Expression of Reaction Selectivity and the Substituent Effect in Ullmann, Suzuki, Hiyama, and Allylic Arylation Reactions Caused by Reducing Catalyst Loading
Abstract
:1. Introduction
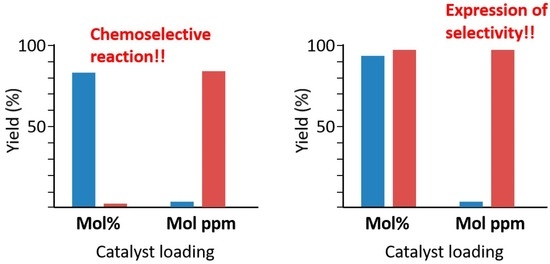
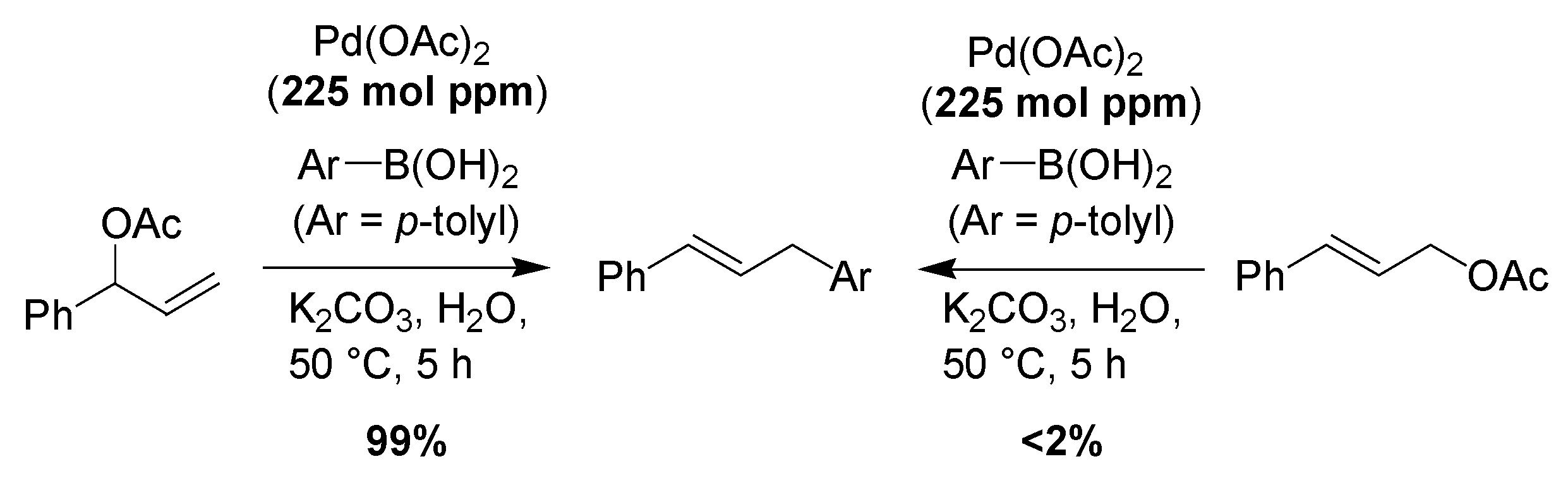
2. Results and Discussion
3. Materials and Methods
3.1. General Comments
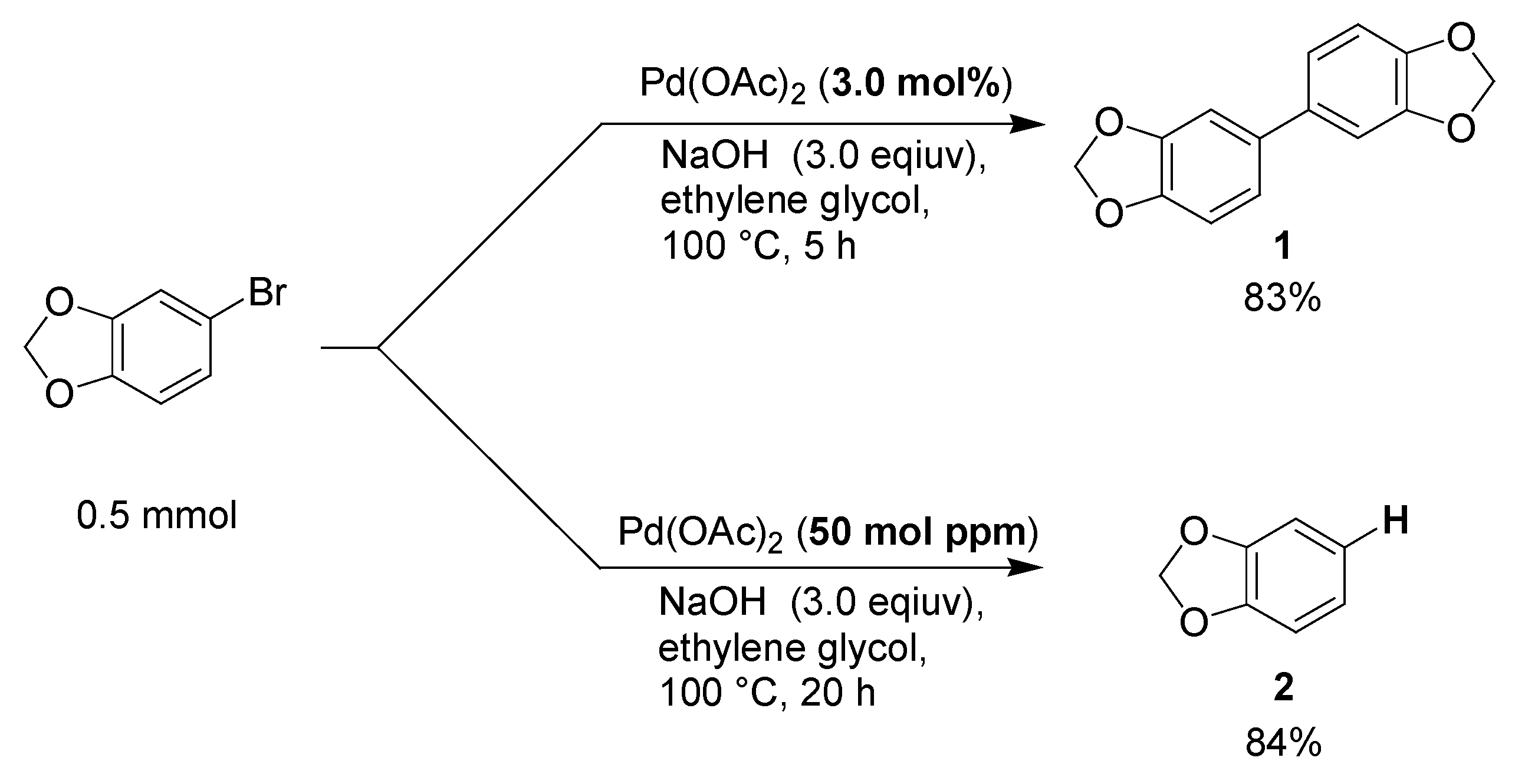
3.2. Procedure for the Selective Reaction of 5-Bromo-1,3-Benzodioxole
3.3. Procedure for the Substrate-Dependent Suzuki Coupling Reaction
3.4. General Procedure for Suzuki Coupling Reaction at Mol Ppm Level of Pd Loading
3.5. General Procedure for Hiyama Coupling Reaction at the Mol Ppm Level of Pd Loading
3.6. General Procedure for Allylic Arylaton at the Mol Ppm Level of Pd Loading
3.7. Compounds Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.-J.; Liu, K.; Yu, J.; Zhang, L.; Gong, L.-Z. Switchable Stereoselectivity in Bromoaminocyclization of Olefins Catalyzed by Brønsted Acids of Anionic Chiral CoIII Complexes. Angew. Chem. Int. Ed. 2017, 56, 11931–11935. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Sakaguchi, S. Inversions in Asymmetric Conjugate Addition Reaction of Cyclic Enones Catalyzed by the Cu/NHC-AgX System: Factors Affecting the Stereoselective Formation of Both Enantiomers. J. Organomet. Chem. 2017, 846, 407–416. [Google Scholar] [CrossRef]
- Fu, S.; Chen, N.-Y.; Liu, X.; Shao, Z.; Luo, S.-P.; Liu, Q. Ligand-Controlled Cobalt-Catalyzed Transfer Hydrogenation of Alkynes: Stereodivergent Synthesis of Z- and E-Alkenes. J. Am. Chem. Soc. 2016, 138, 8588–8594. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, F.; Ma, Z.; Liu, Y.; Zhang, W. Switchable Stereoselectivity: The Effects of Substituents on the D2-Symmetric Biphenyl Backbone of Phosphoramidites in Copper-Catalyzed Asymmetric Conjugate Addition Reactions with Triethylaluminum. Adv. Synth. Catal. 2012, 354, 1941–1947. [Google Scholar] [CrossRef]
- Chen, G.; Gui, J.; Li, L.; Liao, J. Chiral Sulfoxide-Olefin Ligands: Completely Switchable Stereoselectivity in Rhodium-Catalyzed Asymmetric Conjugate Additions. Angew. Chem. Int. Ed. 2011, 50, 7681–7685. [Google Scholar] [CrossRef]
- Scott, N.W.J.; Ford, M.J.; Jeddi, N.; Eyles, A.; Simon, L.; Whitwood, A.C.; Tanner, T.; Willans, C.E.; Fairlamb, I.J.S. A Dichotomy in Cross-Coupling Site Selectivity in a Dihalogenated Heteroarene: Influence of Mononuclear Pd, Pd Clusters, and Pd Nanoparticles-the Case for Exploiting Pd Catalyst Speciation. J. Am. Chem. Soc. 2021, 143, 9682–9693. [Google Scholar] [CrossRef]
- Wu, F.; Lu, S.; Zhu, S. Regioselectivity-Switchable Intramolecular Hydroarylation of Ynone. Adv. Synth. Catal. 2020, 362, 5632–5636. [Google Scholar] [CrossRef]
- Yang, X.-H.; Davison, R.T.; Nie, S.-Z.; Cruz, F.A.; McGinnis, T.M.; Dong, V.M. Catalytic Hydrothiolation: Counterion-Controlled Regioselectivity. J. Am. Chem. Soc. 2019, 141, 3006–3013. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Fu, Y. Mechanistic Study on Ligand-Controlled Cobalt-Catalyzed Regioselectivity-Switchable Hydroarylation of Styrenes. Chem. Eur. J. 2013, 19, 12093–12103. [Google Scholar] [CrossRef]
- Bedford, R.B.; Durrant, S.J.; Montgomery, M. Catalyst-Switchable Regiocontrol in the Direct Arylation of Remote C-H Groups in Pyrazolo [1,5-a]pyrimidines. Angew. Chem. Int. Ed. 2015, 54, 8787–8790. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Chen, Y.; Garrell, S.; Liu, H.; Zhang, L.-K.; Palani, A.; Hughes, G.; Nargund, R. Ligand-Dependent Site-Selective Suzuki Cross-Coupling of 3,5-Dichloropyridazines. J. Org. Chem. 2013, 78, 7758–7763. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Yoshikai, N. Regioselectivity-Switchable Hydroarylation of Styrenes. J. Am. Chem. Soc. 2011, 133, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Dubovyk, I.; Watson, I.D.G.; Yudin, A.K. Chasing the Proton Culprit from Palladium-Catalyzed Allylic Amination. J. Am. Chem. Soc. 2007, 129, 14172–14173. [Google Scholar] [CrossRef]
- Miller, K.M.; Jamison, T.F. Ligand-Switchable Directing Effects of Tethered Alkenes in Nickel-Catalyzed Additions to Alkynes. J. Am. Chem. Soc. 2004, 126, 15342–15343. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.; Xu, S.; Ding, K. Switchable Cobalt-Catalyzed α-Olefination and α-Alkylation of Nitriles with Primary Alcohols. Org. Lett. 2021, 23, 5028–5032. [Google Scholar] [CrossRef]
- Masson-Makdissi, J.; Vandavasi, J.K.; Newman, S.G. Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of Esters. Org. Lett. 2018, 20, 4094–4098. [Google Scholar] [CrossRef]
- Zhu, B.; Yan, L.-K.; Yao, L.-S.; Ren, H.; Li, R.-H.; Guan, W.; Su, Z.-M. Orthogonal Reactivity of Ni(I)/Pd(0) Dual Catalysts for Ullmann C-C Cross-Coupling: Theoretical Insight. Chem. Commun. 2018, 54, 7959–7962. [Google Scholar] [CrossRef]
- Dhital, R.N.; Sen, A.; Sato, T.; Hu, H.; Ishii, R.; Hashizume, D.; Takaya, H.; Uozumi, Y.; Yamada, Y.M.A. Activator-Promoted Aryl Halide-Dependent Chemoselective Buchwald-Hartwig and Suzuki-Miyaura Type Cross-Coupling Reactions. Org. Lett. 2020, 22, 4797–4801. [Google Scholar] [CrossRef]
- Ohtaka, A.; Kotera, T.; Sakon, A.; Ueda, K.; Hamasaka, G.; Uozumi, Y.; Shinagawa, T.; Shimomura, O.; Nomura, R. Fluoride-Free Hiyama Coupling Reaction Catalyzed by Linear Polystyrene-Stabilized PdO Nanoparticles in Water: Specific Reactivity of PdO Nanoparticles over Pd Nanoparticles. Synlett 2016, 27, 1202–1206. [Google Scholar] [CrossRef]
- Hong, X.; Liang, Y.; Houk, K.N. Mechanisms and Origins of Switchable Chemoselectivity of Ni-Catalyzed C(aryl)-O and C(acyl)-O Activation of Aryl Esters with Phosphine Ligands. J. Am. Chem. Soc. 2014, 136, 2017–2025. [Google Scholar] [CrossRef]
- So, C.M.; Yuen, O.Y.; Ng, S.S.; Chen, Z. General Chemoselective Suzuki-Miyaura Coupling of Polyhalogenated Aryl Triflates Enabled by an Alkyl-Heteroaryl-Based Phosphine Ligand. ACS Catal. 2021, 11, 7820–7827. [Google Scholar] [CrossRef]
- Fleige, M.; Janssen-Müller, D.; Daniliuc, C.G.; Glorius, F. Switchable Selectivity in an NHC-Catalysed Dearomatizing Annulation Reaction. Nat. Chem. 2015, 7, 842–847. [Google Scholar] [CrossRef]
- Ishida, J.; Nakatsuji, M.; Nagata, T.; Kawasaki, H.; Suzuki, T.; Obora, Y. Synthesis and Characterization of N,N-Dimethylformamide-Protected Palladium Nanoparticles and Their Use in the Suzuki-Miyaura Cross-Coupling Reaction. ACS Omega 2020, 5, 9598–9604. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.F.; Ellis, P.J.; Fairlamb, I.J.S.; Wilson, K. Surface Catalysed Suzuki-Miyaura Cross-Coupling by Pd Nanoparticles: An Operando XAS Study. Dalton Trans. 2010, 39, 10473–10482. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Aranzaes, J.R.; Astruc, D. “Homeopathic” Catalytic Activity and Atom-Leaching Mechanism in Miyaura-Suzuki Reactions under Ambient Conditions with Precise Dendrimer-Stabilized Pd Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8644–8648. [Google Scholar] [CrossRef] [PubMed]
- Alimardanov, A.; Schmieder-van de Vondervoot, L.; de Vries, A.H.M.; de Vries, J.G. Use of “Homeopathic” Ligand-Free Palladium as Catalyst for Aryl-Aryl Coupling Reactions. Adv. Synth. Catal. 2004, 346, 1812–1817. [Google Scholar] [CrossRef] [Green Version]
- de Vries, A.H.M.; Mulders, J.M.C.A.; Mommers, J.H.M.; Henderickx, H.J.W.; de Vries, J.G. Homeopathic Ligand-Free Palladium as a Catalyst in the Heck Reaction. A Comparison with a Palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Astruc, D. “Homeopathic” Palladium Nanoparticles Catalysis of Cross Carbon-Carbon Coupling Reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef]
- Tubaro, C.; Biffis, A.; Gonzato, C.; Zecca, M.; Basato, M. Reactivity of Chelating Dicarbene Metal Complex Catalysts, I: An Investigation on the Heck Reaction. J. Mol. Catal. A Chem. 2006, 248, 93–98. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Diallo, A.K.; Ruiz, J. Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using “Click” Dendrimer-Stabilized Palladium Nanoparticles. Molecules 2010, 15, 4947–4960. [Google Scholar] [CrossRef] [Green Version]
- Ohtaka, A.; Kawase, M.; Usami, A.; Fukui, S.; Yamashita, M.; Yamaguchi, K.; Sakon, A.; Shiraki, T.; Ishida, T.; Nagata, S.; et al. Mechanistic Study on Allylic Arylation in Water with Linear Polystyrene-Stabilized Pd and PdO Nanoparticles. ACS Omega 2019, 4, 15764–15770. [Google Scholar] [CrossRef] [Green Version]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Jutand, A.; Mosleh, A. Rate and Mechanism of Oxidative Addition of Aryl Triflates to Zerovalent Palladium Complexes. Evidence for the Formation of Cationic (σ-Aryl)palladium Complexes. Organometallics 1995, 14, 1810–1817. [Google Scholar] [CrossRef]
- Hirakawa, T.; Uramoto, Y.; Yanagisawa, S.; Ikeda, T.; Inagaki, K.; Morikawa, Y. First-Principles Molecular Dynamics Analysis of Ligand-Free Suzuki-Miyaura Cross-Coupling in Water: Transmetalation and Reductive Elimination. J. Phys. Chem. C 2017, 121, 19904–19914. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Al-Halaiqa, A.; Smirnov, V.V. Heck Reactions of Alkenes with Aryl Iodides and Aryl Bromides: Rate-Determining Steps Deduced from a Comparative Kinetic Study of Competing and Noncompeting Reactions. Kinet. Catal. 2007, 48, 716–727. [Google Scholar] [CrossRef]
- Witte, F.; Zucker, S.P.; Paulus, B.; Tzschucke, C.C. Unexpected Substituent Effects in Aryl-Aryl Negishi Cross-Coupling Reactions Rationalized by Density Functional Theory and Natural Charges. Organometallics 2021, 40, 591–599. [Google Scholar] [CrossRef]
- Shekhar, S.; Hartwig, J.F. Distinct Electronic Effects on Reductive Eliminations of Symmetrical and Unsymmetrical Bis-Aryl Platinum Complexes. J. Am. Chem. Soc. 2004, 126, 13016–13027. [Google Scholar] [CrossRef]
- Ichii, S.; Hamasaka, G.; Uozumi, Y. The Hiyama Cross-Coupling Reaction at Parts Per Million Levels of Pd: In Situ Formation of Highly Active Spirosilicates in Glycol Solvents. Chem. Asian J. 2019, 14, 3850–3854. [Google Scholar] [CrossRef]
- Amatore, C.; Grimaud, L.; Le Duc, G.; Jutand, A. Three Roles for the Fluoride Ion in Palladium-Catalyzed Hiyama Reactions: Transmetalation of [ArPdFL2] by Ar’Si(OR)3. Angew. Chem. Int. Ed. 2014, 53, 6982–6985. [Google Scholar] [CrossRef]
- Denmark, S.E.; Smith, R.C.; Chang, W.-T.T. Probing the Electronic Demands of Transmetalation in the Palladium-Catalyzed Cross-Coupling of Arylsilanolates. Tetrahedron 2011, 67, 4391–4396. [Google Scholar] [CrossRef] [Green Version]
- Hübner, S.; de Vries, J.G.; Farina, V. Why Does Industry Not Use Immobilized Transition Metal Complexes as Catalysts? Adv. Synth. Catal. 2016, 358, 3–25. [Google Scholar] [CrossRef]
- Horbaczewskyj, C.S.; Fairlamb, I.J.S. Pd-Catalyzed Cross-Couplings: On the Importance of the Catalyst Quantity Descriptors, Mol % and ppm. Org. Process Res. Dev. 2022, 26, 2240–2269. [Google Scholar] [CrossRef] [PubMed]

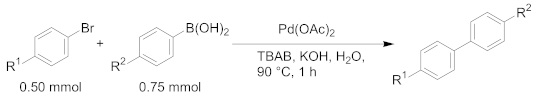 | |||||
|---|---|---|---|---|---|
| Entry | Amount of Pd | Product | R1 | R2 | Yield 2 |
| 1 | 1 mol% | 3a | H | Me | 99% |
| 2 | 3d | MeO | Me | 99% | |
| 3 | 3e | CF3 | Me | 95% | |
| 4 | 3f | H | MeO | 99% | |
| 5 | 3g | H | CF3 | 99% | |
| 6 | 40 mol ppm | 3a | H | Me | 91% |
| 7 | 3d | MeO | Me | 97% | |
| 8 | 3e | CF3 | Me | 98% | |
| 9 | 3f | H | MeO | 99% | |
| 10 | 3g | H | CF3 | 26% | |
| 11 | 10 mol ppm | 3a | H | Me | 65% |
| 12 | 3d | MeO | Me | 41% | |
| 13 | 3e | CF3 | Me | 8% 3 | |
| 14 | 3f | H | MeO | 84% | |
| 15 | 3g | H | CF3 | 7% | |
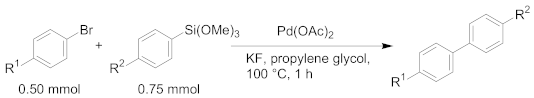 | |||||
|---|---|---|---|---|---|
| Entry | Amount of Pd | Product | R1 | R2 | Yield 2 |
| 1 | 1 mol% | 3a | Me | H | 81% |
| 2 | 3f | MeO | H | 99% | |
| 3 | 3g | CF3 | H | 69% | |
| 4 | 3d | Me | MeO | 70% | |
| 5 | 3h | Me | F | 72% | |
| 6 | 100 mol ppm | 3a | Me | H | 99% |
| 7 | 3f | MeO | H | 99% | |
| 8 | 3g | CF3 | H | 93% | |
| 9 | 3d | Me | MeO | 86% | |
| 10 | 3h | Me | F | <1% 3 | |
| 11 | 10 mol ppm | 3a | Me | H | 90% |
| 12 | 3f | MeO | H | 87% | |
| 13 | 3g | CF3 | H | 2% 4 | |
| 14 | 3d | Me | MeO | 93% | |
| 15 | 3h | Me | F | 0% 3 | |
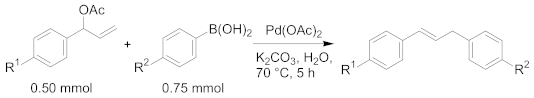 | |||||
|---|---|---|---|---|---|
| Entry | Amount of Pd | Product | R1 | R2 | Yield 2 |
| 1 | 100 mol ppm | 4a | H | Me | 66% |
| 2 | 4b | MeO | Me | 71% | |
| 3 | 4c | CF3 | Me | 58% | |
| 4 | 4d | H | MeO | 86% | |
| 5 | 4e | H | CF3 | 40% | |
| 6 | 40 mol ppm | 4a | H | Me | 17% |
| 7 | 4b | MeO | Me | 21% | |
| 8 | 4c | CF3 | Me | 20% | |
| 9 | 4d | H | MeO | 58% | |
| 10 | 4e | H | CF3 | 0% 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawase, M.; Shibata, T.; Masuu, S.; Yamaguchi, M.; Matsumura, Y.; Shimomura, O.; Ohtaka, A. Expression of Reaction Selectivity and the Substituent Effect in Ullmann, Suzuki, Hiyama, and Allylic Arylation Reactions Caused by Reducing Catalyst Loading. Catalysts 2023, 13, 1115. https://doi.org/10.3390/catal13071115
Kawase M, Shibata T, Masuu S, Yamaguchi M, Matsumura Y, Shimomura O, Ohtaka A. Expression of Reaction Selectivity and the Substituent Effect in Ullmann, Suzuki, Hiyama, and Allylic Arylation Reactions Caused by Reducing Catalyst Loading. Catalysts. 2023; 13(7):1115. https://doi.org/10.3390/catal13071115
Chicago/Turabian StyleKawase, Misa, Tomohiro Shibata, Shouhei Masuu, Masaki Yamaguchi, Yoshimasa Matsumura, Osamu Shimomura, and Atsushi Ohtaka. 2023. "Expression of Reaction Selectivity and the Substituent Effect in Ullmann, Suzuki, Hiyama, and Allylic Arylation Reactions Caused by Reducing Catalyst Loading" Catalysts 13, no. 7: 1115. https://doi.org/10.3390/catal13071115