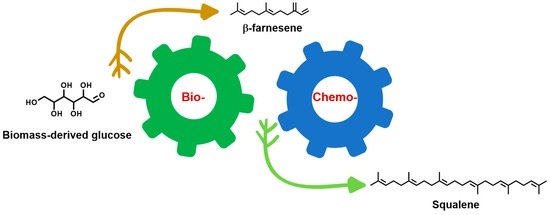
Integrating Fermentation Engineering and Organopalladium Chemocatalysis for the Production of Squalene from Biomass-Derived Carbohydrates as the Starting Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. Establishment of the β-Farnesene Fermentation Process
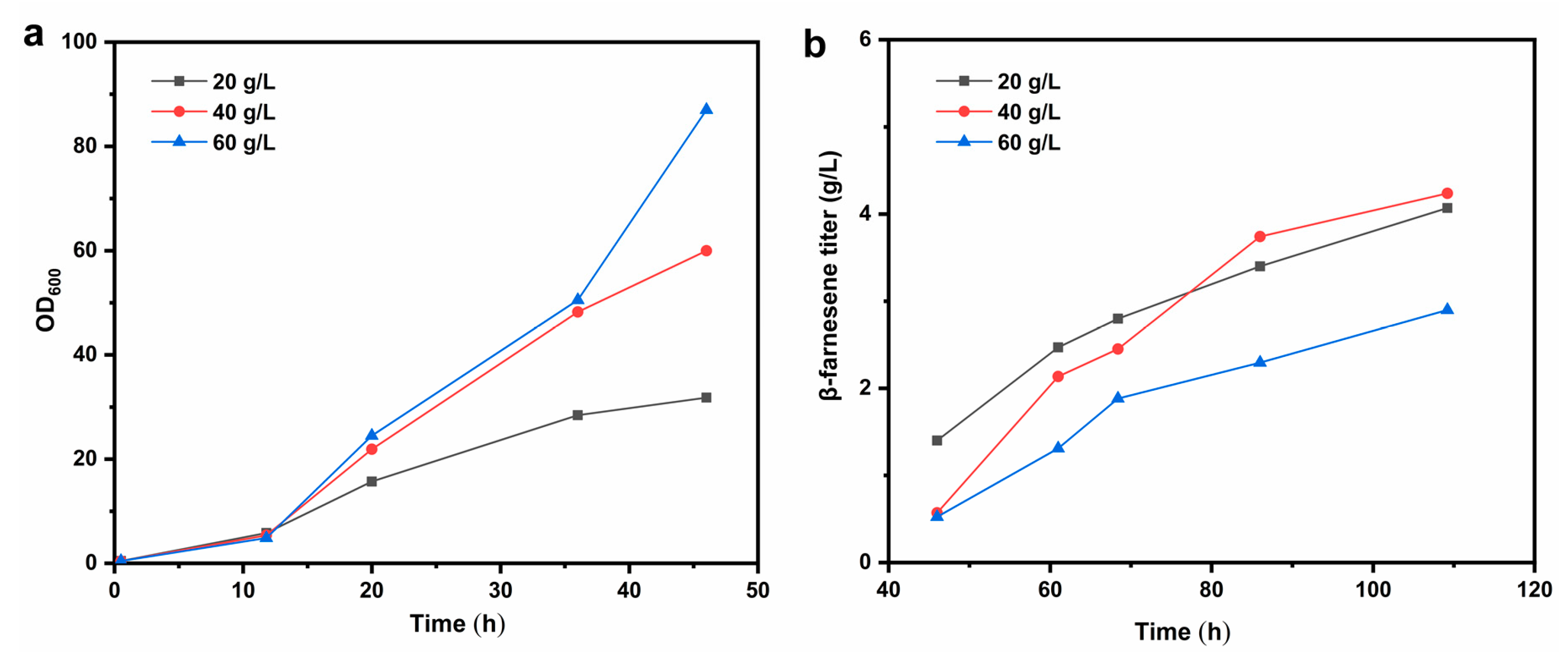
2.1.1. Initial Glucose Concentration
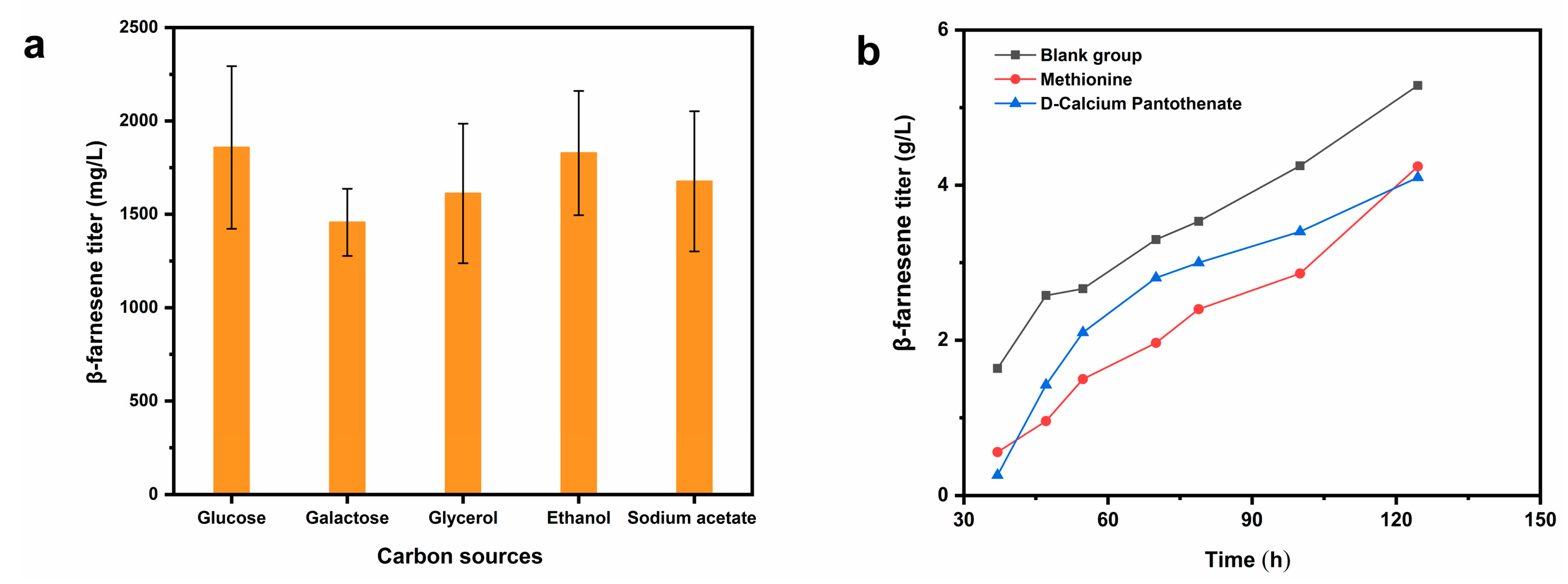
2.1.2. Supplementary Carbon Sources and Substrate Addition Fermentation
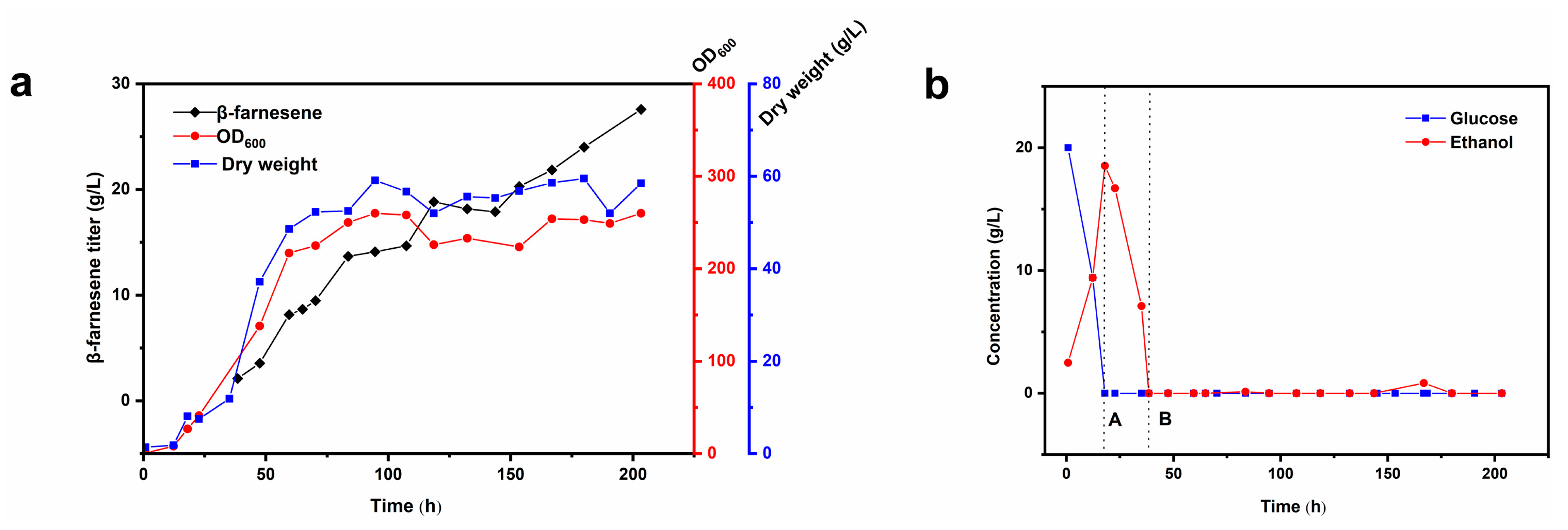
2.1.3. Fed-Batch Fermentation in a 2.5 L Bioreactor
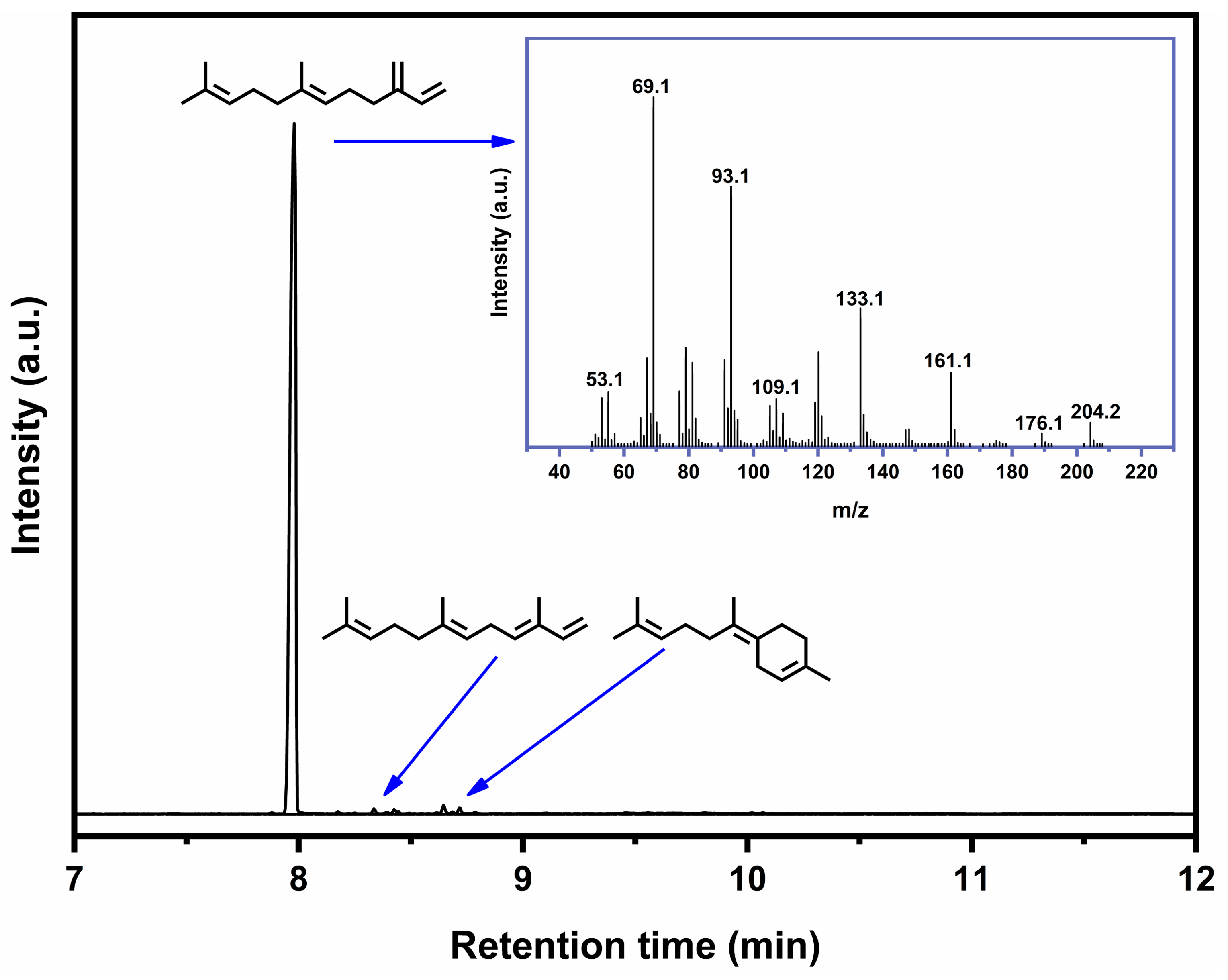
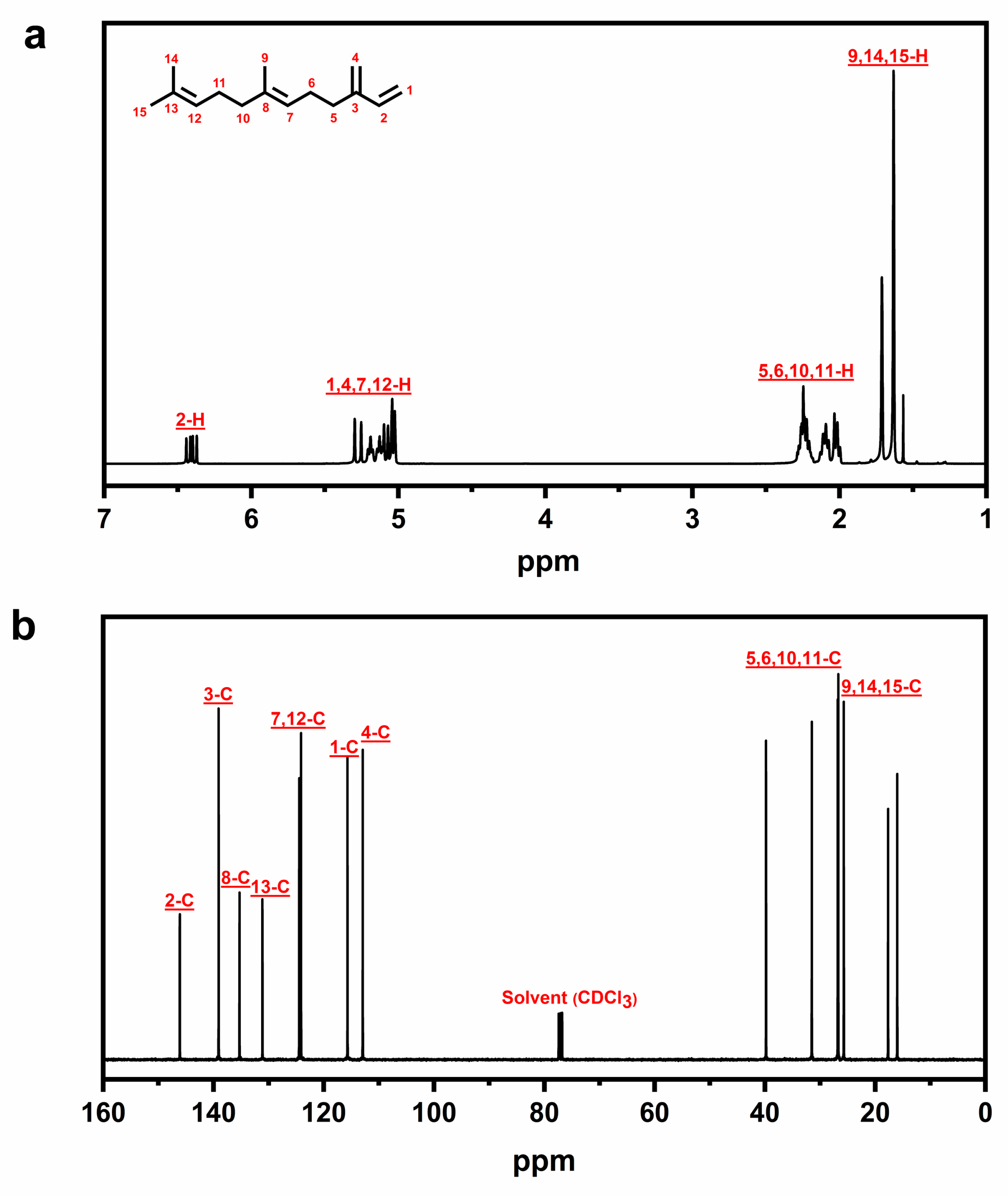
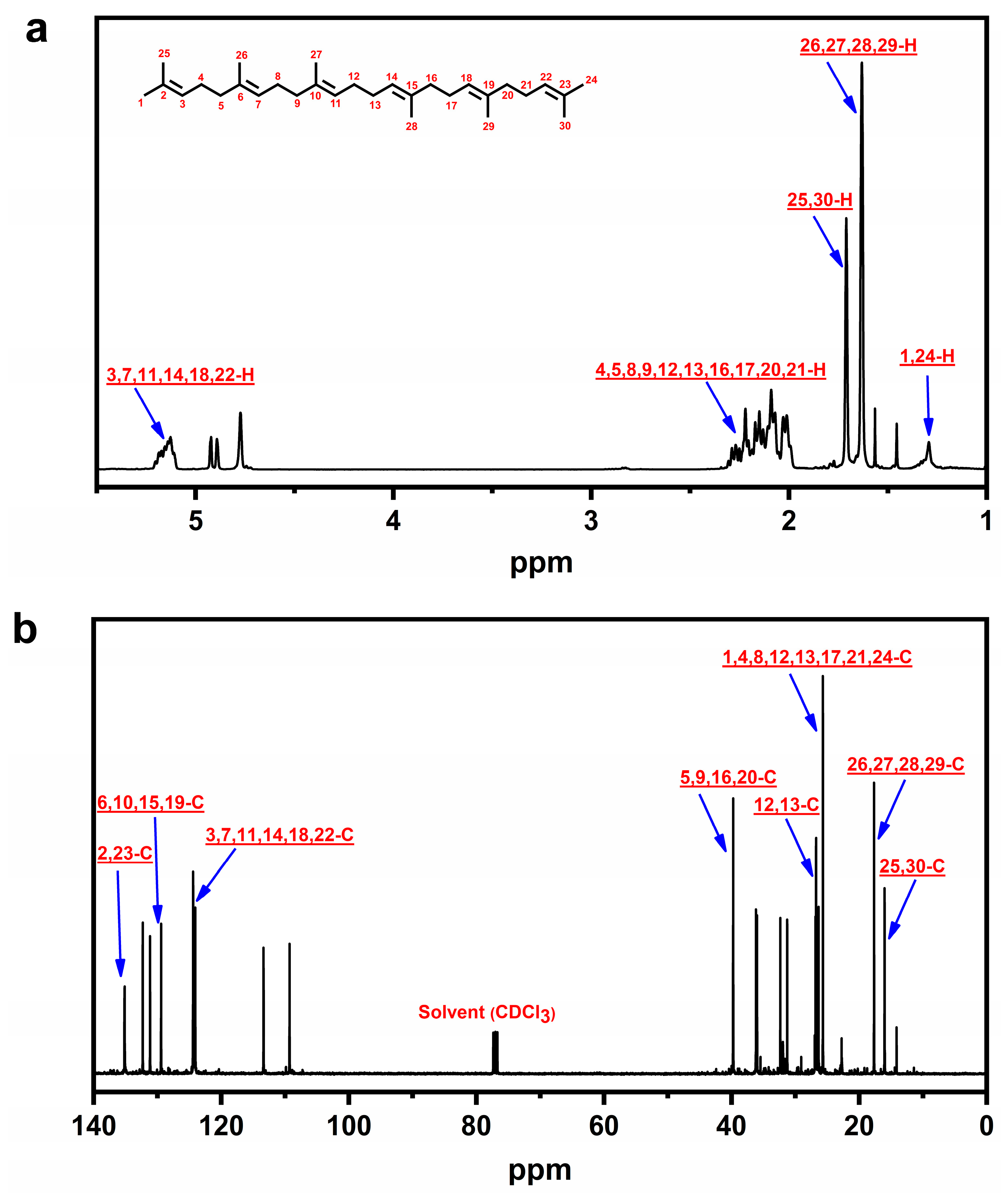
2.2. Characterization and Quantification of β-Farnesene
2.3. Palladium-Catalyzed Reductive Coupling of β-Farnesene
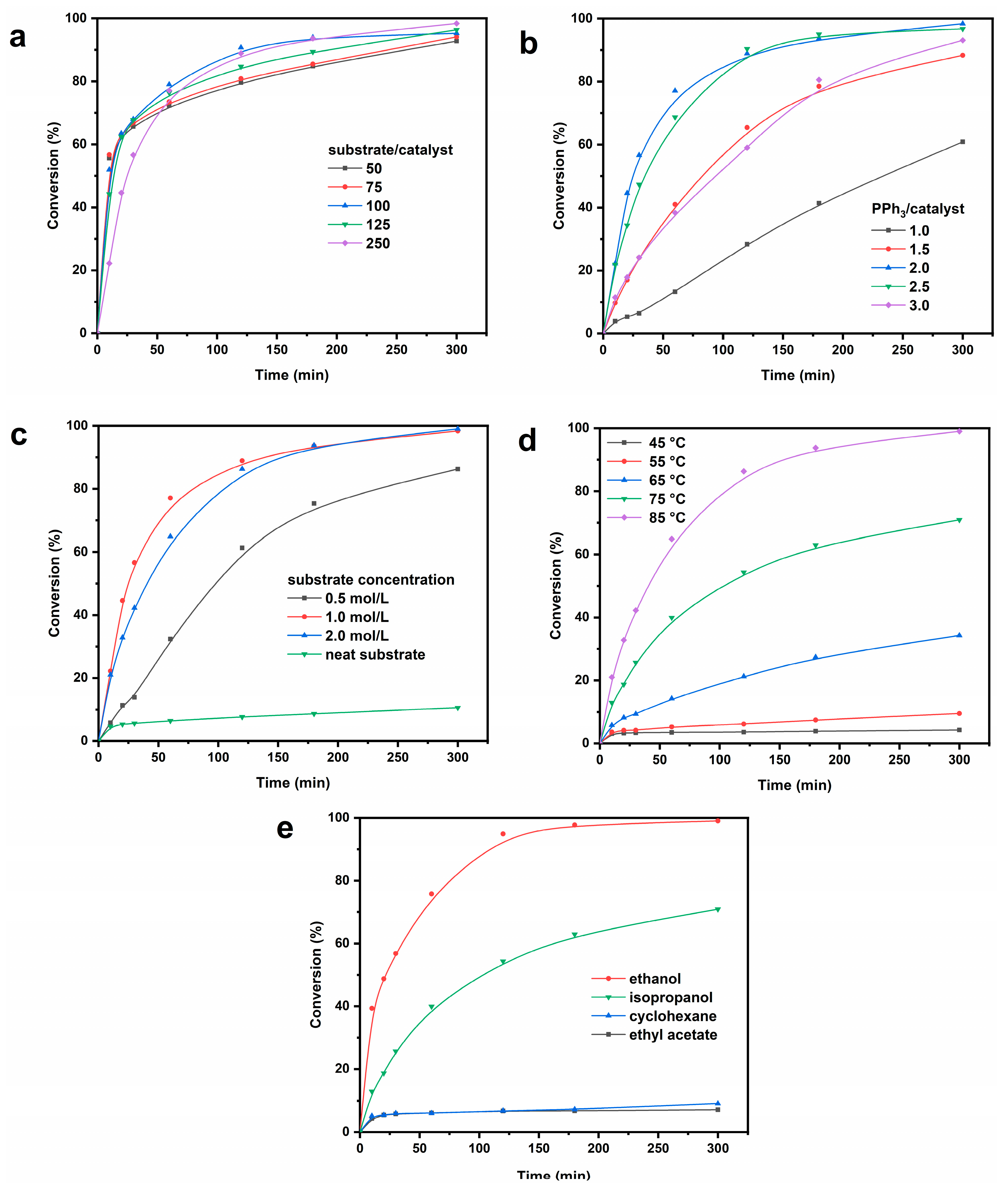
2.3.1. Influence of Catalyst Content on Catalytic Activity
2.3.2. Influence of PPh3 Amount on Catalytic Activity
2.3.3. Influence of Substance Concentration on Catalytic Activity
2.3.4. Influence of Reaction Temperature on Catalytic Activity
2.3.5. Influence of Reaction Solvent on Catalytic Activity
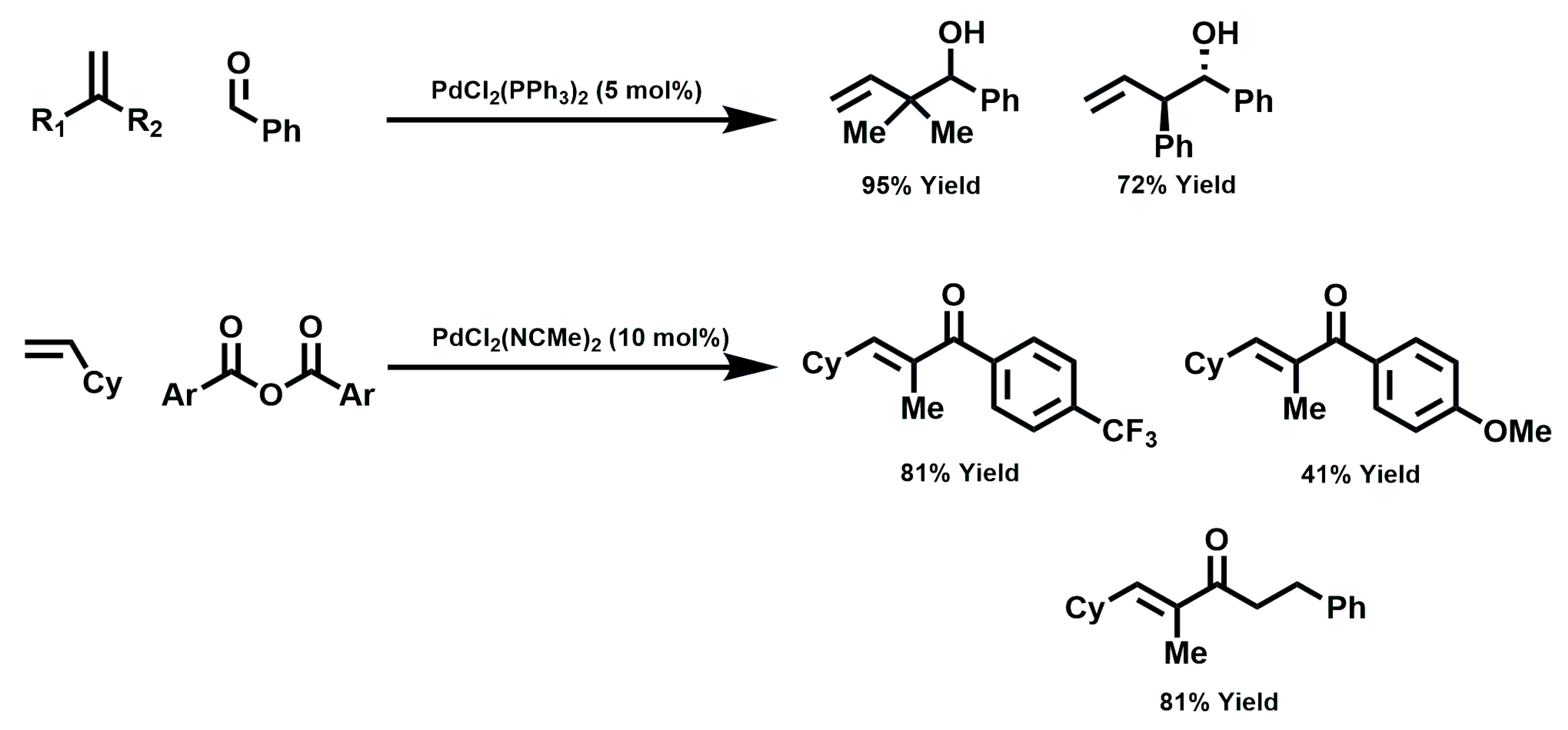
2.4. Reaction Mechanism for Reductive Coupling of β-Farnesene
2.5. Separation and Purification of the Coupling Reaction Products
3. Experimental Section
3.1. Materials and Chemicals
3.2. Product Analysis
3.3. Fed-Batch Fermentation in a 2.5 L Bioreactor
3.4. Separation and Purification Steps of β-Farnesene from the Biphasic Medium
3.5. Palladium-Catalyzed Reductive Coupling of β-Farnesene
3.6. Column Purification of the Coupling Reaction Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Albers, S.C.; Berklund, A.M.; Graff, G.D. The rise and fall of innovation in biofuels. Nat. Biotechnol. 2016, 34, 814–821. [Google Scholar] [CrossRef]
- Ripa, M.; Cadillo-Benalcazar, J.J.; Giampietro, M. Cutting through the biofuel confusion: A conceptual framework to check the feasibility, viability and desirability of biofuels. Energy Strategy Rev. 2021, 35, 100642. [Google Scholar] [CrossRef]
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Kumaniaev, I.; Navare, K.; Crespo Mendes, N.; Placet, V.; Van Acker, K.; Samec, J.S.M. Conversion of birch bark to biofuels. Green Chem. 2020, 22, 2255–2263. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Bryant, N.D.; Pu, Y.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Kalluri, U.C.; Yoo, C.G.; Ragauskas, A.J. Transgenic Poplar Designed for Biofuels. Trends Plant Sci. 2020, 25, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Stafford, W.; De Lange, W.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Bang, J.; Ahn, J.H.; Lee, J.A.; Hwang, C.H.; Kim, G.B.; Lee, J.; Lee, S.Y. Synthetic Formatotrophs for One-Carbon Biorefinery. Adv. Sci. 2021, 8, 2100199. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R. A Tandem for Lignin-First Biorefinery. Joule 2017, 1, 427–428. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Haus, M.; Ding, Y.; Papadokonstantakis, S.; Mondelli, C.; Pérez-Ramírez, J. Environmental and economical perspectives of a glycerol biorefinery. Energy Environ. Sci. 2018, 11, 1012–1029. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological potential of Phaeodactylum tricornutum for biorefinery processes. Fuel 2020, 268, 117357. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335–4382. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Chua, N.K.; Yan, N. The shape of human squalene epoxidase expands the arsenal against cancer. Nat. Commun. 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Pollier, J.; Vancaester, E.; Kuzhiumparambil, U.; Vickers, C.E.; Vandepoele, K.; Goossens, A.; Fabris, M. A widespread alternative squalene epoxidase participates in eukaryote steroid biosynthesis. Nat. Microbiol. 2019, 4, 226–233. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Cui, Z.; Qi, Q.; Hou, J. Progress and perspectives for microbial production of farnesene. Bioresour. Technol. 2022, 347, 126682. [Google Scholar] [CrossRef]
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Strong, P.J.; Xie, S.; Clarke, W.P. Methane as a resource: Can the methanotrophs add value? Environ. Sci. Technol. 2015, 49, 4001–4018. [Google Scholar] [CrossRef]
- Orzolek, B.J.; Rahman, M.A.; Iovine, P.M. Synthesis of Biorenewable Starch–Farnesene Amphiphilic Conjugates via Transesterification of Terpene-Derived Diels–Alder Adducts. ACS Sustain. Chem. Eng. 2018, 6, 13562–13569. [Google Scholar] [CrossRef]
- Ding, J.; You, S.; Ba, W.; Zhang, H.; Chang, H.; Qi, W.; Su, R.; He, Z. Bifunctional utilization of whey powder as a substrate and inducer for beta-farnesene production in an engineered Escherichia coli. Bioresour. Technol. 2021, 341, 125739. [Google Scholar] [CrossRef]
- Wang, J.; Chen, N.; Bian, G.; Mu, X.; Du, N.; Wang, W.; Ma, C.G.; Fu, S.; Huang, B.; Liu, T.; et al. Solar-Driven Overproduction of Biofuels in Microorganisms. Angew. Chem. Int. Ed. 2022, 61, e202207132. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, Y.; Zhang, C.; You, S.; Qi, W.; Su, R.; He, Z. Highly efficient production of FAMEs and β-farnesene from a two-stage biotransformation of waste cooking oils. Energy Convers. Manag. 2019, 199, 112001. [Google Scholar] [CrossRef]
- Tippmann, S.; Nielsen, J.; Khoomrung, S. Improved quantification of farnesene during microbial production from Saccharomyces cerevisiae in two-liquid-phase fermentations. Talanta 2016, 146, 100–106. [Google Scholar] [CrossRef]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlen, M.; Nielsen, J.; Hudson, E.P. Affibody Scaffolds Improve Sesquiterpene Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef]
- Han, J.Y.; Song, J.M.; Seo, S.H.; Wang, C.; Lee, S.G.; Lee, H.; Kim, S.W.; Choi, E.S. Ty1-fused protein-body formation for spatial organization of metabolic pathways in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2018, 115, 694–704. [Google Scholar] [CrossRef]
- Liu, C.-L.; Xue, K.; Yang, Y.; Liu, X.; Li, Y.; Lee, T.S.; Bai, Z.; Tan, T. Metabolic engineering strategies for sesquiterpene production in microorganism. Crit. Rev. Biotechnol. 2022, 42, 73–92. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Oliveira, A.L.S.; Carsanba, E.; Pintado, M.; Oliveira, C. Phenolic compounds modulation in β-farnesene fed-batch fermentation using sugarcane syrup as feedstock. Ind. Crops Prod. 2022, 188, 115721. [Google Scholar] [CrossRef]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.M.; Ayson, M.; Moss, N.; Lieu, B.; Jackson, P.; Gaucher, S.P.; Horning, T.; Dahl, R.H.; Denery, J.R.; Abbott, D.A.; et al. Use of pantothenate as a metabolic switch increases the genetic stability of farnesene producing Saccharomyces cerevisiae. Metab. Eng. 2014, 25, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Narbonne, H.; Wang, C.; Zhou, S.; Abbatt, J.P.D.; Faust, J. Heterogeneous Chlorination of Squalene and Oleic Acid. Environ. Sci. Technol. 2019, 53, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.K.; Coates, H.W.; Brown, A.J. Squalene monooxygenase: A journey to the heart of cholesterol synthesis. Prog. Lipid Res. 2020, 79, 101033. [Google Scholar] [CrossRef]
- Park, J.; Kang, D.H.; Woo, H.M. Microbial Bioprocess for Extracellular Squalene Production and Formulation of Nanoemulsions. ACS Sustain. Chem. Eng. 2021, 9, 14263–14276. [Google Scholar] [CrossRef]
- Kimura, K.; Shiraishi, K.; Kondo, T.; Nakamura, J.; Fujitani, T. Cracking of squalene into isoprene as chemical utilization of algae oil. Green Chem. 2020, 22, 3083–3087. [Google Scholar] [CrossRef]
- Cheng, P.; Okada, S.; Zhou, C.; Chen, P.; Huo, S.; Li, K.; Addy, M.; Yan, X.; Ruan, R.R. High-value chemicals from Botryococcus braunii and their current applications—A review. Bioresour. Technol. 2019, 291, 121911. [Google Scholar] [CrossRef]
- Thapa, H.R.; Naik, M.T.; Okada, S.; Takada, K.; Molnar, I.; Xu, Y.; Devarenne, T.P. A squalene synthase-like enzyme initiates production of tetraterpenoid hydrocarbons in Botryococcus braunii Race L. Nat. Commun. 2016, 7, 11198. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Liu, L.; Ma, X.; Li, W.; Sun, X.; Wang, Y. Improving squalene production by enhancing the NADPH/NADP+ ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol. Biofuels 2019, 12, 68. [Google Scholar] [CrossRef]
- Patel, A.; Bettiga, M.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microbial genetic engineering approach to replace shark livering for squalene. Trends Biotechnol. 2022, 40, 1261–1273. [Google Scholar] [CrossRef]
- Moula Ali, A.M.; Prodpran, T.; Benjakul, S. Effect of squalene rich fraction from shark liver on mechanical, barrier and thermal properties of fish (Probarbus Jullieni) skin gelatin film. Food Hydrocoll. 2019, 96, 123–133. [Google Scholar] [CrossRef]
- Tenllado, D.; Reglero, G.; Torres, C.F. A combined procedure of supercritical fluid extraction and molecular distillation for the purification of alkylglycerols from shark liver oil. Sep. Purif. Technol. 2011, 83, 74–81. [Google Scholar] [CrossRef]
- Fisher, K.J.; Kinsey, R.; Mohamath, R.; Phan, T.; Liang, H.; Orr, M.T.; Lykins, W.R.; Guderian, J.A.; Bakken, J.; Argilla, D.; et al. Semi-synthetic terpenoids with differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions. NPJ Vaccines 2023, 8, 14. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Huang, R.; Huang, H. Palladium-Catalyzed Allyl–Allyl Reductive Coupling of Allylamines or Allylic Alcohols with H2 as Sole Reductant. Org. Lett. 2021, 23, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.; Schwartz, L.A.; Krische, M.J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-Q.; Li, H.; Zhou, A.; Yang, W.-R.; Yin, Q. Palladium-Catalyzed Chemo- and Regioselective C–H Bond Functionalization of Phenols with 1,3-Dienes. J. Org. Chem. 2023, 88, 2599–2604. [Google Scholar] [CrossRef]
- Zargari, N.; de Prevoisin, G.; Kim, Y.; Kaneshiro, K.; Runburg, R.; Park, J.; LaCroix, K.; Narain, R.; Lee, B.D.; Lee, J.H.; et al. Hydroalkenylation: Palladium catalyzed co-dimerization of unactivated alkenes. Tetrahedron Lett. 2016, 57, 815–818. [Google Scholar] [CrossRef]
- Cai, G.; Ding, M.; Wu, Q.; Jiang, H.L. Encapsulating soluble active species into hollow crystalline porous capsules beyond integration of homogeneous and heterogeneous catalysis. Natl. Sci. Rev. 2020, 7, 37–45. [Google Scholar] [CrossRef]
- Lang, R.; Li, T.; Matsumura, D.; Miao, S.; Ren, Y.; Cui, Y.T.; Tan, Y.; Qiao, B.; Li, L.; Wang, A.; et al. Hydroformylation of Olefins by a Rhodium Single-Atom Catalyst with Activity Comparable to RhCl(PPh(3))(3). Angew. Chem. Int. Ed. 2016, 55, 16054–16058. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Duchesne, P.N.; Zhang, P.; Dai, Y.; Yang, H.; Wu, B.; Liu, S.; Xu, C.; Zheng, N. A nanoparticulate polyacetylene-supported Pd(II) catalyst combining the advantages of homogeneous and heterogeneous catalysts. Chin. J. Catal. 2015, 36, 1560–1572. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Tang, M.; Zhao, J.-J.; Song, C.; Yu, S. Enantioselective Reductive Homocoupling of Allylic Acetates Enabled by Dual Photoredox/Palladium Catalysis: Access to C2-Symmetrical 1,5-Dienes. J. Am. Chem. Soc. 2021, 143, 12836–12846. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tian, Y.; Ding, Y.; Li, Z. The use of fermentation liquid of wastewater primary sedimentation sludge as supplemental carbon source for denitrification based on enhanced anaerobic fermentation. Bioresour. Technol. 2016, 219, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Torres, C.I.; Rittmann, B.E. Changes in Glucose Fermentation Pathways as a Response to the Free Ammonia Concentration in Microbial Electrolysis Cells. Environ. Sci. Technol. 2017, 51, 13461–13470. [Google Scholar] [CrossRef] [PubMed]
- Darshi, M.; Tumova, J.; Saliba, A.; Kim, J.; Baek, J.; Pennathur, S.; Sharma, K. Crabtree effect in kidney proximal tubule cells via late-stage glycolytic intermediates. iScience 2023, 26, 106462. [Google Scholar] [CrossRef]
- Malina, C.; Yu, R.; Bjorkeroth, J.; Kerkhoven, E.J.; Nielsen, J. Adaptations in metabolism and protein translation give rise to the Crabtree effect in yeast. Proc. Natl. Acad. Sci. USA 2021, 118, e2112836118. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Xu, X.; Zhang, H.; Wu, L.; Zhu, P.; Li, S.; Xu, H. Two-step economical welan gum production by Sphingomonas sp. HT-1 from sugar industrial by-products. Carbohydr. Polym. 2018, 181, 412–418. [Google Scholar] [CrossRef]
- He, F.; Qin, S.; Yang, Z.; Bai, X.; Suo, Y.; Wang, J. Butyric acid production from spent coffee grounds by engineered Clostridium tyrobutyricum overexpressing galactose catabolism genes. Bioresour. Technol. 2020, 304, 122977. [Google Scholar] [CrossRef]
- Xiberras, J.; Klein, M.; Nevoigt, E. Glycerol as a substrate for Saccharomyces cerevisiae based bioprocesses—Knowledge gaps regarding the central carbon catabolism of this ‘non-fermentable’ carbon source. Biotechnol. Adv. 2019, 37, 107378. [Google Scholar] [CrossRef]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Carbon catabolite repression occurrence in photo fermentation of ethanol-rich substrates. J. Environ. Manag. 2021, 297, 113371. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Cheng, Z.; Li, Y.; Tang, J. Effects of additional fermented food wastes on nitrogen removal enhancement and sludge characteristics in a sequential batch reactor for wastewater treatment. Environ. Sci. Pollut. Res. Int. 2016, 23, 12890–12899. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Webb, M.E.; Smith, A.G.; Abell, C. Biosynthesis of pantothenate. Nat. Prod. Rep. 2004, 21, 695–721. [Google Scholar] [CrossRef] [PubMed]
- Szafranek, B.; Chrapkowska, K.; Pawińska, M.; Szafranek, J. Analysis of Leaf Surface Sesquiterpenes in Potato Varieties. J. Agric. Food Chem. 2005, 53, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Svatoš, A.; Attygalle, A.B. Characterization of Vinyl-Substituted, Carbon-Carbon Double Bonds by GC/FT-IR Analysis. Anal. Chem. 1997, 69, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ren, Z.; Jiang, L.; Zhang, J.; Wang, H.; Zhang, G. Raman and FTIR spectroscopic studies on two hydroxylated tung oils (HTO) bearing conjugated double bonds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 199, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kato, K.; Tsunoda, K.-i.; Maeda, T. Metrological effectiveness of an analytical method for volatile organic compounds standard materials using post-column reaction GC/FID system. Anal. Chim. Acta 2008, 619, 26–29. [Google Scholar] [CrossRef]
- Niculescu, R.; Năstase, M.; Clenci, A. On the determination of the distillation curve of fatty acid methyl esters by gas chromatography. Fuel 2022, 314, 123143. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.; Zhang, S.; Xiao, S.; Zhan, C.; Yang, M.; Lu, X.; You, W. Distinction between PTB7-Th samples prepared from Pd(PPh3)4 and Pd2(dba)3/P(o-tol)3 catalysed stille coupling polymerization and the resultant photovoltaic performance. J. Mater. Chem. A 2018, 6, 179–188. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, K.; Zhuang, Z.; Qian, S.; Yu, J.-Q. From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C–H Functionalization Reactions. Acc. Chem. Res. 2020, 53, 833–851. [Google Scholar] [CrossRef]
- Petters, L.; Burger, S.; Kronawitter, S.; Drees, M.; Kieslich, G. Linear negative thermal expansion in Pd(acac)2. CrystEngComm 2021, 23, 5425–5429. [Google Scholar] [CrossRef]
- Janusson, E.; Zijlstra, H.S.; Nguyen, P.P.T.; MacGillivray, L.; Martelino, J.; McIndoe, J.S. Real-time analysis of Pd2(dba)3 activation by phosphine ligands. Chem. Commun. 2017, 53, 854–856. [Google Scholar] [CrossRef]
- Mikhel, I.S.; Gavrilov, K.N.; Polosukhin, A.I.; Rebrov, A.I. Reactions of chiral phosphoramidites with complexes Pd(COD)Cl2 and Pt(COD)Cl2. Russ. Chem. Bull. 1998, 47, 1585–1588. [Google Scholar] [CrossRef]
- Cong, M.; Fan, Y.; Raimundo, J.-M.; Tang, J.; Peng, L. Pd(dba)2 vs Pd2(dba)3: An in-Depth Comparison of Catalytic Reactivity and Mechanism via Mixed-Ligand Promoted C–N and C–S Coupling Reactions. Org. Lett. 2014, 16, 4074–4077. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Bora, U. An efficient protocol for palladium-catalyzed ligand-free Suzuki–Miyaura coupling in water. Green Chem. 2012, 14, 1873–1876. [Google Scholar] [CrossRef]
- Chen, P.-P.; Wipf, P.; Houk, K.N. How mono- and diphosphine ligands alter regioselectivity of the Rh-catalyzed annulative cleavage of bicyclo[1.1.0]butanes. Nat. Commun. 2022, 13, 7292. [Google Scholar] [CrossRef]
- Krachko, T.; Bispinghoff, M.; Tondreau, A.M.; Stein, D.; Baker, M.; Ehlers, A.W.; Slootweg, J.C.; Grützmacher, H. Facile Phenylphosphinidene Transfer Reactions from Carbene–Phosphinidene Zinc Complexes. Angew. Chem. Int. Ed. 2017, 56, 7948–7951. [Google Scholar] [CrossRef] [PubMed]
- Swansborough-Aston, W.A.; Soltan, A.; Coulson, B.; Pratt, A.; Chechik, V.; Douthwaite, R.E. Efficient photoelectrochemical Kolbe C–C coupling at BiVO4 electrodes under visible light irradiation. Green Chem. 2023, 25, 1067–1077. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. 2-Methyltetrahydrofuran: A Green Solvent for Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2018, 11, 1290–1294. [Google Scholar] [CrossRef]
- Camp, J.E.; Dunsford, J.J.; Cannons, E.P.; Restorick, W.J.; Gadzhieva, A.; Fay, M.W.; Smith, R.J. Glucose-Derived Palladium(0) Nanoparticles as in Situ-Formed Catalysts for Suzuki–Miyaura Cross-Coupling Reactions in Isopropanol. ACS Sustain. Chem. Eng. 2014, 2, 500–505. [Google Scholar] [CrossRef]
- Corulli, C.J.; Groover, E.A.; Attelah, J.D.; Miller, C.B.; Penland, B.B. Rational design of peptides for biomimetic palladium nanoparticle catalysis with Suzuki and Heck coupling in ethanol. Colloid Interface Sci. Commun. 2023, 54, 100708. [Google Scholar] [CrossRef]
- Datta, A.; Ebert, K.; Plenio, H. Nanofiltration for Homogeneous Catalysis Separation: Soluble Polymer-Supported Palladium Catalysts for Heck, Sonogashira, and Suzuki Coupling of Aryl Halides. Organometallics 2003, 22, 4685–4691. [Google Scholar] [CrossRef]
- Ricordi, V.G.; Freitas, C.S.; Perin, G.; Lenardão, E.J.; Jacob, R.G.; Savegnago, L.; Alves, D. Glycerol as a recyclable solvent for copper-catalyzed cross-coupling reactions of diaryl diselenides with aryl boronic acids. Green Chem. 2012, 14, 1030–1034. [Google Scholar] [CrossRef]
- Chang, H.-M.; Cheng, C.-H. Highly Regioselective and Stereoselective Allylation of Aldehydes via Palladium-Catalyzed in Situ Hydrostannylation of Allenes. Org. Lett. 2000, 2, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, T.; Tatsumi, K.; Terao, J.; Tsuji, Y. Palladium-Catalyzed Formal Hydroacylation of Allenes Employing Acid Chlorides and Hydrosilanes. Org. Lett. 2013, 15, 2286–2289. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-W.; Li, R.; Li, J.-F.; Sun, J.; Ye, M. NHC ligand-enabled Ni-catalyzed reductive coupling of alkynes and imines using isopropanol as a reductant. Green Chem. 2019, 21, 2240–2244. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Li, W.-D.; Li, Y.-L.; Cui, K.; Xia, J.-B. Selective Reductive Coupling of Vinyl Azaarenes and Alkynes via Photoredox Cobalt Dual Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202213281. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes-Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manasa, V.; Tumaney, A.W.; Bettadaiah, K.B.; Chaudhari, S.R.; Giridhar, P. Chemical composition, nutraceuticals characterization, NMR confirmation of squalene and antioxidant activities of Basella rubra L. seed oil. RSC Adv. 2020, 10, 31863–31873. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Westfall, P.; Main, A. Methods of developing terpene synthase variants. KR Patent KR20130110226A, 1 February 2012. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Tian, K.; Guo, X.; Fang, Y. Integrating Fermentation Engineering and Organopalladium Chemocatalysis for the Production of Squalene from Biomass-Derived Carbohydrates as the Starting Material. Catalysts 2023, 13, 1392. https://doi.org/10.3390/catal13111392
Wu C, Tian K, Guo X, Fang Y. Integrating Fermentation Engineering and Organopalladium Chemocatalysis for the Production of Squalene from Biomass-Derived Carbohydrates as the Starting Material. Catalysts. 2023; 13(11):1392. https://doi.org/10.3390/catal13111392
Chicago/Turabian StyleWu, Cuicui, Kaifei Tian, Xuan Guo, and Yunming Fang. 2023. "Integrating Fermentation Engineering and Organopalladium Chemocatalysis for the Production of Squalene from Biomass-Derived Carbohydrates as the Starting Material" Catalysts 13, no. 11: 1392. https://doi.org/10.3390/catal13111392