Atomic-Scale Insights into Carbon Dioxide Hydrogenation over Bimetallic Iron–Cobalt Catalysts: A Density Functional Theory Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption of Species Involved in CO2 Hydrogenation to Single Carbon Products on Co(111) and FeCo(111) Surfaces
2.2. H2 Dissociation
2.3. CO2 Dissociation
- (a)
- Direct CO2 Dissociation
- (b)
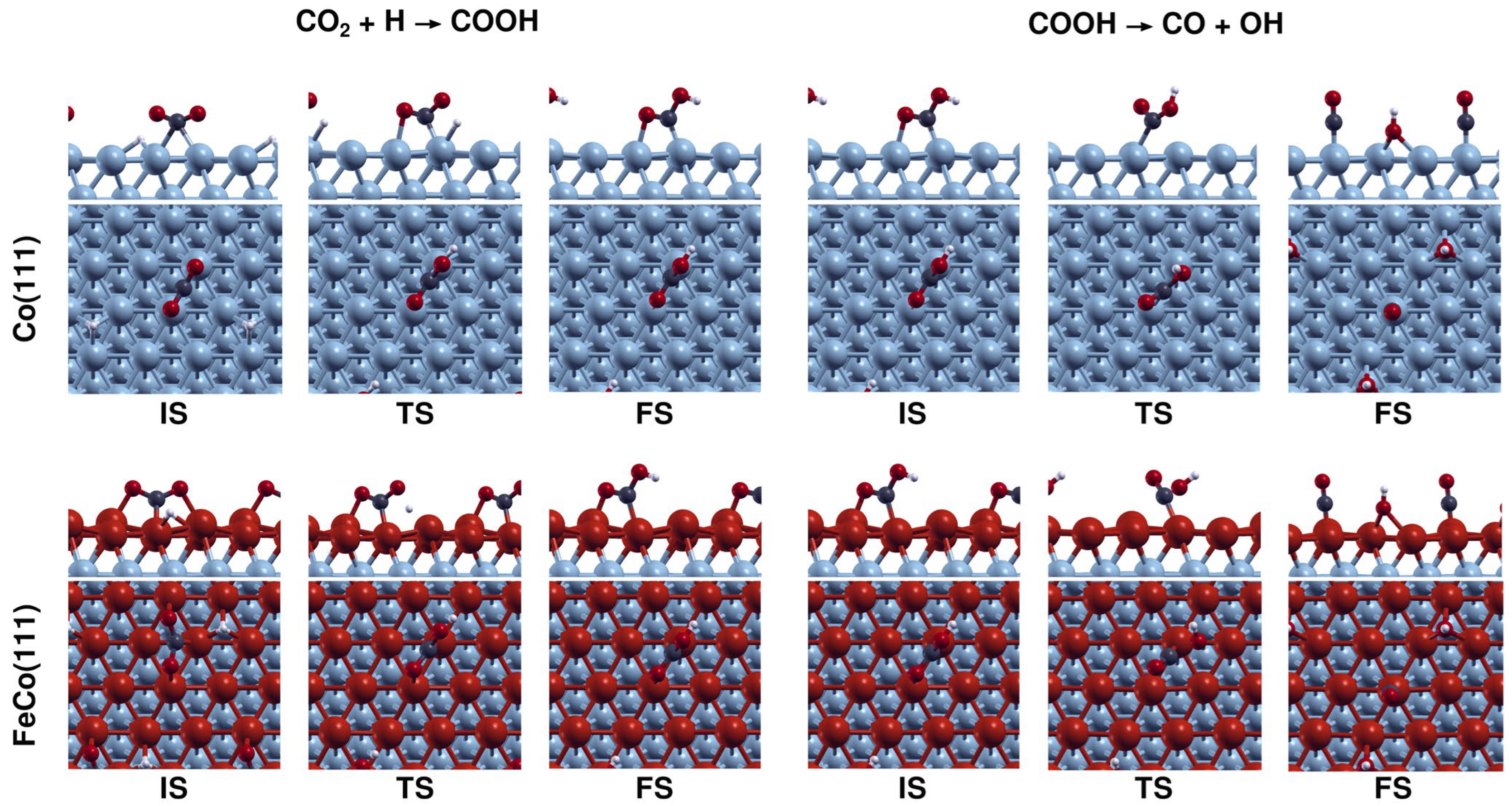
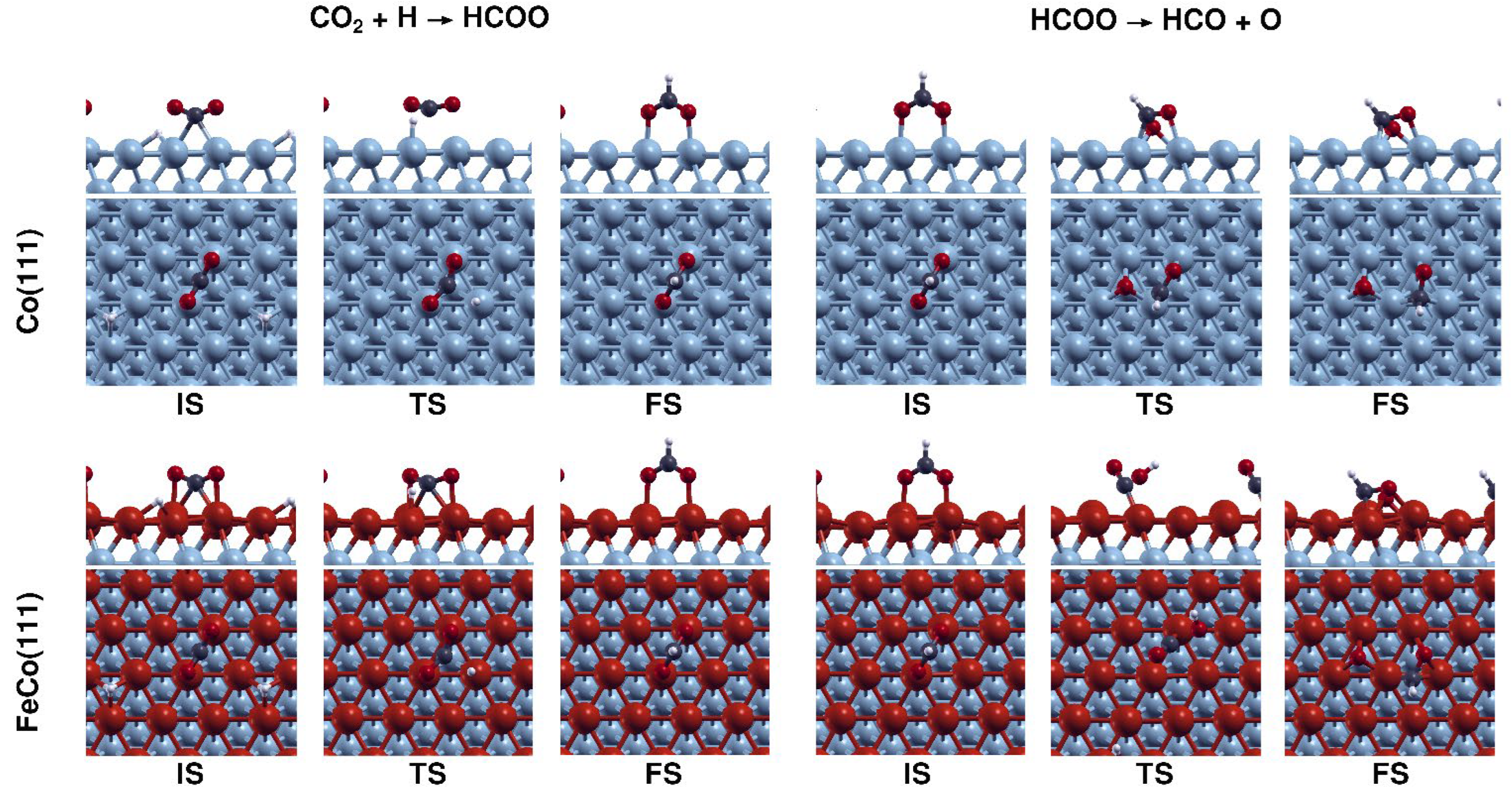
- H-assisted CO2 Dissociation
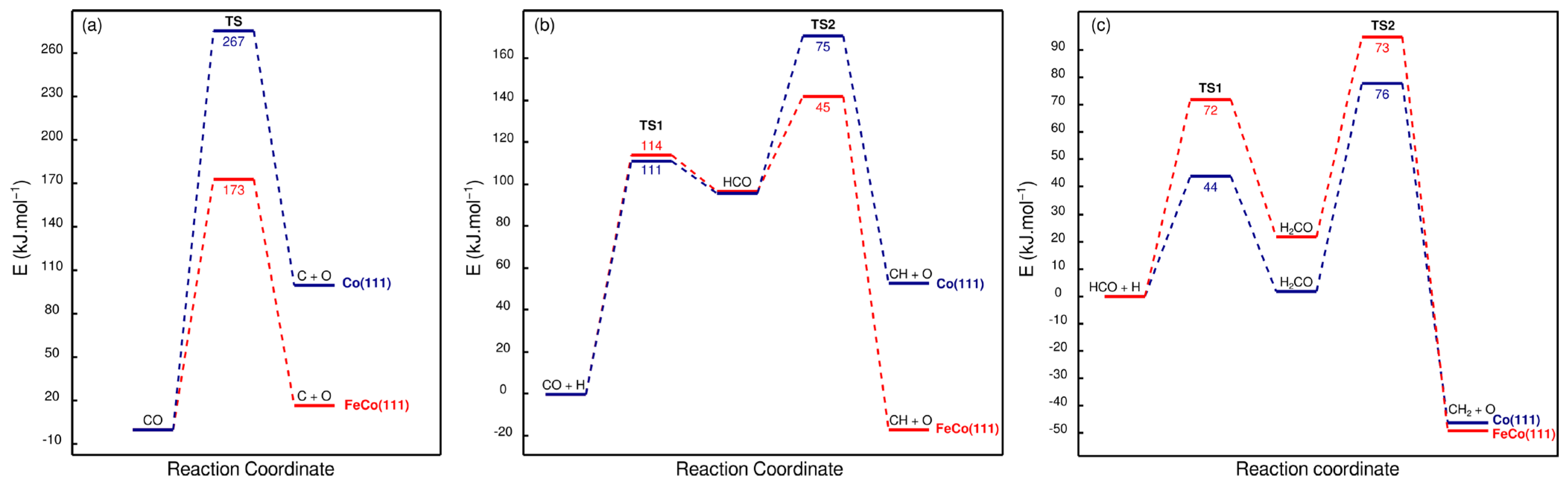
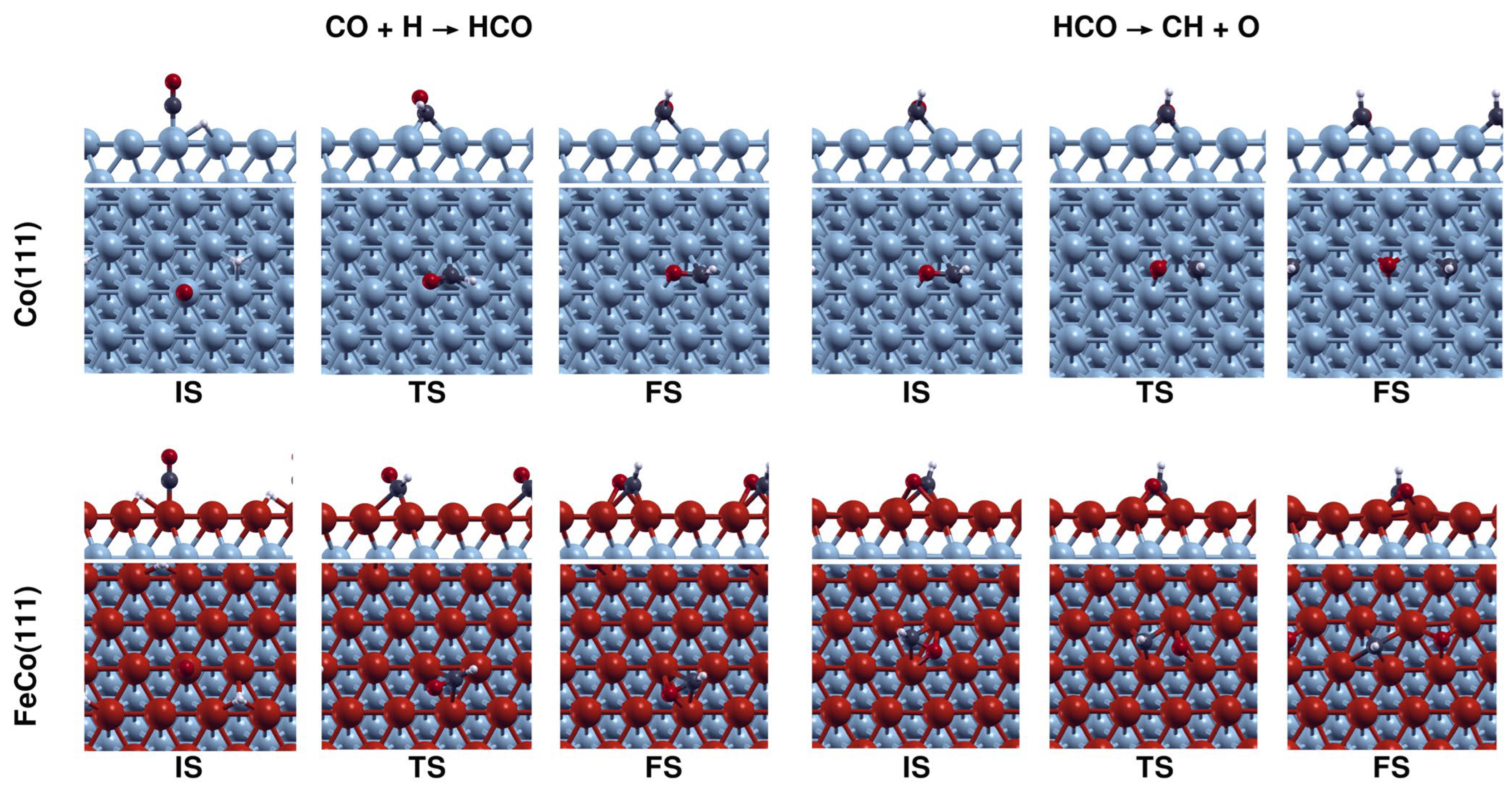
2.4. CO Dissociation
- (a)
- Direct CO Dissociation
- (b)
- H-assisted CO Dissociation
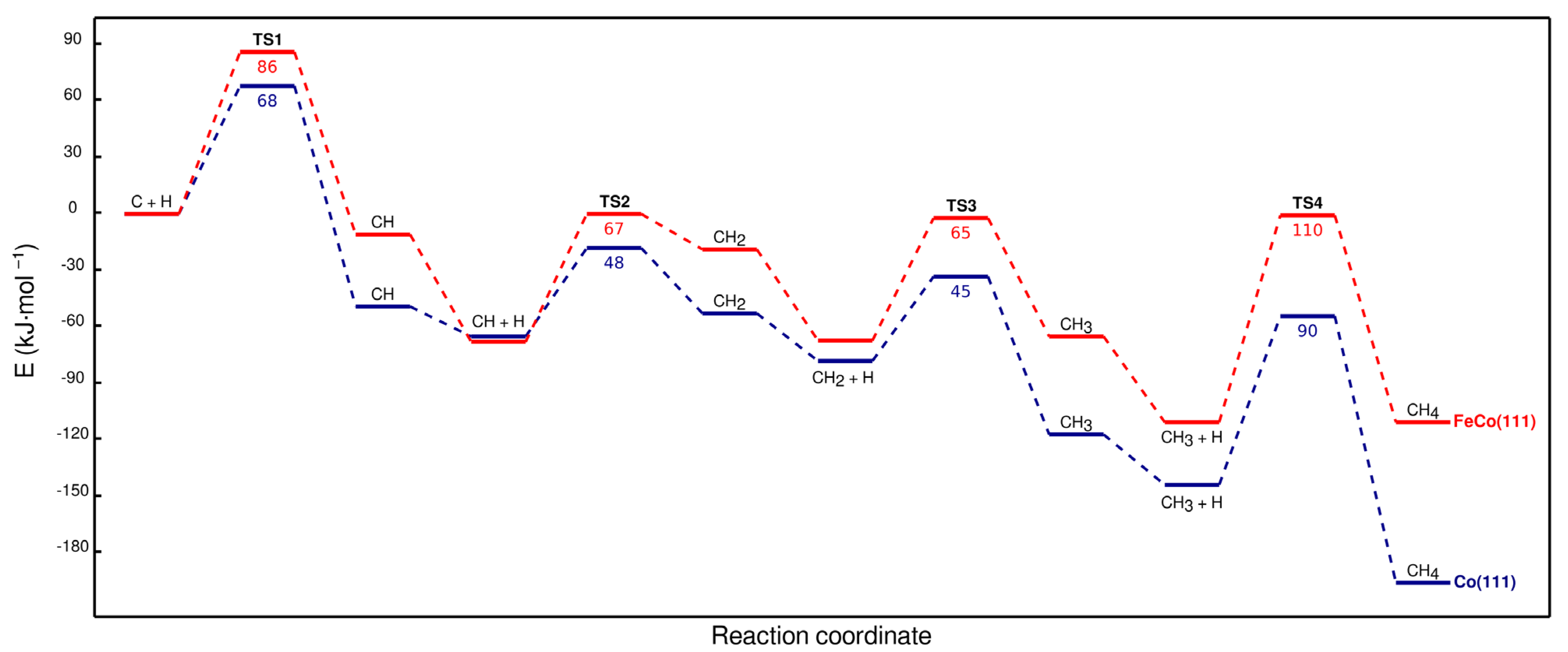
2.5. CH4 Formation
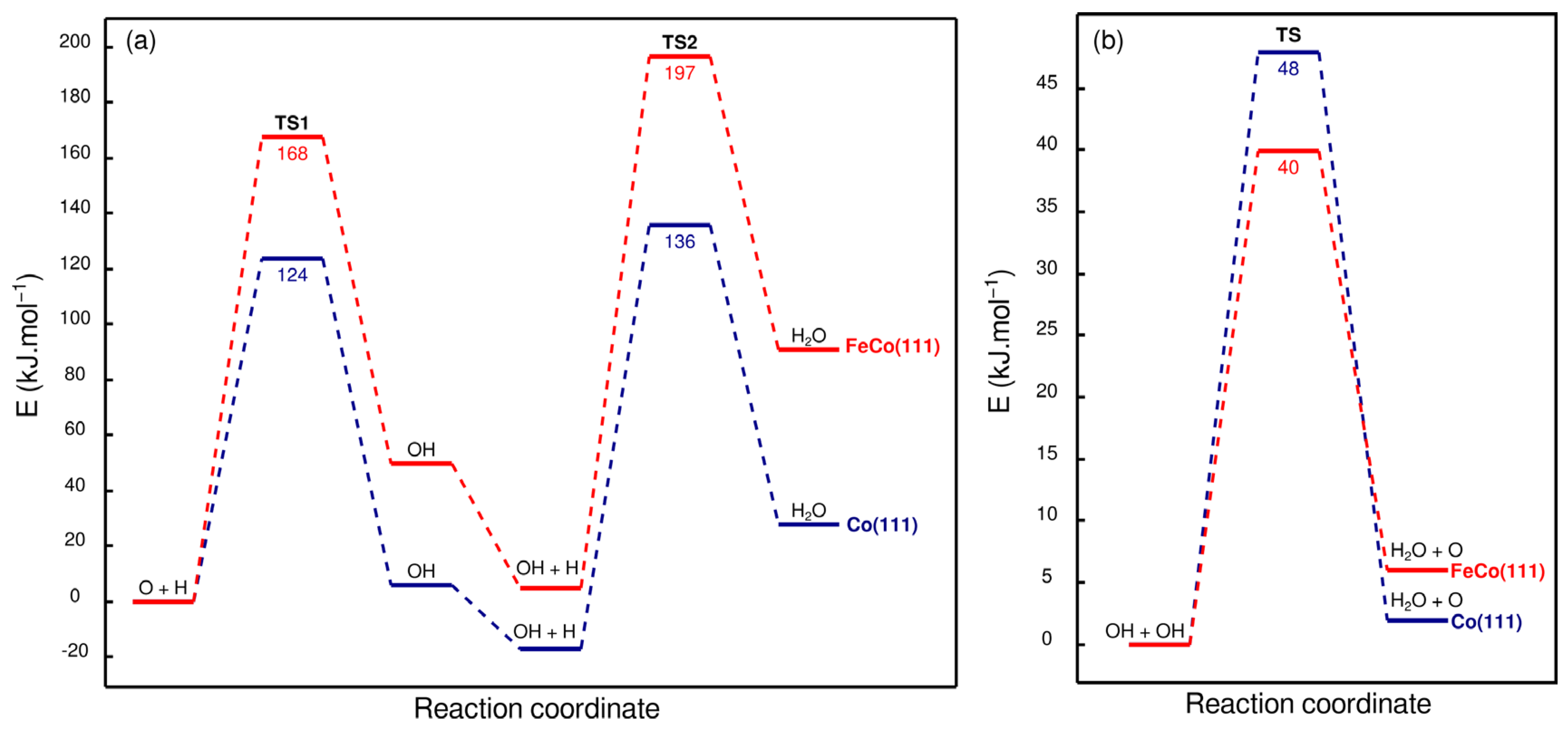
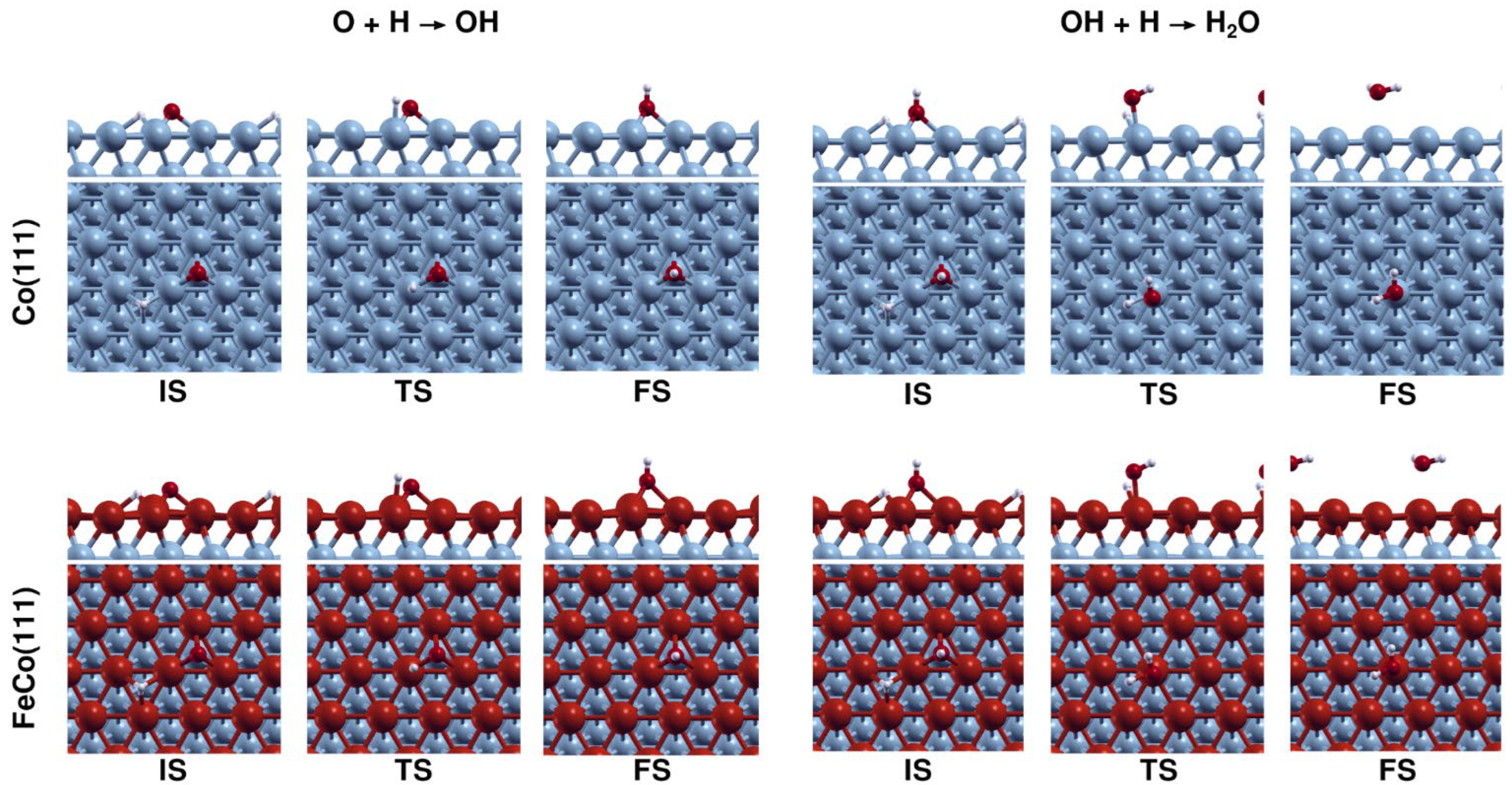
2.6. H2O Formation
2.7. H3COH Formation
2.8. Effect of Fe-Induced Charge Modification on Adsorbates and Elementary Reactions on Bimetallic FeCo(111) Surface
3. Computational Methodology
3.1. Computational Model
3.2. Computational Details
4. Conclusions
- (1)
- Fe doping leads to increased adsorption energies for all surface species, including CO2; CO; and atomic species, such as C, O, and H. However, with respect to the relative increases in adsorption energies, we find that Fe doping mainly leads to increased binding strengths for CO containing species, which can be projected to cause increased COx/H adsorbate ratios on the metallic FeCo(111) surface.
- (2)
- In terms of catalytic activity, FeCo(111) surface leads to decreased activation energies for direct CO2 dissociation, which yields increased surface CO and O coverages. However, due to a slight increase in the activation energy for HCO formation, the rate-limiting step for CO dissociation on FeCo(111) surfaces can be expected to have lower overall activity for CO2 hydrogenation, as previously observed in experimental studies.
- (3)
- In terms of selectivity, FeCo(111) surface hinders the formation of hydrogenated products due to increased activation energies and decreased H coverages. FeCo(111) surface neither promotes CH4 and methanol formation (due to high activation energies) nor CO formation (due to stronger adsorption), which indicates that the bimetallic FeCo surfaces does not play a role in the formation of these species, as experimentally observed on FeCo nanoparticles.
- (4)
- The fundamental cause of increased adsorption energies and increased activation energies for hydrogenation due to Fe doping is linked to the electron-donating ability of Fe atoms to both cobalt atoms and adsorbates. Therefore, the tuning of the electronic charge on the cobalt surface is projected to be a fundamental descriptor, which can adjust the balance between CO2 activation and hydrogenation reactions.
- (5)
- The main effect of the formation of a bimetallic FeCo(111) surface on the CO2 hydrogenation performance of cobalt surfaces is the inhibition of oxygen removal from the catalytic surface. Therefore, the introduction of Fe to cobalt surfaces in FeCo nanoparticles can be expected to lead the structural evolution of the bimetallic surfaces towards iron oxide and mixed iron–cobalt oxide phases, which in turn can lead to a different selectivity behavior of bimetallic FeCo nanoparticles.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Van de Loosdrecht, J.; Botes, F.G.; Ciobica, I.M.; Ferreira, A.; Gibson, P.; Moodley, D.J.; Saib, A.M.; Visagie, J.L.; Weststrate, C.J.; Niemantsverdriet, J.W. Comprehensive Inorganic Chemistry II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Puga, A.V. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170. [Google Scholar] [CrossRef]
- Daga, Y.; Kizilkaya, A.C. Mechanistic Insights into the Effect of Sulfur on the Selectivity of Cobalt-Catalyzed Fischer–Tropsch Synthesis: A DFT Study. Catalysts 2022, 12, 425. [Google Scholar] [CrossRef]
- Jeske, K.; Kizilkaya, A.C.; López-Luque, I.; Pfänder, N.; Bartsch, M.; Concepción, P.; Prieto, G. Design of Cobalt Fischer-Tropsch Catalysts for the Combined Production of Liquid Fuels and Olefin Chemicals from Hydrogen-Rich Syngas. ACS Catal. 2021, 11, 4784–4798. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, B.; Broos, R.J.P.; Chen, W.; Filot, I.A.W.; Hensen, E.J.M. First-principles based microkinetic modeling of transient kinetics of CO hydrogenation on cobalt catalysts. Catal. Today 2020, 342, 131–141. [Google Scholar] [CrossRef]
- Owen, R.E.; O’Byrne, J.P.; Mattia, D.; Plucinski, P.; Pascu, S.I.; Jones, M.D. Cobalt catalysts for the conversion of CO2 to light hydrocarbons at atmospheric pressure. Chem. Commun. 2013, 49, 11683–11685. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, G.; Jiang, X.; Liu, Y.; Zhu, J.; Ding, F.; Liu, Z.; Guo, X.; Song, C. CO2 Hydrogenation on Unpromoted and M-Promoted Co/TiO2 Catalysts (M = Zr, K, Cs): Effects of Crystal Phase of Supports and Metal-Support Interaction on Tuning Product Distribution. ACS Catal. 2019, 9, 2739–2751. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light olefin synthesis from CO2 hydrogenation over K-promoted Fe-Co bimetallic catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Wang, W.; Toshcheva, E.; Ramirez, A.; Shterk, G.; Ahmad, R.; Caglayan, M.; Cerrillo, J.L.; Dokania, A.; Clancy, G.; Shoinkhorova, T.B.; et al. Bimetallic Fe-Co catalysts for the one step selective hydrogenation of CO2 to liquid hydrocarbons. Catal. Sci. Technol. 2023, 13, 1527–1540. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 2018, 6, 787–793. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, J.; Tian, Y.; Zhang, B.; Wu, Y. Preparation and Performances of ZIF-67-Derived FeCo Bimetallic Catalysts for CO2 Hydrogenation toLight Olefins. Catalysts 2020, 10, 455. [Google Scholar] [CrossRef]
- Guo, L.; Cui, Y.; Li, H.; Fang, Y.; Prasert, R.; Wu, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Selective formation of linear-alpha olefins (LAOs) by CO2 hydrogenation over bimetallic Fe/Co-Y catalyst. Catal. Commun. 2019, 130, 105759. [Google Scholar] [CrossRef]
- Liang, J.; Guo, L.; Gao, W.; Wang, C.; Guo, X.; He, Y.; Yang, G.; Tsubaki, N. Direct Conversion of CO2 to Aromatics over K−Zn−Fe/ZSM-5 Catalysts via a Fischer−Tropsch Synthesis Pathway. Ind. Eng. Chem. Res. 2022, 61, 10336–10346. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of Zeolites to C1 Chemistry: Recent Advances, Challenges, and Opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef] [PubMed]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co-Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Ischenko, O.V.; Dyachenko, A.G.; Saldan, I.; Lisnyak, V.V.; Diyuk, V.E.; Vakaliuk, A.V.; Yatsymyrskyi, A.V.; Gaidai, S.V.; Zakharova, T.M.; Makota, O.; et al. Methanation of CO2 on bulk Co–Fe catalysts. Int. J. Hydrogen Energy 2021, 46, 37860–37871. [Google Scholar] [CrossRef]
- Sandupatla, A.S.; Banerjee, A.; Deo, G. Optimizing CO2 hydrogenation to methane over CoFe bimetallic catalyst: Experimental and density functional theory studies. Appl. Surf. Sci. 2019, 485, 441–449. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; van Bavel, A.P.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct conversion of CO2 to a jet fuel over CoFe alloy catalysts. Innovation 2021, 2, 100170. [Google Scholar] [CrossRef]
- Hwang, S.M.; Han, S.J.; Park, H.G.; Lee, H.; An, K.; Jun, K.W.; Kim, S.K. Atomically alloyed Fe-Co catalyst derived from a N-coordinated Co single-atom structure for CO2 hydrogenation. ACS Catal. 2021, 11, 2267–2278. [Google Scholar] [CrossRef]
- Gunasooriya, G.T.K.K.; Van Bavel, A.P.; Kuipers, H.P.C.E.; Saeys, M. Key Role of Surface Hydroxyl Groups in C-O Activation during Fischer-Tropsch Synthesis. ACS Catal. 2016, 6, 3660–3664. [Google Scholar] [CrossRef]
- Chen, S.; Zaffran, J.; Yang, B. Dry reforming of methane over the cobalt catalyst: Theoretical insights into the reaction kinetics and mechanism for catalyst deactivation. Appl. Catal. B Environ. 2020, 270, 118859. [Google Scholar] [CrossRef]
- Huang, H.; Yu, Y.; Zhang, M. Mechanistic insight into methane dry reforming over cobalt: A density functional theory study. Phys. Chem. Chem. Phys. 2020, 22, 27320–27331. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Q.; Wang, G.; Hou, B.; Jia, L.; Li, D. Mechanistic insight into the C2 hydrocarbons formation from Syngas on fcc-Co(111) surface: A DFT study. J. Phys. Chem. C 2016, 120, 9132–9147. [Google Scholar] [CrossRef]
- Van Helden, P.; Weststrate, C.J. Hydrogen Adsorption on Co Surfaces: A Density Functional Theory and Temperature Programmed Desorption Study. ACS Catal. 2012, 2, 1097–1107. [Google Scholar] [CrossRef]
- Zijlstra, B.; Broos, R.J.P.; Chen, W.; Bezemer, G.L.; Filot, I.A.W.; Hensen, E.J.M. The vital role of step-edge sites for both CO activation and chain growth on cobalt fischer-tropsch catalysts revealed through first-principles-based microkinetic modeling including lateral interactions. ACS Catal. 2020, 10, 9376–9400. [Google Scholar] [CrossRef]
- Yu, M.; Liu, L.; Wang, Q.; Jia, L.; Wang, J.; Li, D. Rediscovering Tuning Product Selectivity by an Energy Descriptor: CH4 Formation and C1-C1 Coupling on the FCC Co Surface. J. Phys. Chem. C 2020, 124, 11040–11049. [Google Scholar] [CrossRef]
- Pedersen, E.Ø.; Svenum, I.H.; Blekkan, E.A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins—An experimental and theoretical study. J. Catal. 2018, 361, 23–32. [Google Scholar] [CrossRef]
- Kizilkaya, A.C.; Niemantsverdriet, J.W.; Weststrate, C.J. Oxygen Adsorption and Water Formation on Co(0001). J. Phys. Chem. C 2016, 120, 4833–4842. [Google Scholar] [CrossRef]
- Weststrate, C.J.; Sharma, D.; Rodríguez, D.G.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Water Formation Kinetics on Co(0001) at Low and Near-Ambient Hydrogen Pressures in the Context of Fischer-Tropsch Synthesis. J. Phys. Chem. C 2023, 127, 3452–3461. [Google Scholar] [CrossRef]
- Luo, W.; Asthagiri, A. Density functional theory study of methanol steam reforming on Co(0001) and Co(111) surfaces. J. Phys. Chem. C 2014, 118, 15274–15285. [Google Scholar] [CrossRef]
- Ma, F.F.; Ma, S.H.; Jiao, Z.Y.; Dai, X.Q. Adsorption and decomposition of H2O on cobalt surfaces: A DFT study. Appl. Surf. Sci. 2016, 384, 10–17. [Google Scholar] [CrossRef]
- Ismail, A.S.M.; Casavola, M.; Liu, B.; Gloter, A.; Van Deelen, T.W.; Versluijs, M.; Meeldijk, J.D.; Stéphan, O.; De Jong, K.P.; De Groot, F.M.F. Atomic-Scale Investigation of the Structural and Electronic Properties of Cobalt-Iron Bimetallic Fischer-Tropsch Catalysts. ACS Catal. 2019, 9, 7998–8011. [Google Scholar] [CrossRef]
- Greeley, J.; Norskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, J.R.; Norskov, J.K. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Gambu, T.G.; van Heerden, T.; van Steen, E. Mechanistic pathways for oxygen removal on Pt-doped Co(111) in the Fischer-Tropsch reaction. Catal. Today 2020, 342, 142–151. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Q.; Yi, Z.; Wang, J.; Yang, H. Rational electronic control of carbon dioxide reduction over cobalt oxide. J. Catal. 2020, 387, 119–128. [Google Scholar] [CrossRef]
- Van Helden, P.; Ciobîc, I.M.; Coetzer, R.L.J. The size-dependent site composition of FCC cobalt nanocrystals. Catal. Today 2016, 261, 48–59. [Google Scholar] [CrossRef]
- Rochana, P.; Wilcox, J. A theoretical study of CO adsorption on FeCo(100) and the effect of alloying. Surf. Sci. 2011, 605, 681–688. [Google Scholar] [CrossRef]
- Calderone, V.R.; Shiju, N.R.; Curulla-ferrø, D.; Chambrey, S.; Khodakov, A.; Rose, A.; Thiessen, J.; Jess, A.; Rothenberg, G. De Novo Design of Nanostructured Iron–Cobalt Fischer–Tropsch Catalysts. Angewandte 2013, 52, 4397–4401. [Google Scholar] [CrossRef]
- Moran-Lopez, J.L. Surface Composition of the Ordered Binary Alloy System: Fe-Co. Appl. Surf. Sci. 1984, 19, 167–180. [Google Scholar] [CrossRef]
- Allié, G.; Lauroz, C.; Villemain, P. Leed and aes study of the (100) surface of a 50-50 iron-cobalt monocrystalline alloy. Surf. Sci. 1981, 104, 583–598. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Eren, B.; Torres, D.; Karsl, O.; Liu, Z.; Wu, C.H.; Stacchiola, D.; Bluhm, H.; Somorjai, G.A.; Salmeron, M. Structure of Copper−Cobalt Surface Alloys in Equilibrium with Carbon Monoxide Gas. J. Am. Chem. Soc. 2018, 140, 6575–6581. [Google Scholar] [CrossRef] [PubMed]
- Collinge, G.; Kruse, N.; Mcewen, J. Role of Carbon Monoxide in Catalyst Reconstruction for CO Hydrogenation: First-Principles Study of the Composition, Structure, and Stability of Cu/Co (1012) as a Function of CO Pressure. J. Phys. Chem. C 2017, 121, 2181–2191. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W. Comment on “generalized gradient approximation made simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 22–25. [Google Scholar] [CrossRef]
- Román-Pérez, G.; Soler, J.M. Efficient implementation of a van der waals density functional: Application to double-wall carbon nanotubes. Phys. Rev. Lett. 2009, 103, 096102. [Google Scholar] [CrossRef]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 2010, 22, 022201. [Google Scholar] [CrossRef] [PubMed]
- Gunasooriya, G.T.K.K.; Van Bavel, A.P.; Kuipers, H.P.C.E.; Saeys, M. CO adsorption on cobalt: Prediction of stable surface phases. Surf. Sci. 2015, 642, L6–L10. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
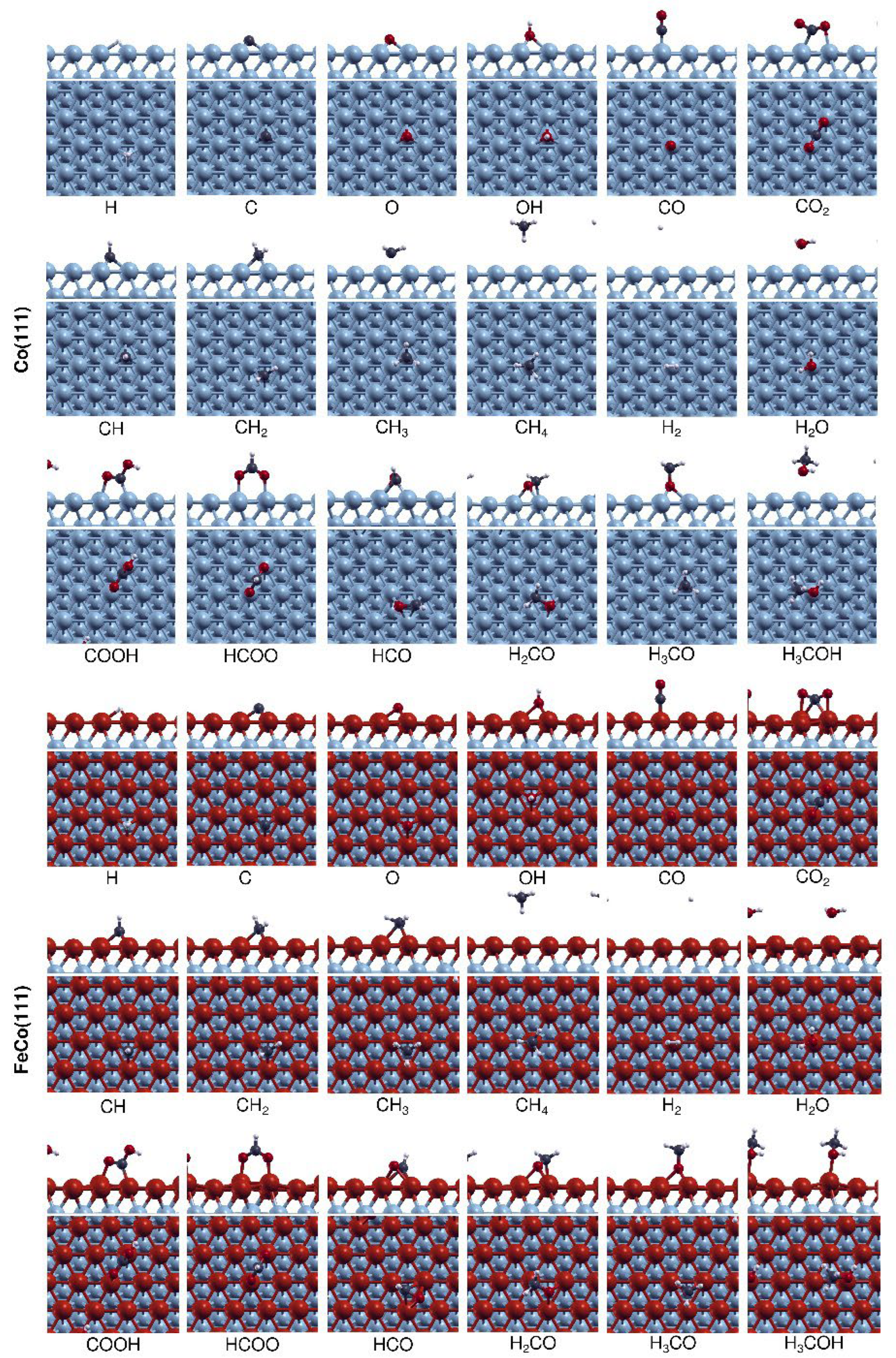










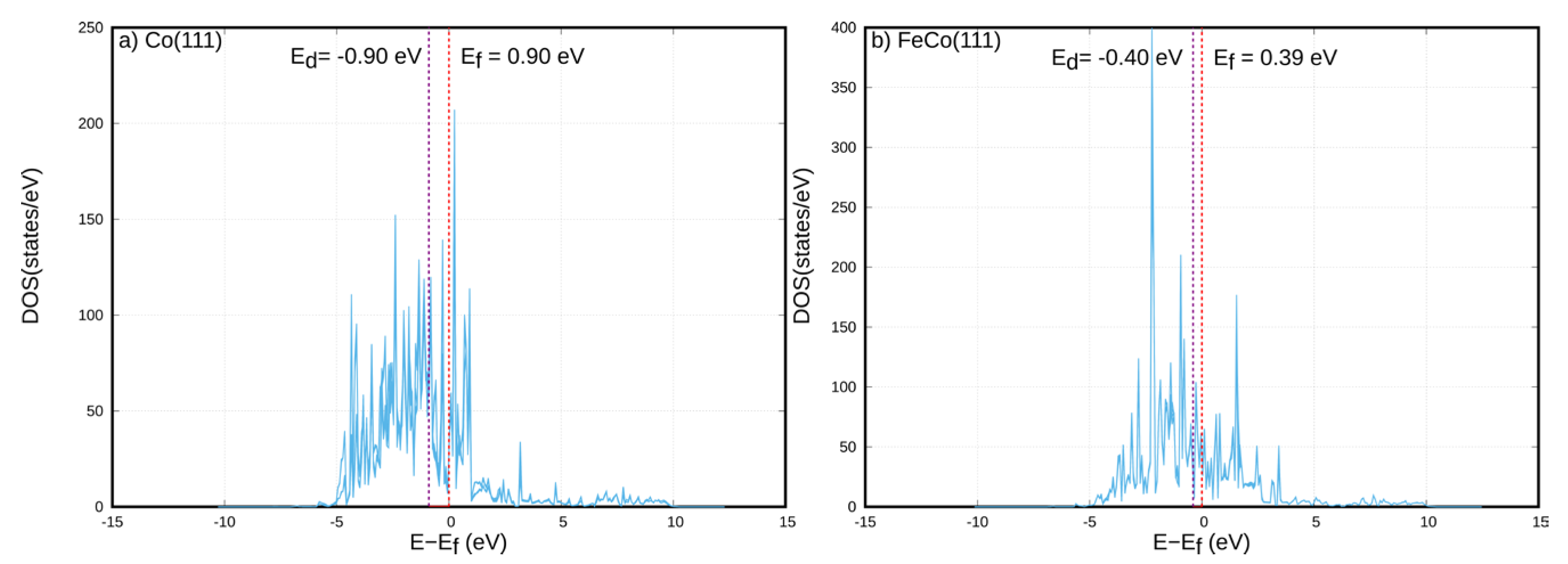
| Species | Co(111) | FeCo(111) | ΔE (%) |
|---|---|---|---|
| H | −278 (fcc) | −302 (fcc) | 9 |
| C | −671 (hcp) | −675 (hcp) | 9 |
| O | −564 (hcp) | −635 (fcc) | 13 |
| CO | −132 (top) | −173 (top) | 31 |
| CO2 | −19 (bridge via C) | −29 (bridge via C) | 53 |
| H2 | −6 (top) | −6 (top) | 0 |
| OH | −329 (hcp) | −368 (fcc) | 12 |
| CH | −561 (hcp) | −615 (fcc) | 10 |
| CH2 | −362 (fcc) | −413 (fcc) | 14 |
| CH3 | −166 (hcp) | −198 (fcc) | 19 |
| CH4 | −14 (top) | −14 (top) | 2 |
| H2O | −28 (top) | −47 (top) | 68 |
| HCO | −187 (bridge via O) | −255 (hcp via O) | 36 |
| COOH | −211 (bridge via C) | −257 (bridge via C) | 22 |
| HCOO | −311 (bridge via C) | −364 (bridge via C) | 17 |
| H2CO | −58 (fcc via O) | −125 (fcc via O) | 116 |
| H3CO | −284 (hcp) | −330 (fcc) | 16 |
| H3COH | −39 (top via O) | −62 (top via O) | 59 |
| No | Elementary Reaction | Ea | ΔE | ||
|---|---|---|---|---|---|
| Co(111) | FeCo(111) | Co(111) | FeCo(111) | ||
| R1 | H2 → H2 | 26 | 14 | 13 | −9 |
| R2 | H2 → H + H | 28 | 8 | −59 | −89 |
| R3 | CO2 → CO + O | 61 | 17 | −77 | −154 |
| R4 | CO2 + H → COOH | 94 | 149 | −18 | 41 |
| R5 | COOH → CO + OH | 53 | 74 | −112 | −142 |
| R6 | CO2 + H → HCOO | 71 | 51 | −74 | −55 |
| R7 | HCOO → HCO + O | 107 | 101 | 52 | 6 |
| R8 | CO → C + O | 267 | 173 | 104 | 17 |
| R9 | CO + H → HCO | 111 | 114 | 96 | 97 |
| R10 | HCO → CH + O | 75 | 45 | −43 | −114 |
| R11 | O + H → OH | 124 | 168 | 6 | 50 |
| R12 | OH + H → H2O | 153 | 178 | 44 | 86 |
| R13 | OH + OH → H2O | 48 | 40 | 2 | 6 |
| R14 | C + H → CH | 68 | 86 | −49 | −11 |
| R15 | CH + H → CH2 | 48 | 67 | 12 | 48 |
| R16 | CH2 + H → CH3 | 45 | 65 | −39 | 2 |
| R17 | CH3 + H → CH4 | 90 | 110 | −52 | 0 |
| R18 | HCO + H → H2CO | 44 | 72 | 2 | 22 |
| R19 | CH2 + O → H2CO | 124 | 150 | 48 | 86 |
| R20 | H2CO → CH2 + O | 76 | 73 | −48 | −71 |
| R21 | H2CO + H → H3CO | 42 | 51 | −83 | −50 |
| R22 | CH3 + O → H3CO | 141 | 166 | −4 | 49 |
| R23 | H3CO + H → H3COH | 160 | 177 | 56 | 94 |
| R24 | CH3 + OH → H3COH | 196 | 238 | 42 | 89 |
| Adsorbate | q on Co(111) (e−) | q on FeCo(111) (e−) | Δq (%) |
|---|---|---|---|
| H | −0.41 | −0.49 | 20 |
| C | −0.90 | −1.10 | 22 |
| O | −1.05 | −1.14 | 9 |
| CO | −0.39 | −0.49 | 26 |
| CO2 | −0.65 | −0.85 | 31 |
| H2 | −0.01 | −0.01 | 0 |
| OH | −0.64 | −0.70 | 9 |
| CH | −0.74 | −0.92 | 24 |
| CH2 | −0.63 | −0.77 | 22 |
| CH3 | −0.43 | −0.50 | 16 |
| CH4 | −0.02 | −0.03 | 50 |
| H2O | −0.02 | −0.30 | 1400 |
| HCO | −0.76 | −0.97 | 28 |
| COOH | −1.02 | −1.30 | 27 |
| HCOO | −0.57 | −0.74 | 30 |
| H2CO | −0.69 | −0.80 | 16 |
| H3CO | −0.63 | −0.68 | 8 |
| H3COH | −0.04 | −0.03 | −25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuncer, D.; Kizilkaya, A.C. Atomic-Scale Insights into Carbon Dioxide Hydrogenation over Bimetallic Iron–Cobalt Catalysts: A Density Functional Theory Study. Catalysts 2023, 13, 1390. https://doi.org/10.3390/catal13111390
Tuncer D, Kizilkaya AC. Atomic-Scale Insights into Carbon Dioxide Hydrogenation over Bimetallic Iron–Cobalt Catalysts: A Density Functional Theory Study. Catalysts. 2023; 13(11):1390. https://doi.org/10.3390/catal13111390
Chicago/Turabian StyleTuncer, Dilan, and Ali Can Kizilkaya. 2023. "Atomic-Scale Insights into Carbon Dioxide Hydrogenation over Bimetallic Iron–Cobalt Catalysts: A Density Functional Theory Study" Catalysts 13, no. 11: 1390. https://doi.org/10.3390/catal13111390






