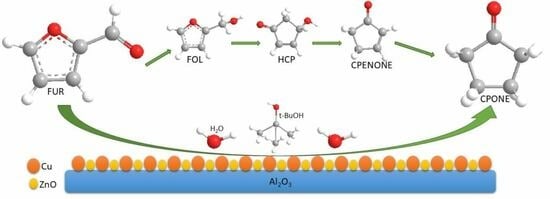
Hydrogenation of Furfural to Cyclopentanone in Tert–Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst
Abstract
:1. Introduction
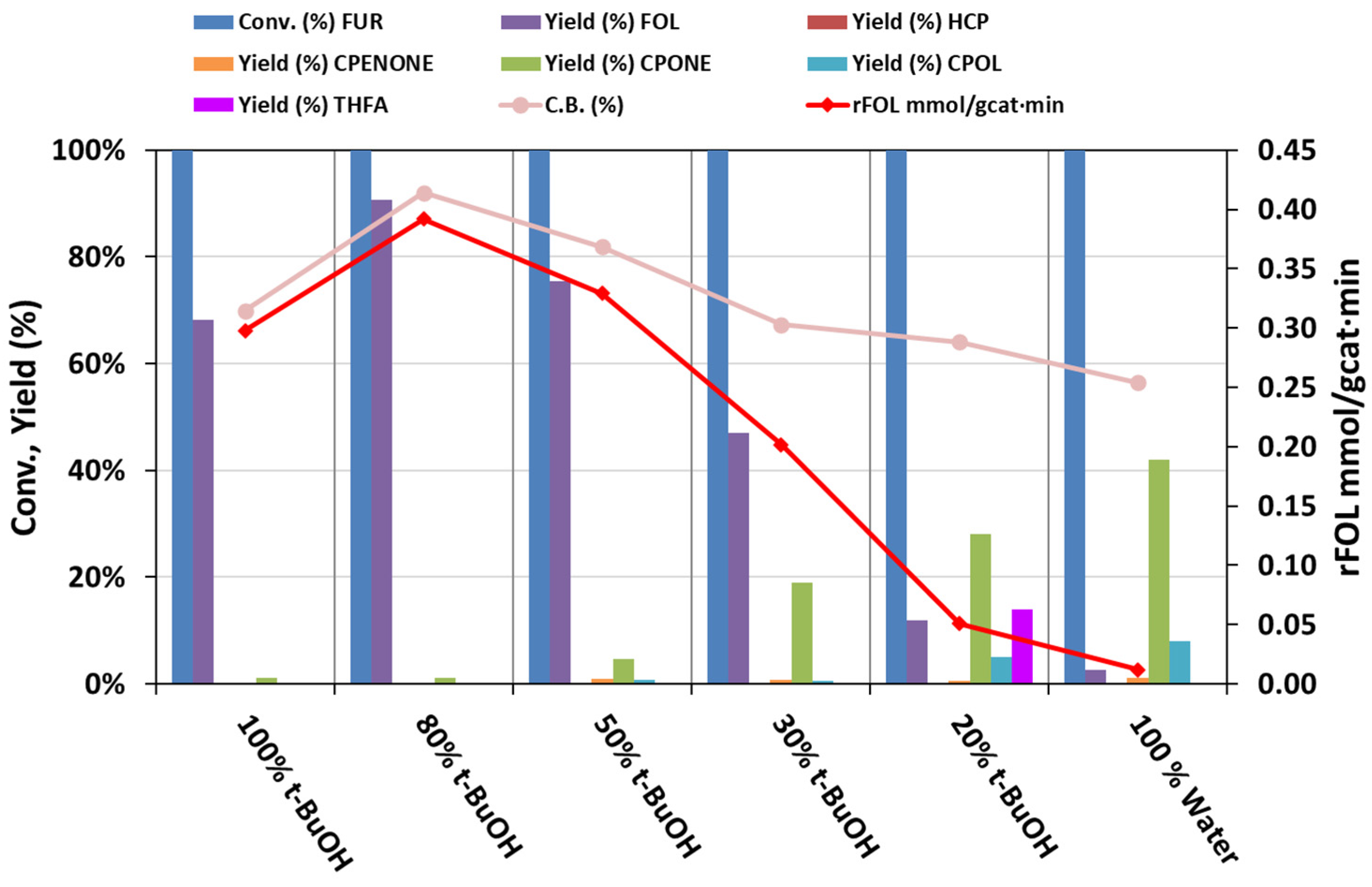
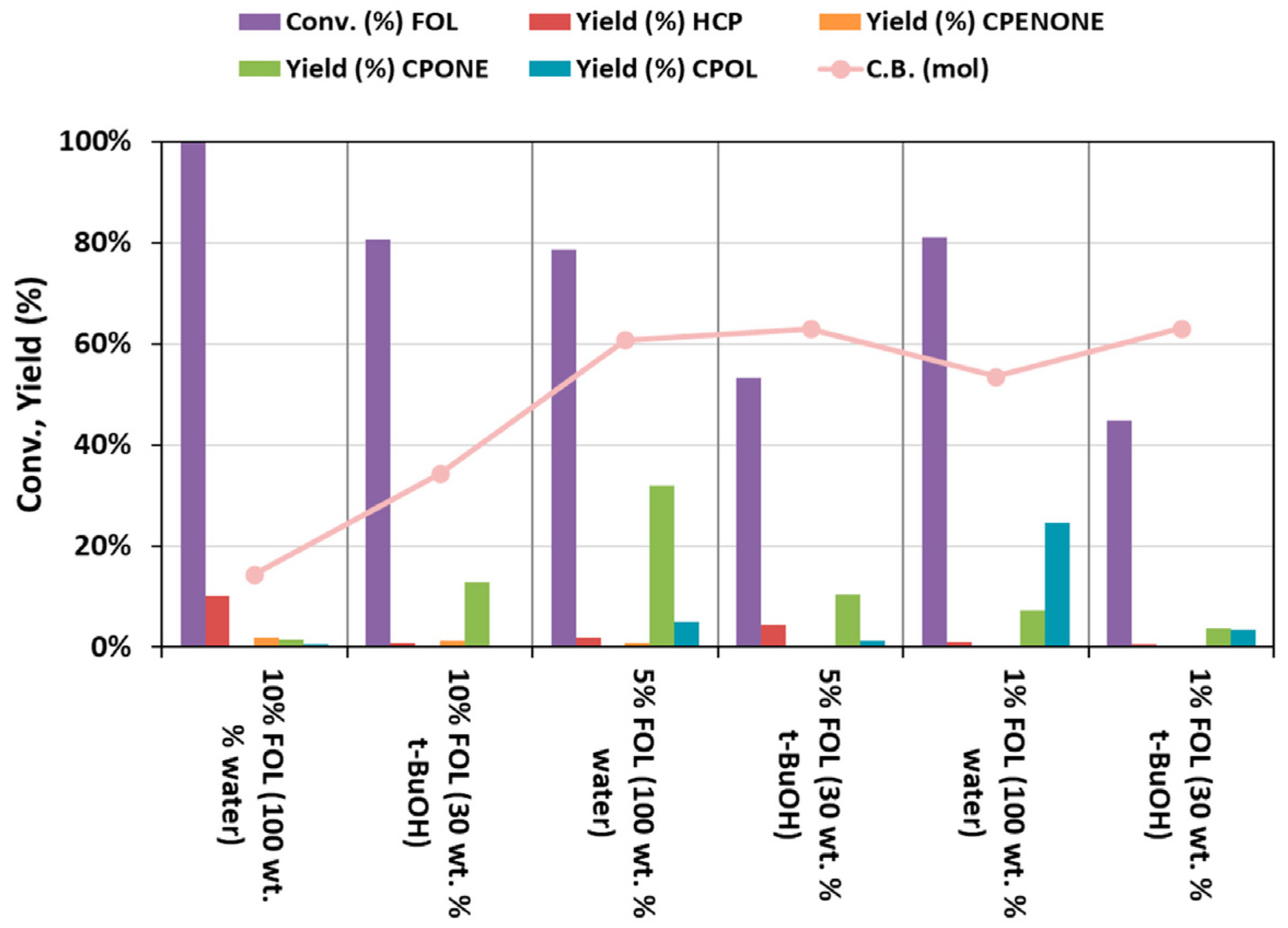
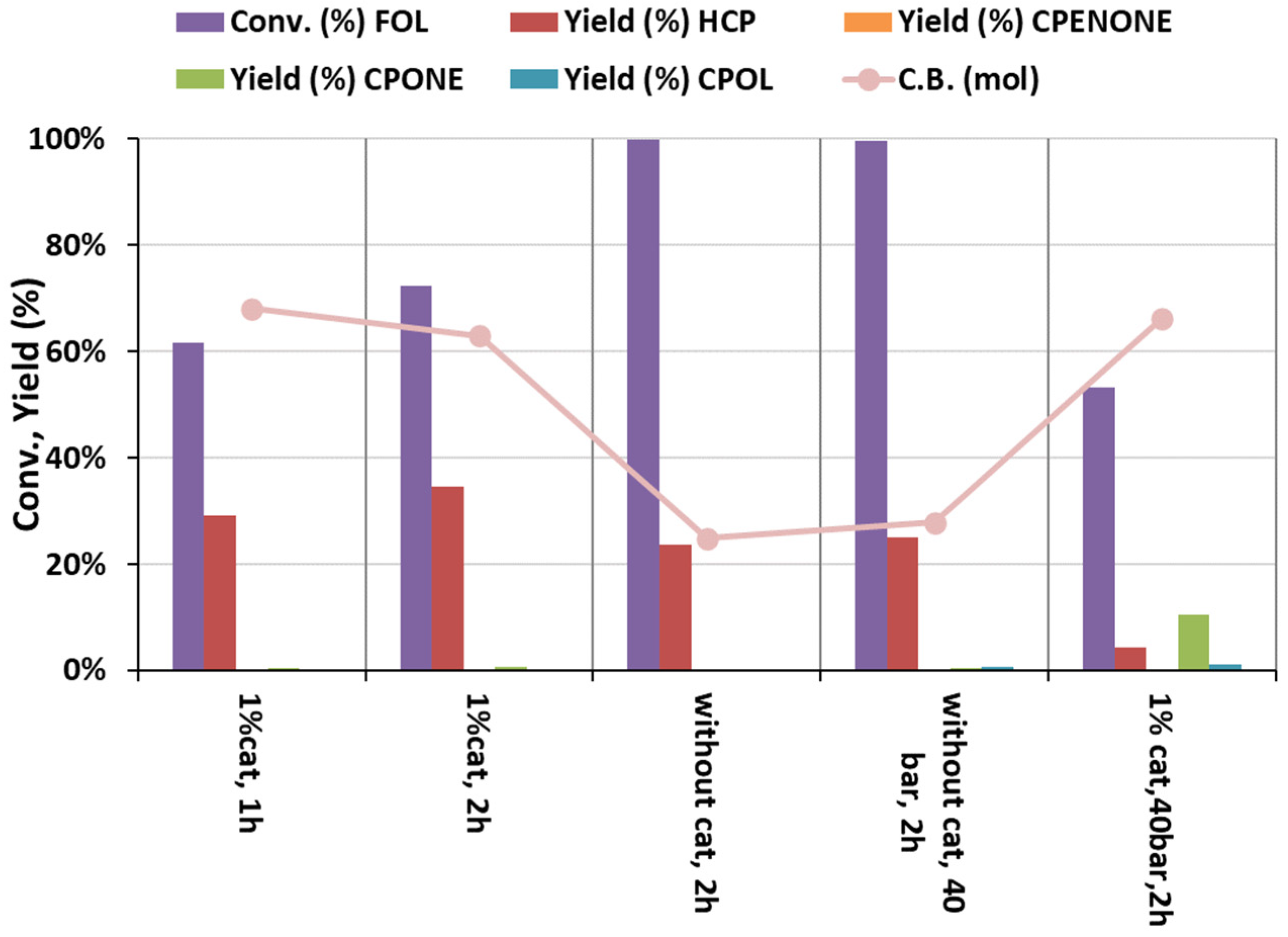
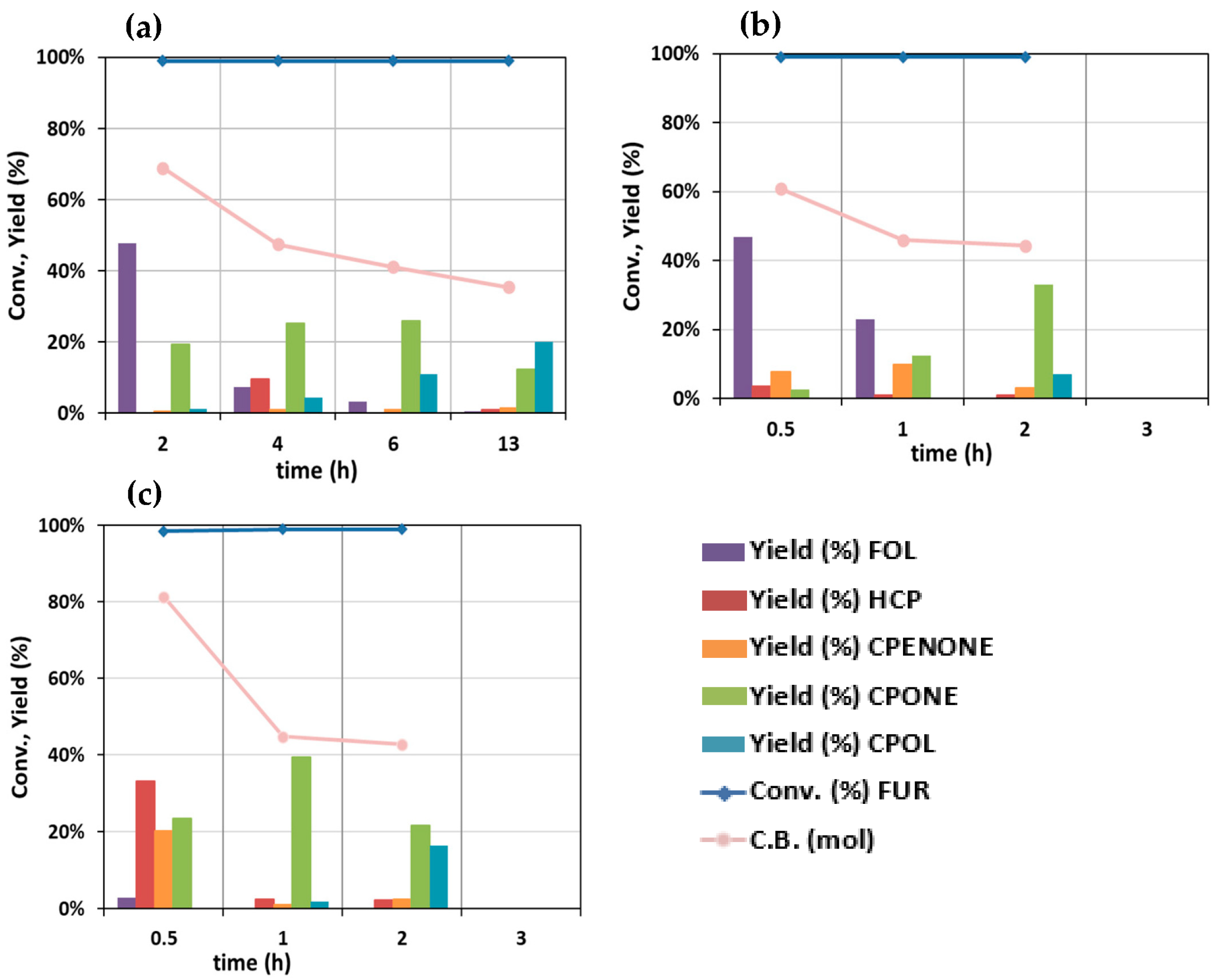
2. Results and Discussion
Catalytic Activity
3. Materials and Methods
3.1. Reagent and Materials
3.2. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, T.W.; Motagamwala, A.H.; Dumesic, J.A.; Huber, G.W. Fundamental Catalytic Challenges to Design Improved Biomass Conversion Technologies. J. Catal. 2019, 369, 518–525. [Google Scholar] [CrossRef]
- Corma Canos, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, J.; Wu, D. Highly Efficient NiCu/SiO2 Catalyst Induced by Ni(Cu)-Silica Interaction for Aqueous-Phase Furfural Hydrogenation. Catal. Lett. 2022, 153, 1543–1555. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation Processes: Advances on Catalytic Transformations of Biomass-Derived Platform Chemicals into Hydrocarbon Fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Janulaitis, N.; Gulotty, R.J.; Kastner, J.R. Continuous Hydrogenation of Aqueous Furfural Using a Metal-Supported Activated Carbon Monolith. ACS Omega 2020, 5, 7836–7849. [Google Scholar] [CrossRef]
- Bertolini, G.R.; Jim, C.P.; Cecilia, J.A.; Maireles-torres, P. Gas-Phase Hydrogenation of Furfural to Furfuryl Alcohol over Cu-ZnO-Al2O3 Catalysts Prepared from Layered Double Hydroxides. Catalysts 2020, 10, 486. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Piancatelli, G.; Sceitm, A. The Synthetic Utility of a 2-Furfuryl Steroid. Tetrahedrom 1977, 33, 69–79. [Google Scholar] [CrossRef]
- López Granados, M.; Martín Alonso, D. Furfural an Entry Point of Lignocellulose in Biorefineries to Produce Renewable Chemicals, Polymers, and Biofuels. Chapter 3. Renewable Chemicals, Biofuels and Resins from Furfural; World Scientific Publishing Co.: Singapore, 2018; Volume 2, pp. 157–168. ISBN 9781786344878. [Google Scholar]
- Khzouz, M.; Wood, J.; Pollet, B.; Bujalski, W. Characterization and Activity Test of Commercial Ni/Al2O3, Cu/ZnO/Al2O3 and Prepared Ni-Cu/Al2O3 Catalysts for Hydrogen Production from Methane and Methanol Fuels. Int. J. Hydrogen Energy 2013, 38, 1664–1675. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Vasiliadou, E.S.; Lemonidou, A.A. Glycerol Hydro-Deoxygenation Aided by: In Situ H2 Generation via Methanol Aqueous Phase Reforming over a Cu-ZnO-Al2O3 Catalyst. Catal. Sci. Technol. 2016, 6, 5415–5426. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Rempel, G.L.; Ng, F.T.T. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with in Situ Hydrogen from Methanol Steam Reforming Using a Cu/Zno/Al2O3 Catalyst. Catalysts 2019, 9, 412. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, Z.; Zhang, W.; Sun, H.; Liu, S. Cu-Zn/Al2O3 Catalyst for the Hydrogenation of Esters to Alcohols. Cuihua Xuebao/Chin. J. Catal. 2010, 31, 769–775. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Liptaj, T. Effect of Catalyst and Solvent on the Furan Ring Rearrangement to Cyclopentanone. Appl. Catal. A Gen. 2012, 437–438, 104–111. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly Selective Hydrogenation of Furfural to Cyclopentanone over a NiFe Bimetallic Catalyst in a Methanol/Water Solution with a Solvent Effect. ACS Sustain. Chem. Eng. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
- Dohade, M.; Dhepe, P.L. Efficient Method for Cyclopentanone Synthesis from Furfural: Understanding the Role of Solvents and Solubility in a Bimetallic Catalytic System. Catal. Sci. Technol. 2018, 8, 5259–5269. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Lu, X.; Yin, J.; Wu, P. Comparison of Titanosilicates with Different Topologies as Liquid-Phase Oxidation Catalysts. Catal. Today 2018, 347, 48–55. [Google Scholar] [CrossRef]
- Kim, T.; Assary, R.S.; Marshall, C.L.; Gosztola, D.J.; Curtiss, L.A.; Stair, P.C. Acid-Catalyzed Furfuryl Alcohol Polymerization: Characterizations of Molecular Structure and Thermodynamic Properties. ChemCatChem 2011, 3, 1451–1458. [Google Scholar] [CrossRef]
- Li, D.; Tian, Z.; Cai, X.; Li, Z.; Zhang, C.; Zhang, W.; Song, Y.; Wang, H.; Li, C. Nature of Polymeric Condensates during Furfural to Cyclopentanone and cyclopentanol over Cu-Based Catalysts. New J. Chem. 2021, 45, 22767. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K.; Vávra, I.; Soták, T.; Dobročka, E.; Mičušík, M. Carbon Supported Pd-Cu Catalysts for Highly Selective Rearrangement of Furfural to Cyclopentanone. Appl. Catal. B 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Guo, T.; Guo, Q.; Li, S.; Hu, Y.; Yun, S.; Qian, Y. Effect of Surface Basicity over the Supported Cu-ZnO Catalysts on Hydrogenation of CO2 to Methanol. J. Catal. 2022, 407, 312–321. [Google Scholar] [CrossRef]
- Zhang, S. Selective Tandem Hydrogenation and Rearrangement of Furfural to Cyclopentanone over CuNi Bimetallic Catalyst in Water. Chin. J. Catal. 2021, 42, 2216–2224. [Google Scholar] [CrossRef]
- Balaga, R.; Balla, P.; Zhang, X.; Ramineni, K.; Du, H.; Lingalwar, S.; Perupogu, V.; Zhang, Z.C. Enhanced Cyclopentanone Yield from Furfural Hydrogenation: Promotional Effect of Surface Silanols on Ni-Cu/m-Silica Catalyst. Catalysts 2023, 13, 580. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, H.; Wang, G.; Zhao, H. In Situ Synthesis of Highly Dispersed Cu-Co Bimetallic Nanoparticles for Tandem Hydrogenation/Rearrangement of Bioderived Furfural in Aqueous-Phase. ACS Sustain. Chem. Eng. 2018, 6, 14919–14925. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Z.; Guo, W.; Liu, J.; Li, R.; Chen, R.; Huang, J. Hydrogenation and Hydrolysis of Furfural to Furfuryl Alcohol, Cyclopentanone, and Cyclopentanol with a Heterogeneous Copper Catalyst in Water. Ind. Eng. Chem. Res. 2019, 58, 3988–3993. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, C.; Yang, L.; Fan, G.; Li, F. Robust Structured Cu-Based Film Catalysts with Greatly Enhanced Catalytic Hydrogenation Property. Appl. Surf. Sci. 2020, 504, 144364. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Wang, K.; Xia, H.; Xu, J.; Jiang, J. Selective Conversion of Furfural to Cyclopentanone over CNT-Supported Cu Based Catalysts: Model Reaction for Upgrading of Bio-Oil. Fuel 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, G.; Yang, L.; Li, F. Efficient Conversion of Furfural into Cyclopentanone over High Performing and Stable Cu/ZrO2 Catalysts. Appl. Catal. A Gen. 2018, 561, 117–126. [Google Scholar] [CrossRef]
- Neto, R.M.; Lenzi, G.G.; Pimenta, J.L.C.W.; Fornari, A.C.; Dos Santos, O.A.A.; De Matos Jorge, L.M. Analysis of Carbon Monoxide Production in Methanol Steam Reforming Reactor for Generating Hydrogen. Acta Sci. Technol. 2019, 41, e39926. [Google Scholar] [CrossRef]



| Reactions Rate (mmol/gcat·min) | FUR | FOL | HCP | CPENONE | CPONE |
|---|---|---|---|---|---|
| rFUR | 0.48 (0.65) | ||||
| rFOL | 0.19 (0.54) | 0.23 (0.14) | |||
| rHCP | 0.10 (0.01) | 0.04 (0.01) | 0.77 (0.37) | ||
| rCPENONE | 0.12 (0.01) | 0.02 (0.02) | 0.00 (0.30) | 0.84 (0.75) | |
| rCPONE | 0.07 (0.09) | 0.16 (0.11) | 0.51 (0.07) | 0.77 (0.72) | 0.63 (0.15) |
| rCPOL | 0.00 (0.00) | 0.01 (0.00) | 0.26 (0.00) | 0.07 (0.03) | 0.63 (0.15) |
| Reactions Rate (mmol/gcat·min) | 3 wt.% | 2 wt.% | 1 wt.% |
|---|---|---|---|
| rFOL | 0.08 | 0.14 | 0.34 |
| rHCP | 0.04 | 0.07 | 0.17 |
| Entry | Catalyst | FUR (wt.%) | cat./FUR (wt.%) | P (MPa) | T (K) | Time (h) | XFUR (%) | YCPONE (%) | Prod. (gprod·h−1·gcat−1) | Prod. (gprod·s−1·molCu−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni5Cu15/mSiO2 | 3 | 34 | 3 | 413 | 4 | 100 | 90 | 58 | 7 | [24] |
| 2 | NiCu/SiO2-AE-450 | 4 | 13 | 2 | 423 | 6 | 100 | 95 | 106 | 7 | [4] |
| 3 | CuNi/Al-MCM-41 | 5 | 13 | 2 | 433 | 5 | 98 | 68 | 95 | 38 | [25] |
| 4 | CuCo0.8@C-500 | 1 | 35 | 0.5 | 423 | 9 | 100 | 90 | 25 | 2 | [26] |
| 5 | Cu0.4Mg5.6Al2 | 4 | 67 | 0.2 | 453 | 5 | 100 | 98 | 26 | 24 | [27] |
| 6 | CuZn/CNT | 5 | 40 | 4 | 413 | 10 | 95 | 85 | 19 | 2 | [28] |
| 7 | Cu-4-F | 1 | 34 | 2 | 413 | 2 | 100 | 87 | 110 | - | [29] |
| 8 | Cu/ZrO2-500 | 3 | 10 | 1.5 | 423 | 4 | 100 | 91 | 192 | 13 | [30] |
| 9 | Cu/Zn/Al2O3 | 5 | 20 | 6 | 473 | 0.5 | 100 | 47 | 411 | 14 | pw |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Saumell, A.; Mariscal, R.; Vila, F.; López Granados, M.; Alonso, D.M. Hydrogenation of Furfural to Cyclopentanone in Tert–Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst. Catalysts 2023, 13, 1394. https://doi.org/10.3390/catal13111394
Orozco-Saumell A, Mariscal R, Vila F, López Granados M, Alonso DM. Hydrogenation of Furfural to Cyclopentanone in Tert–Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst. Catalysts. 2023; 13(11):1394. https://doi.org/10.3390/catal13111394
Chicago/Turabian StyleOrozco-Saumell, Ana, Rafael Mariscal, Francisco Vila, Manuel López Granados, and David Martín Alonso. 2023. "Hydrogenation of Furfural to Cyclopentanone in Tert–Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst" Catalysts 13, no. 11: 1394. https://doi.org/10.3390/catal13111394