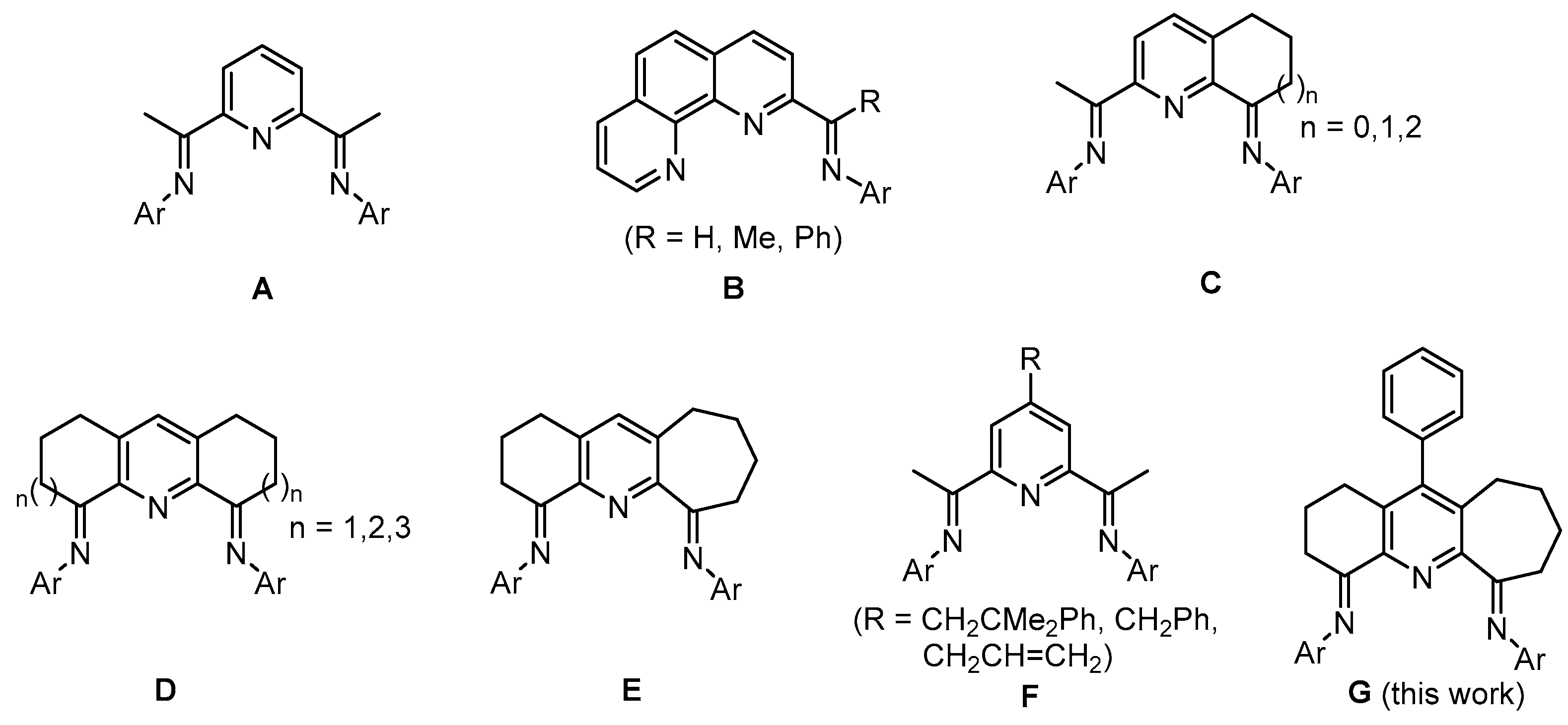
:1. Introduction
2. Results
2.1. Synthesis and Characterization of Co1–Co5
2.2. Ethylene Polymerization Studies
2.2.1. Ethylene Polymerization Evaluation Using Co1–Co5 with MAO as Activator
2.2.2. Ethylene Polymerization Evaluation Using Co1–Co5 with MMAO as Activator
2.2.3. Microstructural Analysis of the Polyethylene
3. Experimental Section
3.1. General Procedures
3.2. Synthesis of 11-phenyl-1,2,3,4,6,7,8,9,10-nonahydrocyclohepta[b]quinoline
3.3. Synthesis of 11-phenyl-1,2,3,7,8,9,10-heptahydrocyclohepta[b]quinoline-4,6-dione
3.4. Synthesis of 4,6-di(arylimino)-11-phenyl-1,2,3,7,8,9,10-heptahydrocycloghept[b]quinoline-cobalt(II) Chloride (Co1–Co5)
3.4.1. Aryl = 2,6-dimethylphenyl (Co1)
3.4.2. Aryl = 2,6-diethylphenyl (Co2)
3.4.3. Aryl = 2,6-diisopropylphenyl (Co3)
3.4.4. Aryl = 2,4,6-trimethylphenyl (Co4)
3.4.5. Aryl = 2,6-diethyl-4-methylphenyl (Co5)
3.5. Polymerization Study
3.5.1. Ethylene Polymerization at 5 or 10 atm Ethylene Pressure
3.5.2. Ethylene Polymerization at 1 atm Ethylene Pressure
3.6. X-ray Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Wang, Z.; Mahmood, Q.; Zhang, W.; Sun, W.-H. Recent progress on the tridentate iron complex catalysts for ethylene oligo-/polymerization. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 79, pp. 41–86. [Google Scholar]
- Chirik, P.J. Carbon-Carbon Bond Formation in a Weak Ligand Field: Leveraging Open-Shell First-Row Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2017, 56, 5170–5181. [Google Scholar] [CrossRef]
- Humphries, M.J.; Tellmann, K.P.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Investigations into the Mechanism of Activation and Initiation of Ethylene Polymerization by Bis(imino)pyridine Cobalt Catalysts: Synthesis, Structures, and Deuterium Labeling Studies. Organometallics 2005, 24, 2039–2050. [Google Scholar] [CrossRef]
- Sun, W.-H. Novel Polyethylenes via Late Transition Metal Complex Pre-catalysts. In Polyolefins: 50 years after Ziegler and Natta II: Polyolefins by Metallocenes and Other Single-Site Catalysts; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 163–178. [Google Scholar]
- Champouret, Y.; Hashmi, O.H.; Visseaux, M. Discrete iron-based complexes: Applications in homogeneous coordination-insertion polymerization catalysis. Coord. Chem. Rev. 2019, 390, 127–170. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J.; Britovsek, G.J.P.; Kimberley, B.S.; Maddox, P.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly Reactive Ligands and a Gateway to New Families of Catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Ma, J.; Feng, C.; Wang, S.L.; Zhao, K.Q.; Sun, W.-H.; Redshaw, C.; Solan, G.A. Bi- and tri-dentate imino-based iron and cobalt pre-catalysts for ethylene oligo-/polymerization. Inorg. Chem. Front. 2014, 1, 14–34. [Google Scholar] [CrossRef]
- Sun, W.-H.; Jie, S.; Zhang, S.; Zhang, W.; Song, Y.; Ma, H. Iron Complexes Bearing 2-Imino-1,10-phenanthrolinyl Ligands as Highly Active Catalysts for Ethylene Oligomerization. Organometallics 2006, 25, 666–677. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Wedeking, K.; Zhang, W.; Ma, H.; Lu, X.; Deng, Y.; Sun, W.-H. Cobalt(II) complexes bearing 2-imino-1,10-phenanthroline ligands: Synthesis, characterization and ethylene oligomerization. C. R. Chim. 2006, 9, 1500–1509. [Google Scholar] [CrossRef]
- Pelletier, J.D.A.; Champouret, Y.D.M.; Cadarso, J.; Clowes, L.; Gañete, M.; Singh, K.; Thanarajasingham, V.; Solan, G.A. Electronically variable imino-phenanthrolinyl-cobalt complexes; synthesis, structures and ethylene oligomerisation studies. J. Organomet. Chem. 2006, 691, 4114–4123. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Sun, W.-H.; Kuang, X.; Liu, T.; Guo, J. Iron(II) complexes ligated by 2-imino-1,10-phenanthrolines: Preparation and catalytic behavior toward ethylene oligomerization. J. Mol. Catal. A Chem. 2007, 269, 85–96. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Sun, W.-H. 2-Arylimino-9-phenyl-1,10-phenanthrolinyl-iron, -cobalt and -nickel Complexes: Synthesis, Characterization and Ethylene Oligomerization Behavior. Eur. J. Inorg. Chem. 2007, 2007, 5584–5598. [Google Scholar] [CrossRef]
- Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8-arylimino-5,6,7-trihydroquinolylcobalt dichloride: Synthesis and polyethylene wax formation. Appl. Catal. A-Gen. 2012, 447–448, 67–73. [Google Scholar] [CrossRef]
- Zhang, W.; Chai, W.; Sun, W.-H.; Hu, X.; Redshaw, C.; Hao, X. 2-(1-(Arylimino)ethyl)-8-arylimino-5,6,7-trihydroquinoline Iron(II) Chloride Complexes: Synthesis, Characterization, and Ethylene Polymerization Behavior. Organometallics 2012, 31, 5039–5048. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Baugh, S.P.D.; Hoarau, O.; Gibson, V.C.; Wass, D.F.; White, A.J.P.; Williams, D.J. The role of bulky substituents in the polymerization of ethylene using late transition metal catalysts: A comparative study of nickel and iron catalyst systems. Inorg. Chim. Acta 2003, 345, 279–291. [Google Scholar] [CrossRef]
- Zabel, D.; Schubert, A.; Wolmershäuser, G.; Jones, R.L.; Thiel, W.R. Iron and Cobalt Complexes of Tridentate N-Donor Ligands in Ethylene Polymerization: Efficient Shielding of the Active Sites by Simple Phenyl Groups. Eur. J. Inorg. Chem. 2008, 2008, 3648–3654. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Lappalainen, K.; Repo, T.; Piironen, U.; Leskela, M. New bis(imino)pyridine-iron(II)- and cobalt(II)-based catalysts: Synthesis, characterization and activity towards polymerization of ethylene. J. Organomet. Chem. 2002, 648, 55–61. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Liang, T.; Redshaw, C.; Li, Y.; Sun, W.-H. Synthesis, characterization and catalytic behavior toward ethylene of 2-[1-(4,6-dimethyl-2-benzhydrylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylmetal (iron or cobalt) chlorides. Dalton Trans. 2013, 42, 9188–9197. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.; Wang, L.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-Dibenzhydryl-4-methylphenylimino)ethyl]-6-[1-(arylimino)ethyl]pyridylcobalt(II) dichlorides: Synthesis, characterization and ethylene polymerization behavior. Dalton Trans. 2011, 40, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, W.; Cao, X.-P.; Liang, T.; Redshaw, C.; Sun, W.-H. 2-[1-(2,6-dibenzhydryl-4-chlorophenylimino)ethyl]-6-[1-aryliminoethyl]pyridyl cobalt dichlorides: Synthesis, characterization and ethylene polymerization behavior. J. Organomet. Chem. 2012, 713, 209–216. [Google Scholar] [CrossRef]
- Mahmood, Q.; Ma, Y.; Hao, X.; Sun, W.-H. Substantially enhancing the catalytic performance of bis(imino)pyridylcobaltous chloride pre-catalysts adorned with benzhydryl and nitro groups for ethylene polymerization. Appl. Organomet. Chem. 2019, 33, e4857. [Google Scholar] [CrossRef]
- Bariashir, C.; Zhang, R.; Vignesh, A.; Ma, Y.; Liang, T.; Sun, W.-H. Enhancing Ethylene Polymerization of NNN-Cobalt(II) Precatalysts Adorned with a Fluoro-substituent. ACS Omega 2021, 6, 4448–4460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Han, M.; Xiang, J.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Fluorinated cobalt catalysts and their use in forming narrowly dispersed polyethylene waxes of high linearity and incorporating vinyl functionality. Catal. Sci. Technol. 2021, 11, 656–670. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Ma, Y.; Solan, G.A.; Liang, T.; Sun, W.-H. Cobalt Catalysts Bearing ortho-(4,4′-Dichlorobenzhydryl) Substituents and Their Use in Generating Narrowly Dispersed Polyethylene of High Linearity. Eur. J. Inorg. Chem. 2022, 2022, e202200396. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, S.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. CF3O-Functionalized Bis(arylimino)pyridine–Cobalt Ethylene Polymerization Catalysts: Harnessing Solvent Effects on Performance and Polymer Properties. Organometallics 2022, 41, 3237–3248. [Google Scholar] [CrossRef]
- Han, M.; Oleynik, I.I.; Liu, M.; Ma, Y.; Oleynik, I.V.; Solan, G.A.; Liang, T.; Sun, W.-H. Ring size enlargement in an ortho -cycloalkyl-substituted bis(imino)pyridine-cobalt ethylene polymerization catalyst and its impact on performance and polymer properties. Appl. Organomet. Chem. 2021, 36, e6529. [Google Scholar] [CrossRef]
- Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. Constrained formation of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopentapyridines and their cobalt(II) chloride complexes: Synthesis, characterization and ethylene polymerization. RSC Adv. 2015, 5, 32720–32729. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Controlling the molecular weights of polyethylene waxes using the highly active precatalysts of 2-(1-aryliminoethyl)-9-arylimino-5,6,7,8-tetrahydrocycloheptapyridylcobalt chlorides: Synthesis, characterization, and catalytic behavior. Dalton Trans. 2016, 45, 657–666. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Zhang, W.; Oleynik, I.I.; Vignesh, A.; Oleynik, I.V.; Hu, X.; Sun, Y.; Sun, W.-H. Highly Linear Polyethylenes Achieved Using Thermo-Stable and Efficient Cobalt Precatalysts Bearing Carbocyclic-Fused NNN-Pincer Ligand. Molecules 2019, 24, 1176. [Google Scholar] [CrossRef] [PubMed]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I. Iron and Cobalt Complexes of 2,3,7,8-Tetrahydroacridine-4,5(1H,6H)-diimine Sterically Modulated by Substituted Aryl Rings for the Selective Oligomerization to Polymerization of Ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Du, S.; Zhang, W.; Yue, E.; Huang, F.; Liang, T.; Sun, W.-H. α,α′-Bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridylcobalt Chlorides: Synthesis, Characterization, and Ethylene Polymerization Behavior. Eur. J. Inorg. Chem. 2016, 2016, 1748–1755. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Mahmood, Q.; Liu, Q.; Ma, Y.; Hao, X.; Sun, W.-H. Bis(imino)pyridines Incorporating Doubly Fused Eight-Membered Rings as Conformationally Flexible Supports for Cobalt Ethylene Polymerization Catalysts. Organometallics 2018, 37, 380–389. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Guo, J.; Liu, Q.; Solan, G.A.; Liang, T.; Sun, W.-H. Bis(imino)pyridines fused with 6- and 7-membered carbocylic rings as N,N,N-scaffolds for cobalt ethylene polymerization catalysts. Dalton Trans. 2019, 48, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Cámpora, J.; Naz, A.M.; Palma, P.; Rodríguez-Delgado, A.; Álvarez, E.; Tritto, I.; Boggioni, L. Iron and Cobalt Complexes of 4-Alkyl-2,6-diiminopyridine Ligands: Synthesis and Ethylene Polymerization Catalysis. Eur. J. Inorg. Chem. 2008, 2008, 1871–1879. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, N.; Xiang, J.; Solan, G.A.; Suo, H.; Ma, Y.; Liang, T.; Sun, W.-H. Bis-cycloheptyl-fused bis(imino)pyridine-cobalt catalysts for PE wax formation: Positive effects of fluoride substitution on catalytic performance and thermal stability. Dalton Trans. 2020, 49, 9425–9437. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivalingam, K.; Karnam Jayarampillai, R.P.; Wang, W.L.; Salas, C.O. Friedländer's synthesis of quinolines as a pivotal step in the development of bioactive heterocyclic derivatives in the current era of medicinal chemistry. Chem. Biol. Drug. Des. 2022, 100, 1042–1085. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Nikcheh, M.S. Oxalic Acid: An Efficient and Cost-Effective Organic Catalyst for the Friedländer Quinoline Synthesis under Solvent-Free Conditions. Monatsh. Chem. 2007, 138, 1249–1252. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Ma, N.; Liu, S.; Han, M.; Yan, X.; Liu, Q.; Solan, G.A.; Sun, W.-H. Direct synthesis of ring-fused quinolines and pyridines catalyzed by NNHY-ligated manganese complexes (Y = NR2 or SR). Catal. Sci. Technol. 2021, 11, 8026–8036. [Google Scholar] [CrossRef]
- Bell, T.W.; Khasanov, A.B.; Drew, M.G.B. Role of Pyridine Hydrogen-Bonding Sites in Recognition of Basic Amino Acid Side Chains. J. Am. Chem. Soc. 2002, 124, 14092–14103. [Google Scholar] [CrossRef] [PubMed]
- Vierhapper, F.W.; Eliel, E.L. Selective Hydrogenation of Quinoline and Its Homologs, Isoquinoline, and Phenyl-Substituted Pyridines in the Benzene Ring. J. Org. Chem. 1975, 40, 2729–2734. [Google Scholar] [CrossRef]
- Thummel, R.P.; Lefoulon, F.; Cantu, D.; Mahadevan, R. Polyaza Cavity-Shaped Molecules. Annelated Derivatives of 2- (2'-Py ridyl)-1,8-naphthyridine and 2,2'-Bi-1,8-naphthyridine. J. Org. Chem. 1984, 49, 2208–2212. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Seitz, M.; Milius, W.; Alt, H.G. Iron(II) coordination compounds with ω-alkenyl substituted bis(imino)pyridine ligands: Self-immobilizing catalysts for the polymerization of ethylene. J. Mol. Catal. A Chem. 2007, 261, 246–253. [Google Scholar] [CrossRef]
- Pelascini, F.; Wesolek, M.; Peruch, F.; Lutz, P.J. Modified Pyridine-Bis(imine) Iron and Cobalt Complexes: Synthesis, Structure, and Ethylene Polymerization Study. Eur. J. Inorg. Chem. 2006, 2006, 4309–4316. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, W.; Sun, Y.; Hu, X.; Solan, G.A.; Sun, W.-H. Thermally stable and highly active cobalt precatalysts for vinyl-polyethylenes with narrow polydispersities: Integrating fused-ring and imino-carbon protection into ligand design. New J. Chem. 2016, 40, 8012–8023. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Q.; Rempel, G.L. Solubility of ethylene in toluene and toluene/styrene–butadiene rubber solutions. J. Appl. Polym. Sci. 2005, 96, 645–649. [Google Scholar] [CrossRef]
- Gibson, V.C.; Solan, G.A. Olefin Oligomerizations and Polymerizations Catalyzed by Iron and Cobalt Complexes Bearing Bis(imino)pyridine Ligands. In Catalysis without Precious Metals; Bullock, R.M., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 111–141. [Google Scholar]
- Gibson, V.C.; Solan, G.A. Iron-Based and Cobalt-Based Olefin Polymerisation Catalysts. In Metal Catalysts in Olefin Polymerization; Guan, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 107–158. [Google Scholar]
- Small, B.L.; Brookhart, M. Iron-Based Catalysts with Exceptionally High Activities and Selectivities for Oligomerization of Ethylene to Linear α-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Mastroianni, S.; Solan, G.A.; Baugh, S.P.D.; Redshaw, C.; Gibson, V.C.; White, A.J.P.; Williams, D.J.; Elsegood, M.R.J. Oligomerisation of Ethylene by Bis(imino)pyridyliron and -cobalt Complexes. Chem. Eur. J. 2000, 6, 2221–2231. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Jordan, R.F. (α-Diimine)nickel Complexes That Contain Menthyl Substituents: Synthesis, Conformational Behavior, and Olefin Polymerization Catalysis. Organometallics 2017, 36, 2784–2799. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Stromberg, S.; et al. Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, Z.; Ma, Y.; Liang, T.; Yang, X.; Sun, W.-H. Fluorinated 2,6-bis(arylimino)pyridyl iron complexes targeting bimodal dispersive polyethylenes: Probing chain termination pathways via a combined experimental and DFT study. Dalton Trans. 2022, 51, 8290–8302. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Liu, B.; Yue, E.; Liu, Q.; Yang, X.; Wang, Z.; Sun, W.-H. A Ruthenium Catalyst with Unprecedented Effectiveness for the Coupling Cyclization of γ-Amino Alcohols and Secondary Alcohols. ACS Catal. 2016, 6, 1247–1253. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]

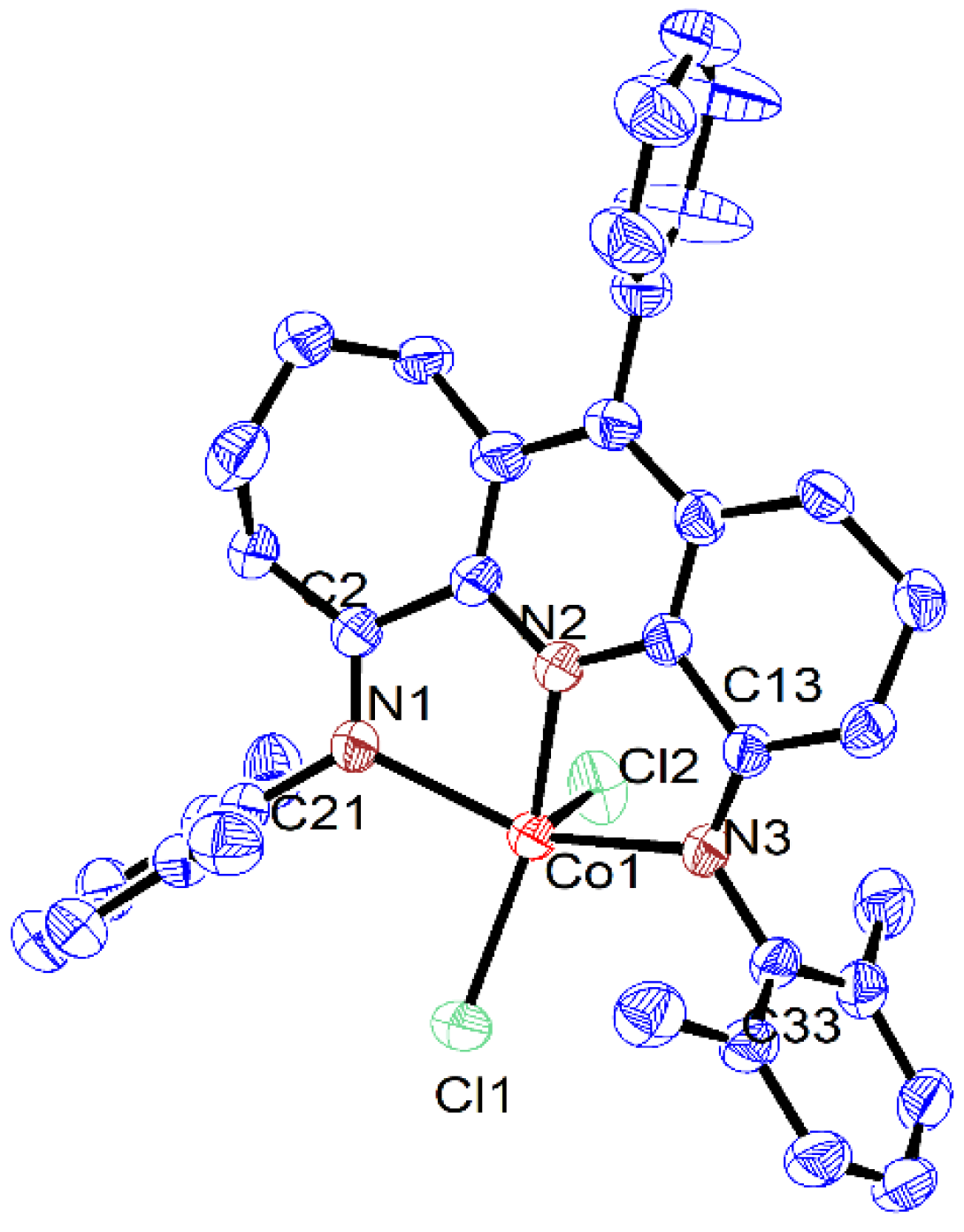
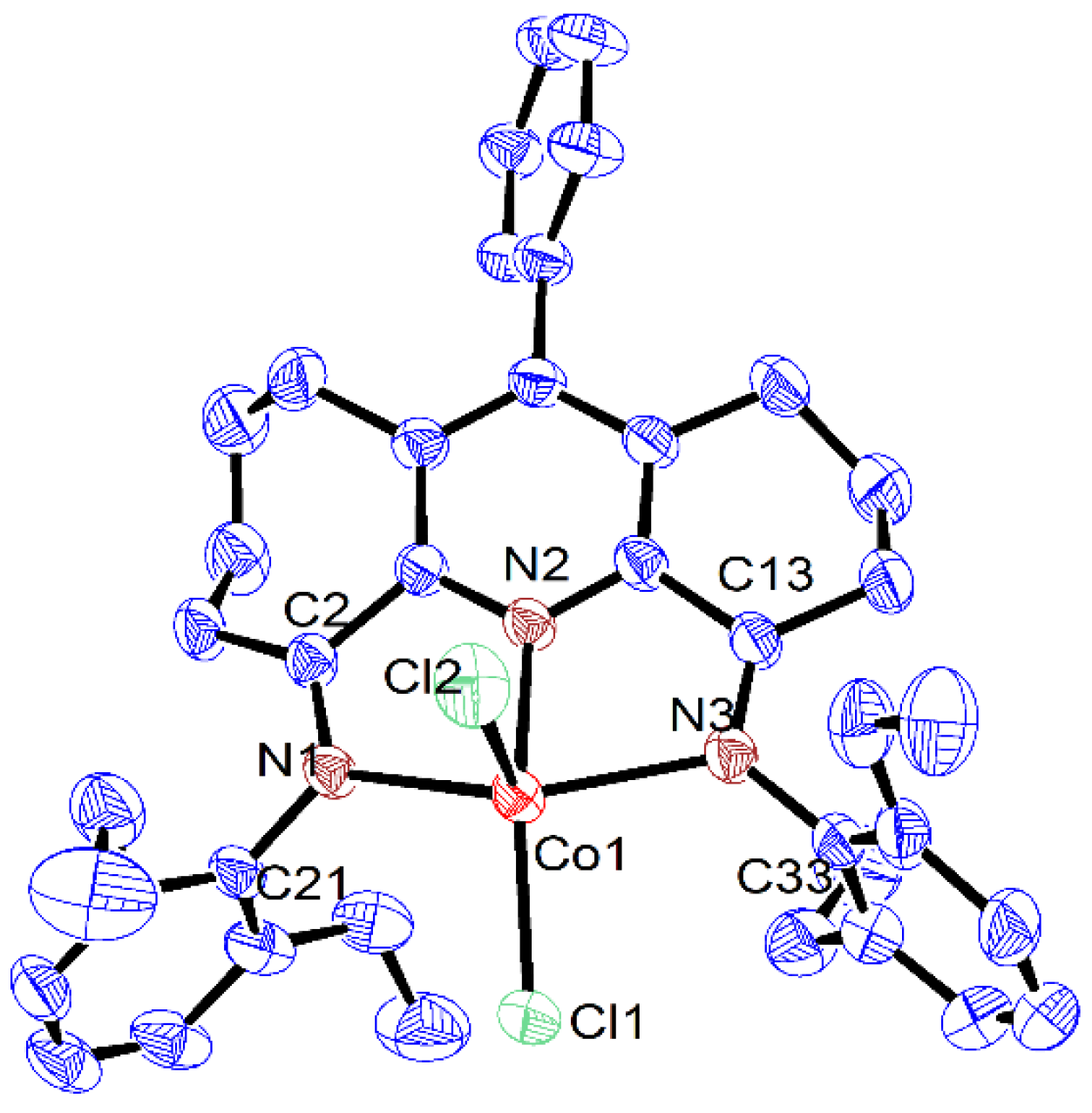
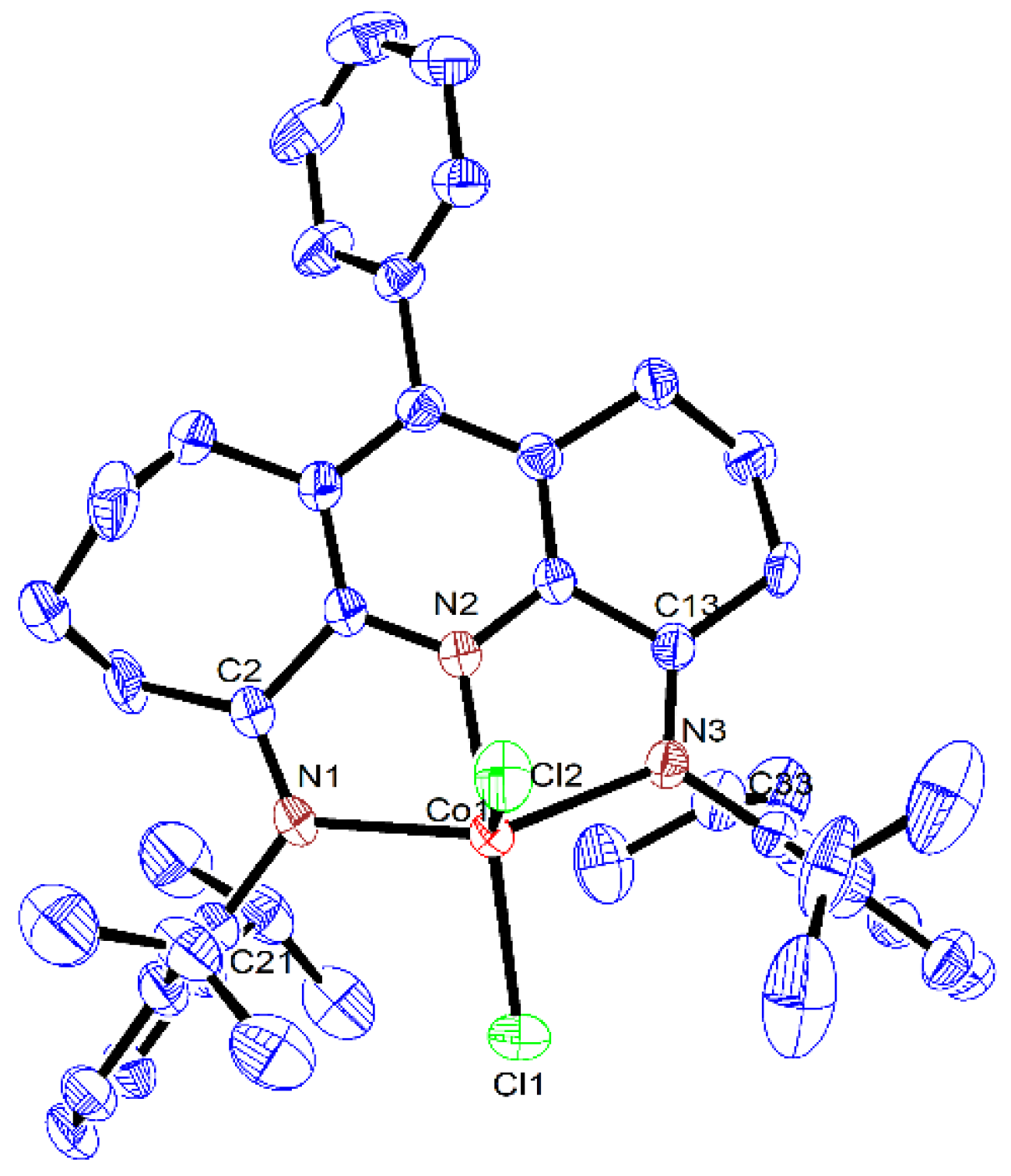

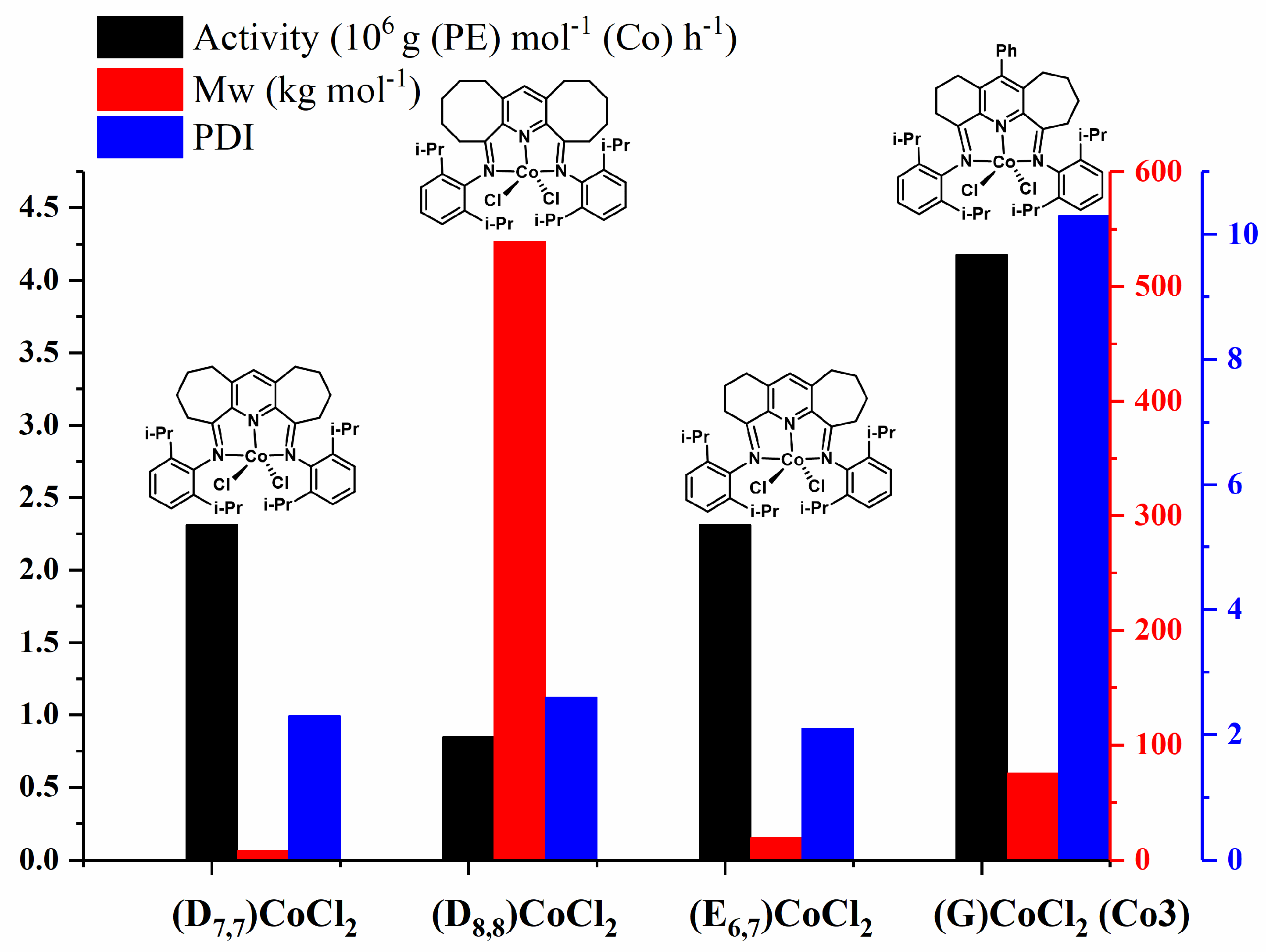


| Co1 | Co2 | Co3 | |
|---|---|---|---|
| Bond lengths (Å) | |||
| Co(1)-N(2) | 2.056(2) | 2.050(2) | 2.0616(19) |
| Co(1)-N(1) | 2.202(2) | 2.195(2) | 2.1600(18) |
| Co(1)-N(3) | 2.183(2) | 2.221(2) | 2.2211(18) |
| Co(1)-Cl(1) | 2.2427(7) | 2.2450(8) | 2.2471(7) |
| Co(1)-Cl(2) | 2.2967(8) | 2.2824(9) | 2.3043(7) |
| N(1)-C(2) | 1.276(3) | 1.286(4) | 1.278(3) |
| N(1)-C(21) | 1.432(3) | 1.445(3) | 1.441(3) |
| N(3)-C(13) | 1.281(3) | 1.285(4) | 1.284(3) |
| N(3)-C(33) | 1.435(3) | 1.435(4) | 1.442(3) |
| Bond angles (°) | |||
| Cl(1)-Co(1)-Cl(2) | 114.01(3) | 118.71(4) | 115.15(3) |
| N(2)-Co(1)-Cl(1) | 149.05(7) | 149.63(7) | 154.97(6) |
| N(2)-Co(1)-Cl(2) | 96.77(7) | 91.66(7) | 89.88(6) |
| N(1)-Co(1)-Cl(1) | 96.31(6) | 99.17(6) | 99.78(6) |
| N(1)-Co(1)-Cl(2) | 101.06(6) | 98.98(7) | 101.07(6) |
| N(3)-Co(1)-Cl(1) | 101.36(6) | 97.96(7) | 99.14(5) |
| N(3)-Co(1)-Cl(2) | 100.83(6) | 101.20(7) | 101.96(5) |
| N(3)-Co(1)-N(1) | 143.14(8) | 142.88(9) | 140.24(8) |
| N(2)-Co(1)-N(1) | 73.62(8) | 74.23(9) | 74.188(7) |
| N(2)-Co(1)-N(3) | 74.64(8) | 74.36(9) | 73.92(7) |
| Entry | Precat. | Al:Co | T (°C) | t (min) | Activity b | Mw c | Mw/Mn c | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|
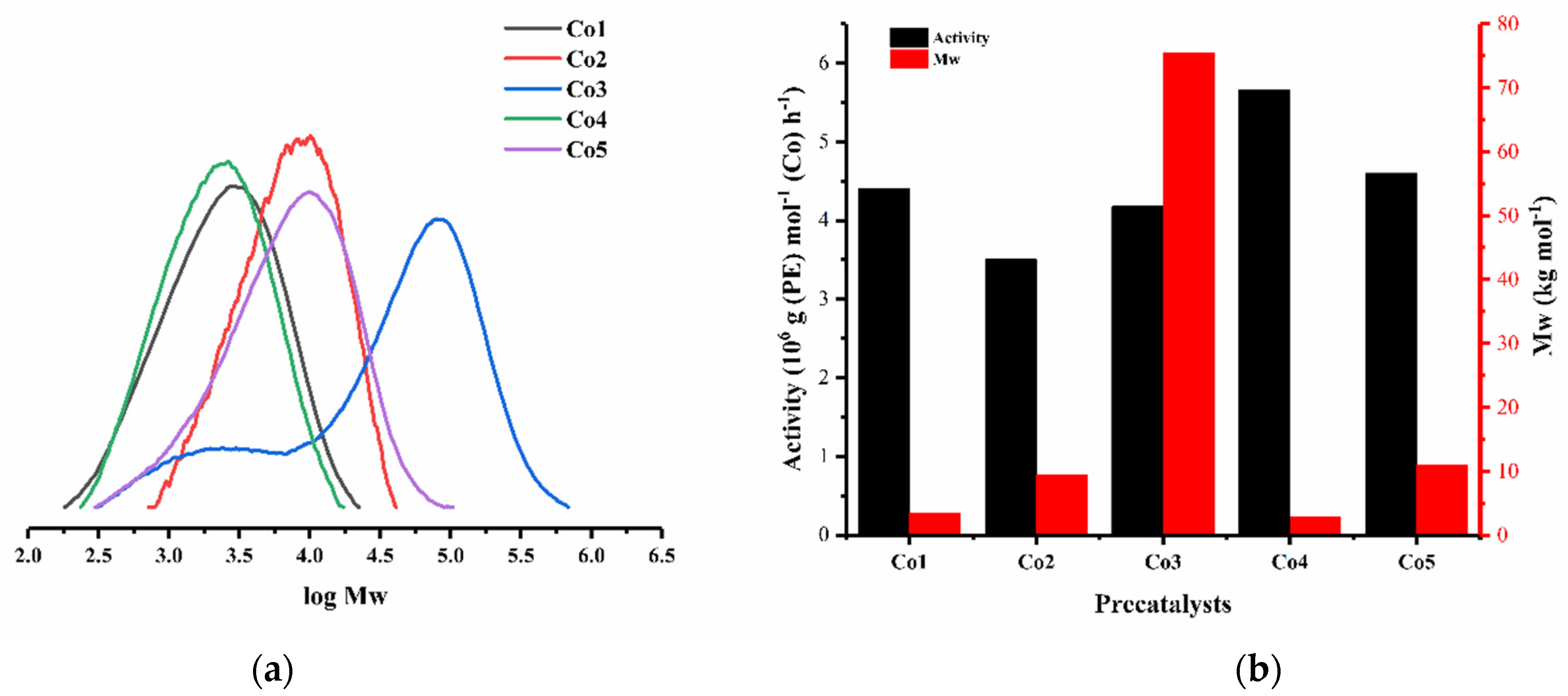
| 1 | Co3 | 2500 | 30 | 30 | 3.57 | 65.6 | 10.7 | 133.0 |
| 2 | Co3 | 3000 | 30 | 30 | 3.62 | 70.8 | 10.9 | 131.9 |
| 3 | Co3 | 3500 | 30 | 30 | 3.95 | 72.4 | 10.5 | 131.6 |
| 4 | Co3 | 4000 | 30 | 30 | 4.18 | 75.5 | 10.3 | 132.1 |
| 5 | Co3 | 4500 | 30 | 30 | 4.07 | 82.6 | 11.6 | 132.0 |
| 6 | Co3 | 5000 | 30 | 30 | 3.65 | 73.9 | 11.6 | 132.2 |
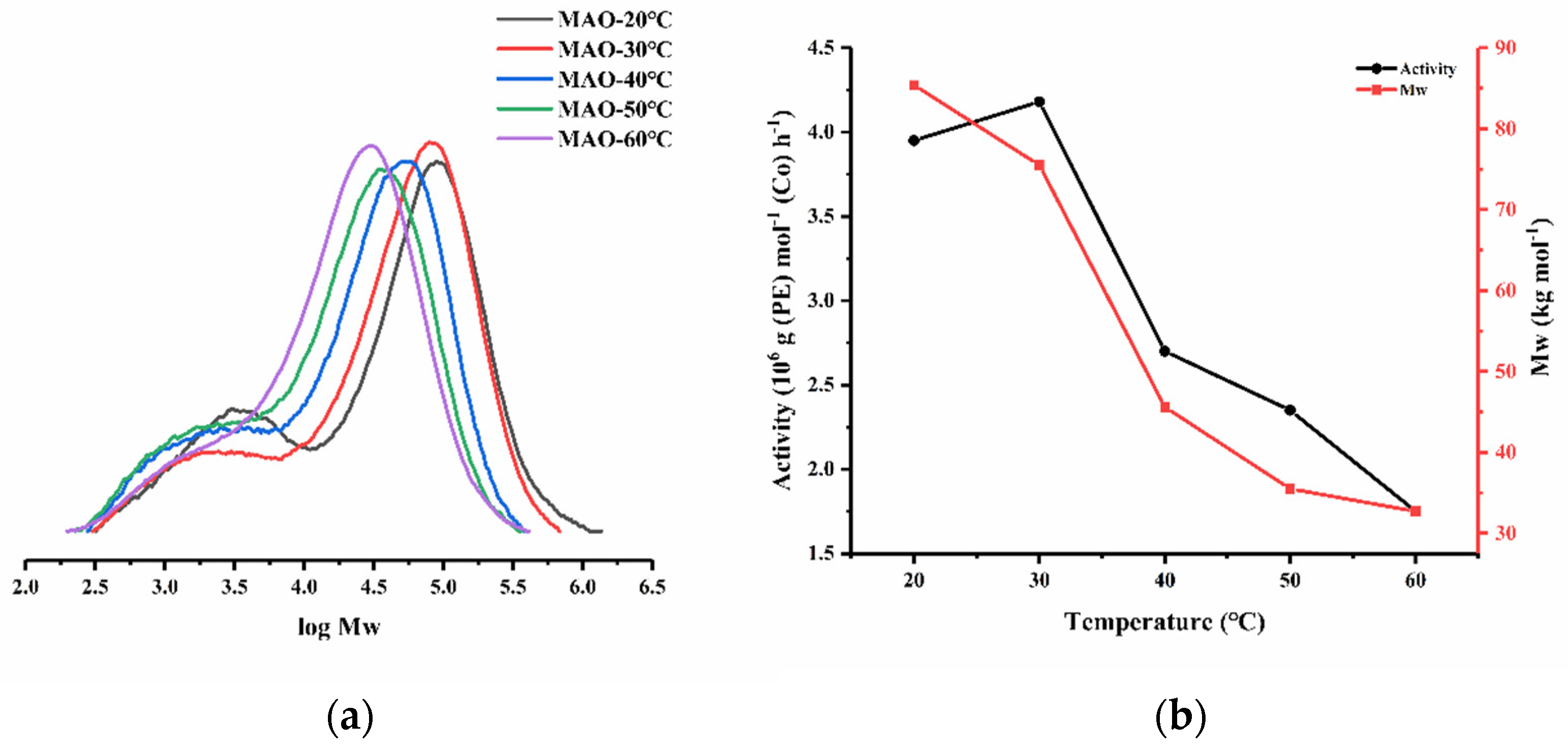
| 7 | Co3 | 4000 | 20 | 30 | 3.95 | 85.4 | 12.3 | 133.2 |
| 8 | Co3 | 4000 | 40 | 30 | 2.70 | 45.6 | 8.6 | 132.3 |
| 9 | Co3 | 4000 | 50 | 30 | 2.35 | 35.5 | 7.6 | 131.4 |
| 10 | Co3 | 4000 | 60 | 30 | 1.75 | 32.7 | 5.7 | 131.3 |
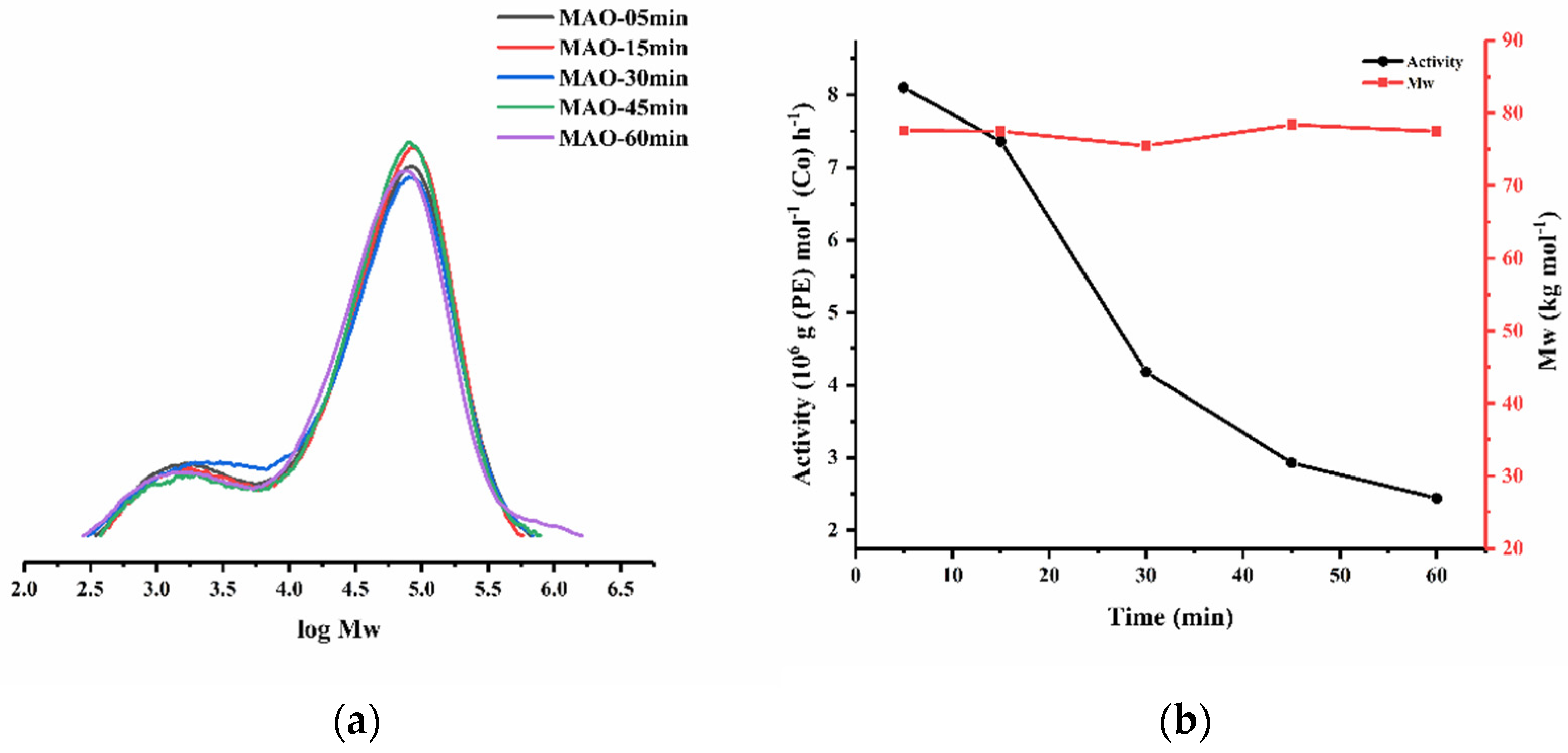
| 11 | Co3 | 4000 | 30 | 5 | 8.10 | 77.6 | 10.0 | 132.3 |
| 12 | Co3 | 4000 | 30 | 15 | 7.36 | 77.5 | 9.1 | 131.8 |
| 13 | Co3 | 4000 | 30 | 45 | 2.93 | 78.4 | 8.8 | 131.4 |
| 14 | Co3 | 4000 | 30 | 60 | 2.44 | 77.5 | 9.6 | 131.4 |
| 15 e | Co3 | 4000 | 30 | 30 | 0.60 | 56.3 | 6.9 | 131.6 |
| 16 f | Co3 | 4000 | 30 | 30 | 2.82 | 70.3 | 10.8 | 131.6 |
| 17 | Co1 | 4000 | 30 | 30 | 4.40 | 3.4 | 2.2 | 123.8 |
| 18 | Co2 | 4000 | 30 | 30 | 3.50 | 9.4 | 1.8 | 129.8 |
| 19 | Co4 | 4000 | 30 | 30 | 5.66 | 2.9 | 2.0 | 122.7 |
| 20 | Co5 | 4000 | 30 | 30 | 4.60 | 10.9 | 2.7 | 128.3 |
| Entry | Precat. | Al:Co | T (°C) | t (min) | Activity b | Mw c | Mw/Mn c | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|
| 1 | Co3 | 1500 | 30 | 30 | 2.75 | 66.6 | 9.6 | 131.8 |
| 2 | Co3 | 2000 | 30 | 30 | 3.07 | 63.6 | 8.9 | 131.5 |
| 3 | Co3 | 2500 | 30 | 30 | 3.24 | 72.9 | 9.9 | 131.9 |
| 4 | Co3 | 3000 | 30 | 30 | 3.05 | 71.0 | 10.2 | 131.6 |
| 5 | Co3 | 3500 | 30 | 30 | 2.45 | 64.4 | 10.4 | 131.2 |
| 6 | Co3 | 2500 | 20 | 30 | 3.56 | 89.3 | 5.0 | 132.8 |
| 7 | Co3 | 2500 | 40 | 30 | 1.96 | 44.8 | 8.5 | 131.4 |
| 8 | Co3 | 2500 | 50 | 30 | 1.45 | 38.5 | 5.6 | 131.7 |
| 9 | Co3 | 2500 | 60 | 30 | 0.80 | 33.7 | 5.2 | 131.1 |
| 10 | Co3 | 2500 | 20 | 5 | 7.02 | 66.5 | 13.5 | 131.1 |
| 11 | Co3 | 2500 | 20 | 15 | 5.10 | 77.0 | 14.3 | 131.6 |
| 12 | Co3 | 2500 | 20 | 45 | 2.40 | 91.7 | 15.9 | 131.5 |
| 13 | Co3 | 2500 | 20 | 60 | 1.85 | 97.6 | 12.7 | 132.4 |
| 14 e | Co3 | 2500 | 20 | 30 | 0.53 | 66.2 | 8.6 | 131.5 |
| 15 f | Co3 | 2500 | 20 | 30 | 2.96 | 79.5 | 13.3 | 131.8 |
| 16 | Co1 | 2500 | 20 | 30 | 2.75 | 3.7 | 2.5 | 123.9 |
| 17 | Co2 | 2500 | 20 | 30 | 2.24 | 13.1 | 3.3 | 129.1 |
| 18 | Co4 | 2500 | 20 | 30 | 2.20 | 3.8 | 2.7 | 124.0 |
| 19 | Co5 | 2500 | 20 | 30 | 3.03 | 13.6 | 3.2 | 129.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Zhang, Q.; Zou, S.; Ma, Y.; Solan, G.A.; Zhang, W.; Sun, W.-H. Exploring Long Range para-Phenyl Effects in Unsymmetrically Fused bis(imino)pyridine-Cobalt Ethylene Polymerization Catalysts. Catalysts 2023, 13, 1387. https://doi.org/10.3390/catal13101387
Wang Y, Wang Z, Zhang Q, Zou S, Ma Y, Solan GA, Zhang W, Sun W-H. Exploring Long Range para-Phenyl Effects in Unsymmetrically Fused bis(imino)pyridine-Cobalt Ethylene Polymerization Catalysts. Catalysts. 2023; 13(10):1387. https://doi.org/10.3390/catal13101387
Chicago/Turabian StyleWang, Yizhou, Zheng Wang, Qiuyue Zhang, Song Zou, Yanping Ma, Gregory A. Solan, Wenjuan Zhang, and Wen-Hua Sun. 2023. "Exploring Long Range para-Phenyl Effects in Unsymmetrically Fused bis(imino)pyridine-Cobalt Ethylene Polymerization Catalysts" Catalysts 13, no. 10: 1387. https://doi.org/10.3390/catal13101387