Catalytic Hydrodeoxygenation of Solar Energy Produced Bio-Oil in Supercritical Ethanol with Mo2C/CNF Catalysts: Effect of Mo Concentration
Abstract
:1. Introduction
2. Results
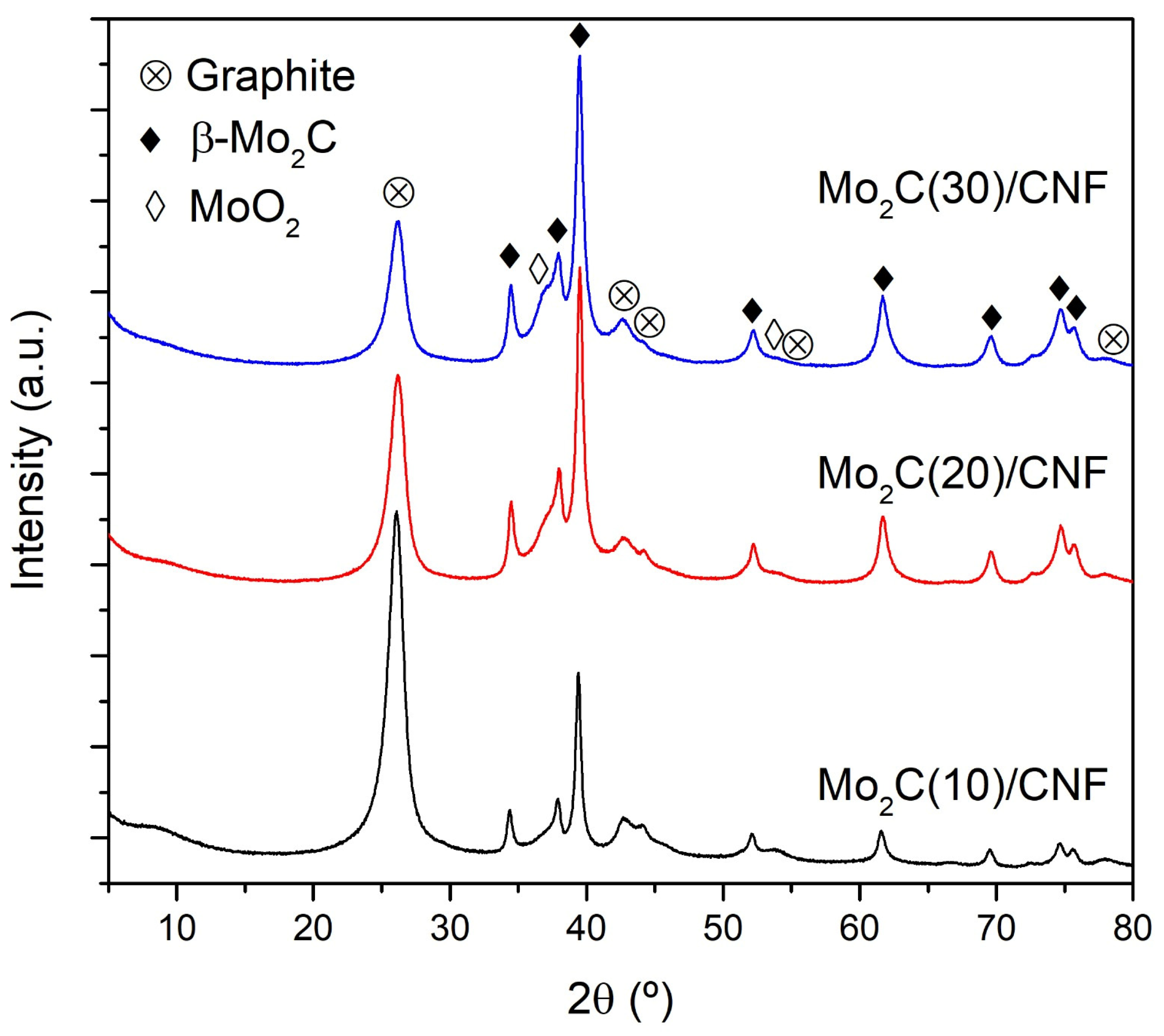
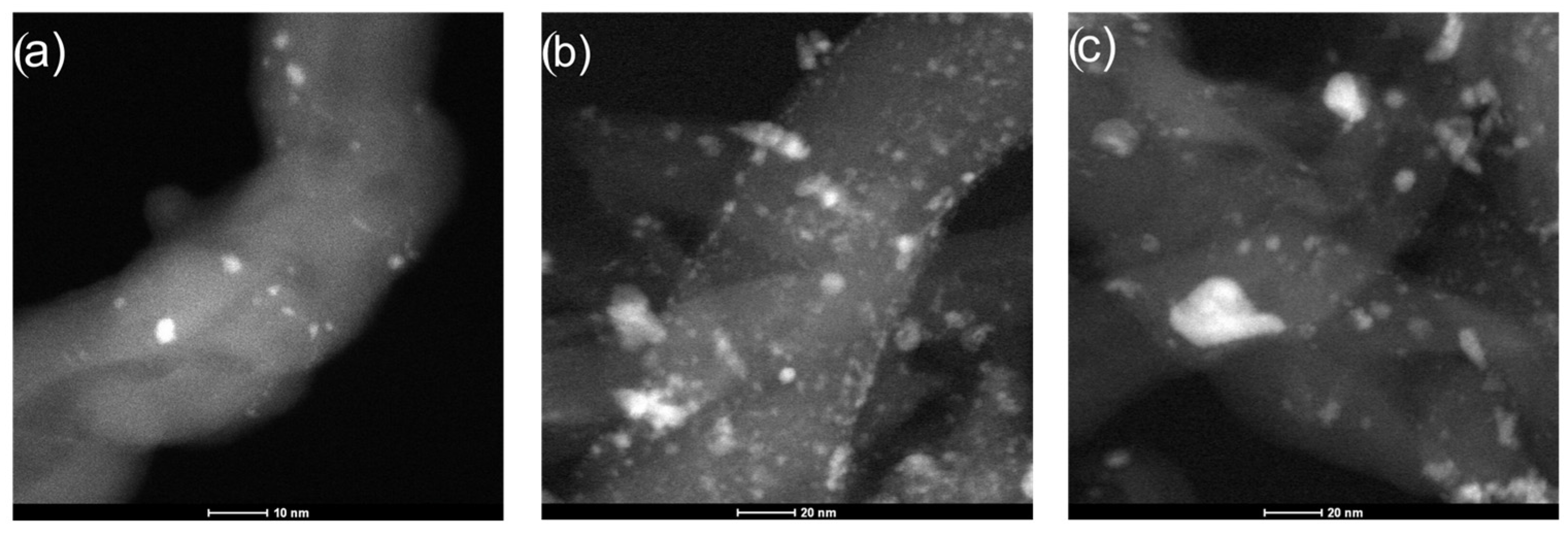
2.1. Characterization of the Mo2C Catalysts
2.2. Effect of the Catalyst Mo Loading on the Product Yields
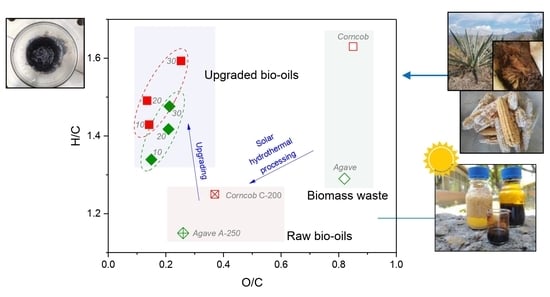
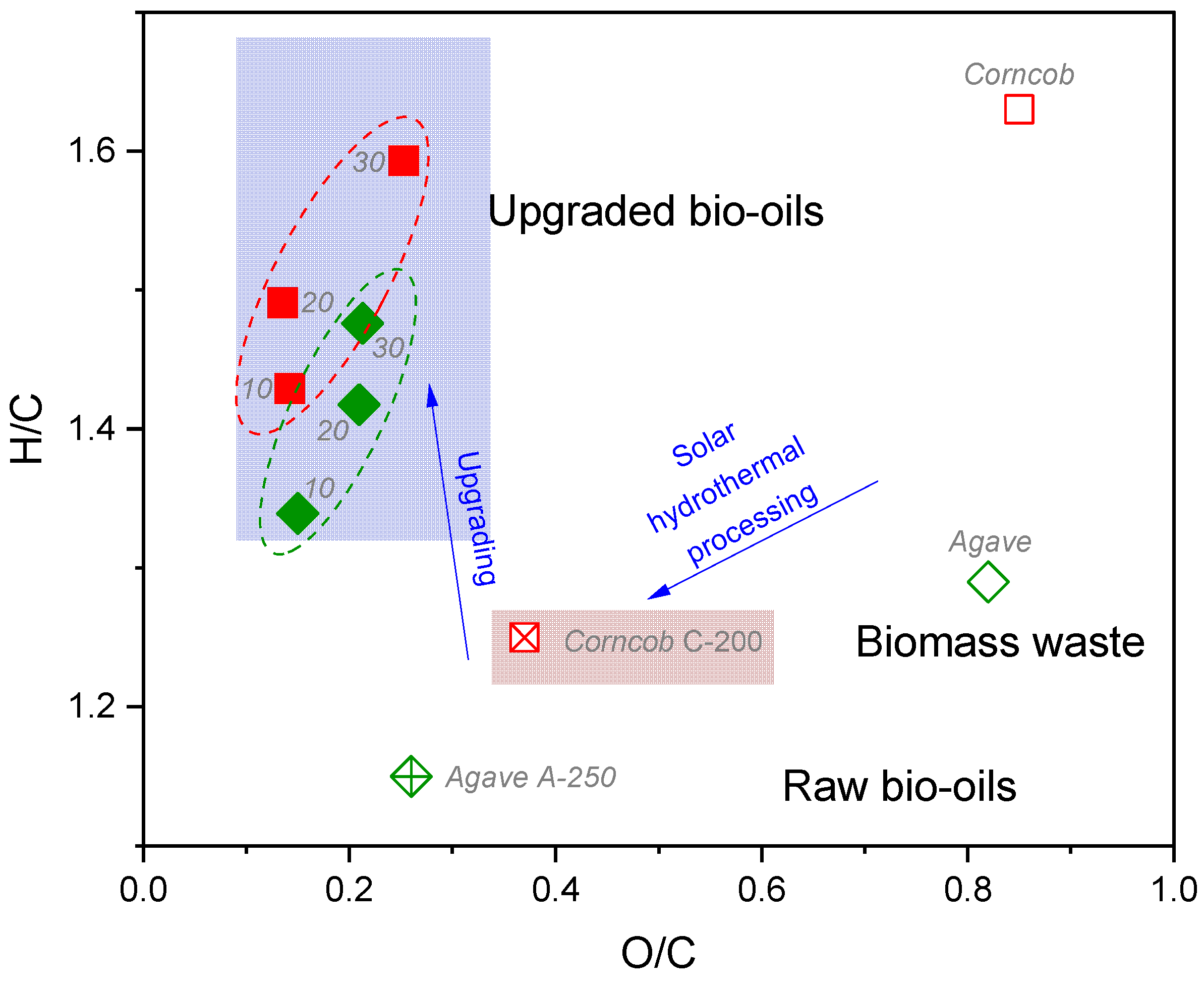
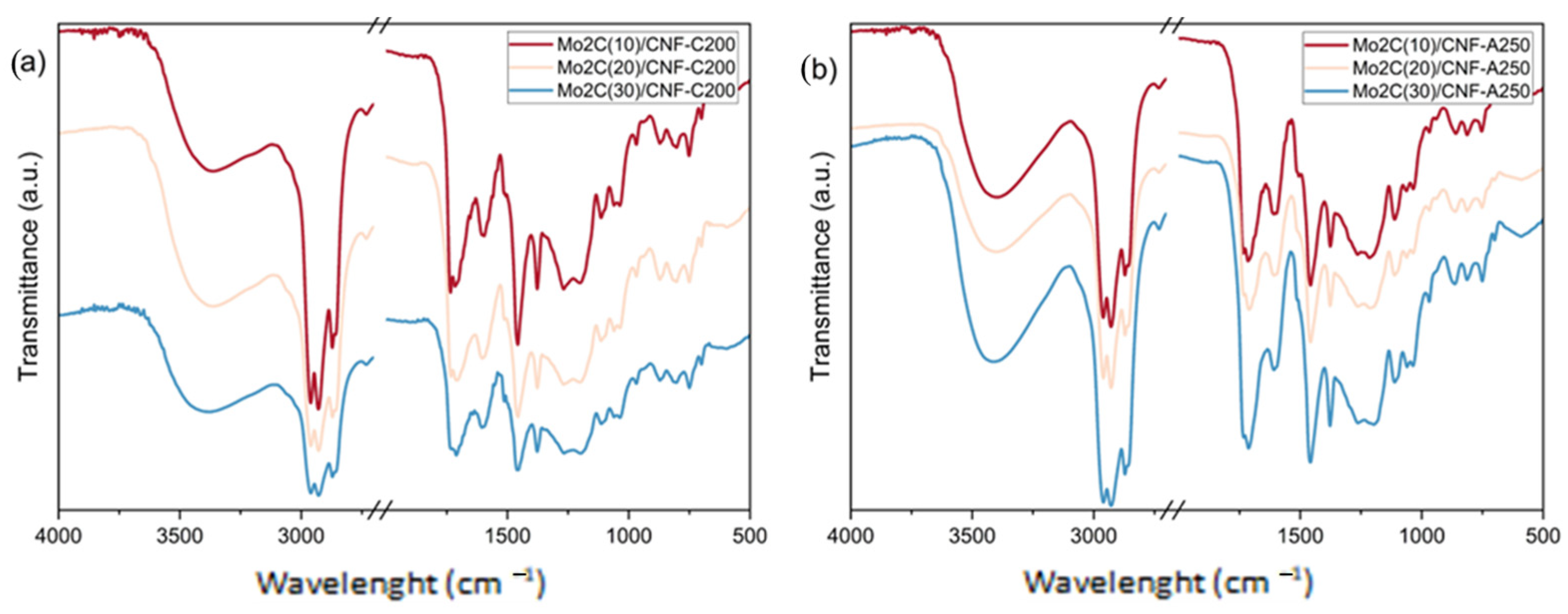
2.3. Effect of the Catalyst Mo Loading on the Upgraded Bio-Oil Properties
2.4. Exhaust Gas Analysis
2.5. Comparison of Upgraded Bio-Oils Using Mo Catalysts from Solar and Conventional Heating
3. Methodology
3.1. Materials
3.2. Catalyst Synthesis
3.3. Hydrodeoxygenation of Bio-Oils
3.4. Analytical Methods
3.5. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R.; Cao, Y.; Boakye, E.; Raynie, D.; Gu, Z. Hydrodeoxygenation upgrading of pine sawdust bio-oil using zinc metal with zero valency. J. Taiwan Inst. Chem. Eng. 2017, 74, 146–153. [Google Scholar] [CrossRef]
- Valle, B.; Remiro, A.; García-Gómez, N.; Gayubo, A.G.; Bilbao, J. Recent research progress on bio-oil conversion into bio-fuels and raw chemicals: A review. J. Chem. Technol. Biotechnol. 2019, 94, 670–689. [Google Scholar] [CrossRef]
- Wang, H.; Feng, M.; Yang, B. Catalytic hydrodeoxygenation of anisole: An insight into the role of metals in transalkylation reactions in bio-oil upgrading. Green Chem. 2017, 19, 1668–1673. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, G.; Yang, L.; Zheng, L.; Li, F. Cooperative Effects between Ni-Mo Alloy Sites and Defective Structures over Hierarchical Ni-Mo Bimetallic Catalysts Enable the Enhanced Hydrodeoxygenation Activity. ACS Sustain. Chem. Eng. 2021, 9, 11604–11615. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Jongerius, A.L.; Gosselink, R.W.; Dijkstra, J.; Bitter, J.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Carbon Nanofiber Supported Transition-Metal Carbide Catalysts for the Hydrodeoxygenation of Guaiacol. ChemCatChem 2013, 5, 2964–2972. [Google Scholar] [CrossRef]
- Pang, J.; Sun, J.; Zheng, M.; Li, H.; Wang, Y.; Zhang, T. Transition metal carbide catalysts for biomass conversion: A review. Appl. Catal. B Environ. 2019, 254, 510–522. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Chen, B.; Kuhn, A.N.; Ma, D.; Yang, H. Progress in hydrogen production over transition metal carbide catalysts: Challenges and opportunities. Curr. Opin. Chem. Eng. 2018, 20, 68–77. [Google Scholar] [CrossRef]
- Sullivan, M.M.; Chen, C.J.; Bhan, A. Catalytic deoxygenation on transition metal carbide catalysts. Catal. Sci. Technol. 2016, 6, 602–616. [Google Scholar] [CrossRef]
- Shilov, I.N.; Smirnov, A.A.; Bulavchenko, O.A.; Yakovlev, V.A. Effect of Ni–Mo carbide catalyst formation on furfural hydrogenation. Catalysts 2018, 8, 560. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Smith, K.J.; Kim, C.S. Hydrodeoxygenation of 2-Methoxyphenol over Ru, Pd, and Mo2C Catalysts Supported on Carbon. Energy Fuels 2017, 31, 6378–6388. [Google Scholar] [CrossRef]
- Ochoa, E.; Torres, D.; Moreira, R.; Pinilla, J.L.; Suelves, I. Carbon nanofiber supported Mo2C catalysts for hydrodeoxygenation of guaiacol: The importance of the carburization process. Appl. Catal. B Environ. 2018, 239, 463–474. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Rocha, A.S.; Souza, L.A.; Oliveira, R.R.; Rocha, A.B.; Teixeira da Silva, V. Hydrodeoxygenation of acrylic acid using Mo2C/Al2O3. Appl. Catal. A Gen. 2017, 531, 69–78. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoon, D.; Lee, S.-C.; Kim, J. Mo2C/Graphene Nanocomposite As a Hydrodeoxygenation Catalyst for the Production of Diesel Range Hydrocarbons. ACS Catal. 2015, 5, 3292–3303. [Google Scholar] [CrossRef]
- Lee, W.S.; Kumar, A.; Wang, Z.; Bhan, A. Chemical Titration and Transient Kinetic Studies of Site Requirements in Mo2C-Catalyzed Vapor Phase Anisole Hydrodeoxygenation. ACS Catal. 2015, 5, 4104–4114. [Google Scholar] [CrossRef]
- Chen, C.J.; Lee, W.S.; Bhan, A. Mo2C catalyzed vapor phase hydrodeoxygenation of lignin-derived phenolic compound mixtures to aromatics under ambient pressure. Appl. Catal. A Gen. 2016, 510, 42–48. [Google Scholar] [CrossRef]
- Ochoa, E.; Torres, D.; Pinilla, J.L.; Suelves, I. On the hydrothermal-enhanced synthesis of highly selective Mo2C catalysts to fully deoxygenated products in the guaiacol HDO reaction. J. Environ. Chem. Eng. 2021, 9, 105146. [Google Scholar] [CrossRef]
- Remón, J.; Ochoa, E.; Foguet, C.; Pinilla, J.L.; Suelves, I. Towards a sustainable bio-fuels production from lignocellulosic bio-oils: Influence of operating conditions on the hydrodeoxygenation of guaiacol over a Mo2C/CNF catalyst. Fuel Process. Technol. 2019, 191, 111–120. [Google Scholar] [CrossRef]
- Grilc, M.; Veryasov, G.; Likozar, B.; Jesih, A.; Levec, J. Hydrodeoxygenation of solvolysed lignocellulosic biomass by unsupported MoS2, MoO2, Mo2C and WS2 catalysts. Appl. Catal. B Environ. 2015, 163, 467–477. [Google Scholar] [CrossRef]
- Remón, J.; Casales, M.; Gracia, J.; Callén, M.S.; Pinilla, J.L.; Suelves, I. Sustainable production of liquid biofuels and value-added platform chemicals by hydrodeoxygenation of lignocellulosic bio-oil over a carbon–neutral Mo2C/CNF catalyst. Chem. Eng. J. 2021, 405, 126705. [Google Scholar] [CrossRef]
- Ranga, C.; Alexiadis, V.I.; Lauwaert, J.; Lødeng, R.; Thybaut, J.W. Effect of Co incorporation and support selection on deoxygenation selectivity and stability of (Co)Mo catalysts in anisole HDO. Appl. Catal. A Gen. 2019, 571, 61–70. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Shi, H.; Li, M.; Chu, Y.; Pan, Z.; Yu, X. Regulating product distribution in deoxygenation of methyl laurate on silica-supported Ni-Mo phosphides: Effect of Ni/Mo ratio. Fuel 2014, 129, 1–10. [Google Scholar] [CrossRef]
- López, M.; Hernández, D.; Laverde, J.; Pérez, S.; López, D. Catalytic Upgrading of Residual Biomass Derived Bio-oil over Molybdenum Carbide. Waste Biomass Valorization 2020, 11, 2849–2856. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Abdullah, B. The effect of metal loading over Ni/γ-Al2O3 and Mo/γ-Al2O3 catalysts on reaction routes of hydrodeoxygenation of rubber seed oil for green diesel production. Catal. Today 2020, 355, 51–64. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, P.; Duan, J.; Han, J.; Lou, H.; Zheng, X.; Hong, H. Carbon nanofibers supported molybdenum carbide catalysts for hydrodeoxygenation of vegetable oils. RSC Adv. 2013, 3, 17485–17491. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.-M.; Lee, I.-G.; Jeon, J.-K.; Jung, S.-C.; Chung, J.D.; Choi, W.G.; Park, Y.-K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Zhai, Y.; Liu, Y.; Zhang, R.; Tang, X. Upgrading of Bio-Oil Using Supercritical 1-Butanol over a Ru/C Heterogeneous Catalyst: Role of the Solvent. Energy Fuels 2014, 28, 4611–4621. [Google Scholar] [CrossRef]
- Philippov, A.; Nesterov, N.; Pakharukova, V.; Kozhevnikov, I.; Martyanov, O. Advanced High-Loaded Ni–Cu Catalysts in Transfer Hydrogenation of Anisole: Unexpected Effect of Cu Addition. Catalysts 2022, 12, 1307. [Google Scholar] [CrossRef]
- Kennema, M.; De Castro, I.B.D.; Meemken, F.; Rinaldi, R. Liquid-Phase H-Transfer from 2-Propanol to Phenol on Raney Ni: Surface Processes and Inhibition. ACS Catal. 2017, 7, 2437–2445. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Yu, C.; Li, G.; Yang, Y.; Zhang, H. Upgrading of bio-oil in supercritical ethanol: Catalysts screening, solvent recovery and catalyst stability study. J. Supercrit. Fluids 2014, 95, 387–393. [Google Scholar] [CrossRef]
- Dang, Q.; Luo, Z.; Zhang, J.; Wang, J.; Chen, W.; Yang, Y. Experimental study on bio-oil upgrading over Pt/SO42−/ZrO2/SBA-15 catalyst in supercritical ethanol. Fuel 2013, 103, 683–692. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Dang, Q.; Wang, J.; Chen, W. Upgrading of Bio-oil over Bifunctional Catalysts in Supercritical Monoalcohols. Energy Fuels 2012, 26, 2990–2995. [Google Scholar] [CrossRef]
- Jiang, Y.; Jones, S.B.; Zhu, Y.; Snowden-Swan, L.; Schmidt, A.J.; Billing, J.M.; Anderson, D. Techno-economic uncertainty quantification of algal-derived biocrude via hydrothermal liquefaction. Algal Res. 2019, 39, 101450. [Google Scholar] [CrossRef]
- Giaconia, A.; Caputo, G.; Ienna, A.; Mazzei, D.; Schiavo, B.; Scialdone, O.; Galia, A. Biorefinery process for hydrothermal liquefaction of microalgae powered by a concentrating solar plant: A conceptual study. Appl. Energy 2017, 208, 1139–1149. [Google Scholar] [CrossRef]
- Pearce, M.; Shemfe, M.; Sansom, C. Techno-economic analysis of solar integrated hydrothermal liquefaction of microalgae. Appl. Energy 2016, 166, 19–26. [Google Scholar] [CrossRef]
- Oshikawa, K.; Nagai, M.; Omi, S. Characterization of molybdenum carbides for methane reforming by TPR, XRD, and XPS. J. Phys. Chem. B 2001, 105, 9124–9131. [Google Scholar] [CrossRef]
- Guo, H.; Song, Y.; Chen, P.; Lou, H.; Zheng, X. Effects of graphitization of carbon nanospheres on hydrodeoxygenation activity of molybdenum carbide. Catal. Sci. Technol. 2018, 8, 4199–4208. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Wichern, M.; Lübken, M. Hydrothermal carbonization of the filter bed remained after filtration of olive mill wastewater on olive stones for biofuel application. Biomass Convers. Biorefinery 2022, 12, 1237–1247. [Google Scholar] [CrossRef]
- Luo, Y.; Guda, V.K.; Hassan, E.B.; Steele, P.H.; Mitchell, B.; Yu, F. Hydrodeoxygenation of oxidized distilled bio-oil for the production of gasoline fuel type. Energy Convers. Manag. 2016, 112, 319–327. [Google Scholar] [CrossRef]
- Grilc, M.; Likozar, B.; Levec, J. Hydrotreatment of solvolytically liquefied lignocellulosic biomass over NiMo/Al2O3 catalyst: Reaction mechanism, hydrodeoxygenation kinetics and mass transfer model based on FTIR. Biomass Bioenergy 2014, 63, 300–312. [Google Scholar] [CrossRef]
- Junming, X.; Jianchun, J.; Yunjuan, S.; Yanju, L. Bio-oil upgrading by means of ethyl ester production in reactive distillation to remove water and to improve storage and fuel characteristics. Biomass Bioenergy 2008, 32, 1056–1061. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Arcelus-Arrillaga, P.; Millan, M.; Arancibia-Bulnes, C.A.; Valadés-Pelayo, P.J.; Villafán-Vidales, H.I. Solar integrated hydrothermal processes: A review. Renew. Sustain. Energy Rev. 2021, 139, 110575. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, Y.; Cen, K.; Cao, X.; Ma, Z.; Li, Y. Comparative study on the pyrolysis behaviors of rice straw under different washing pretreatments of water, acid solution, and aqueous phase bio-oil by using TG-FTIR and Py-GC/MS. Fuel 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, C.; Liu, R. GC-MS and FT-IR analysis of the bio-oil with addition of ethyl acetate during storage. Front. Energy Res. 2014, 2, 3. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nishu; Sarker, M.; Chai, M.; Li, C.; Liu, R.; Cai, J. Potentiality of combined catalyst for high quality bio-oil production from catalytic pyrolysis of pinewood using an analytical Py-GC/MS and fixed bed reactor. J. Energy Inst. 2020, 93, 1737–1746. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yuan, Z.; Rohani, S.; Xu, C. Effects of nano-structured CoMo catalysts on hydrodeoxygenation of fast pyrolysis oil in supercritical ethanol. Catal. Today 2016, 269, 182–194. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Rabnawaz, M. Upgrading pyrolysis bio-oil through hydrodeoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy Convers. Manag. 2017, 150, 331–342. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Arcelus-Arrillaga, P.; Millan, M.; Okoye, P.U.; Arancibia-Bulnes, C.A.; Pacheco-Catalán, D.E.; Villafán-Vidales, H.I. Solar hydrothermal processing of agave bagasse: Insights on the effect of operational parameters. Renew. Energy 2022, 192, 14–23. [Google Scholar] [CrossRef]
- Ochoa, E.; Torres, D.; Pinilla, J.L.; Suelves, I. Influence of carburization time on the activity of Mo2C/CNF catalysts for the HDO of guaiacol. Catal. Today 2020, 357, 240–247. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Wang, J.; Abdelouahed, L.; Jabbour, M.; Taouk, B. Catalytic hydro-deoxygenation of acetic acid, 4-ethylguaiacol, and furfural from bio-oil over Ni2P/HZSM-5 catalysts. Comptes Rendus. Chim. 2021, 24, 131–147. [Google Scholar] [CrossRef]
- Oh, S.; Kim, U.-J.; Choi, I.-G.; Choi, J.W. Solvent effects on improvement of fuel properties during hydrodeoxygenation process of bio-oil in the presence of Pt/C. Energy 2016, 113, 116–123. [Google Scholar] [CrossRef]



| Catalyst | Bulk Mo (wt.%) | Surface Mo (wt.%) | Ratio Mo/C (wt.%) | BET Surface Area (m2/g) |
|---|---|---|---|---|
| Mo2C(10)/CNF | 13.0 | 16.8 | 0.24 | 55 |
| Mo2C(20)/CNF | 20.0 | 19.6 | 0.29 | 36 |
| Mo2C(30)/CNF | 27.0 | 30.1 | 0.55 | 29 |
| Bio-Oil | C (wt.%) | H (wt.%) | O (wt.%) | N (wt.%) | HHV (MJ/kg) | DOD (wt.%) | EE (%) |
|---|---|---|---|---|---|---|---|
| C200 | 61.0 | 6.4 | 30.8 | 1.8 | 25.6 | - | - |
| Mo2C(10)/CNF-C200 | 75.1 | 9.0 | 14.2 | 1.7 | 35.3 | 72.1 | 83.2 |
| Mo2C(20)/CNF-C200 | 75.4 | 9.43 | 13.61 | 1.5 | 36.0 | 74.5 | 81.0 |
| Mo2C(30)/CNF-C200 | 67.3 | 8.9 | 22.6 | 1.0 | 31.7 | 50.4 | 83.4 |
| A250 | 68.4 | 6.6 | 24.3 | 0.7 | 29.1 | - | - |
| Mo2C(10)/CNF-A250 | 75.7 | 8.5 | 15.1 | 0.7 | 34.8 | 69.0 | 59.6 |
| Mo2C(20)/CNF-A250 | 71.1 | 8.4 | 19.8 | 0.6 | 32.7 | 56.9 | 59.2 |
| Mo2C(30)/CNF-A250 | 70.7 | 8.7 | 20.0 | 0.5 | 32.9 | 48.4 | 70.7 |
| Corncob Bio-Oils | Agave Bio-Oils | |||||||
|---|---|---|---|---|---|---|---|---|
| C-200 | Mo2C(10)/CNF-C-200 | Mo2C(20)/CNF-C200 | Mo2C(30)/CNF-C200 | A-250 | Mo2C(10)/CNF-A250 | Mo2C(20)/CNF-A250 | Mo2C(30)/CNF-A250 | |
| Phenolic compounds | 18.1 | 24.3 | 20.5 | 30.7 | 29.1 | 27 | 18.0 | 18.8 |
| Esters | 1.5 | 41.9 | 43.7 | 43.5 | 1.8 | 48.8 | 39.0 | 42.3 |
| Carboxylic acids | 10.7 | 2.1 | n.d. | 2.0 | 2.2 | n.d. | n.d. | 3.3 |
| N-compounds | 46.2 | 11 | 14.8 | 4.9 | 24.9 | 3.3 | 8.1 | 3.1 |
| S-compounds | 2.6 | 1.7 | 0.9 | 0.9 | 3.2 | 1.0 | 0.7 | 1.7 |
| Ketones | 2.7 | 7.3 | 6.1 | 5.7 | 0.7 | 6.9 | 13.2 | 5.9 |
| Epoxides | 1.9 | 2.0 | 1.5 | n.d. | n.d. | 0.3 | n.d. | n.d. |
| Alcohols | 1.2 | 1.1 | 0.6 | 1.8 | n.d. | 6.6 | 3.4 | 9.6 |
| Other O-compounds | 13.2 | 7.3 | 9.3 | 8.2 | 32.4 | 5.2 | 14.9 | 14.1 |
| Aldehydes | 0.4 | 1.4 | 1.5 | 1.8 | 2.4 | n.d. | n.d. | 0.5 |
| Alkanes | 1.6 | 0.8 | n.d. | n.d. | 3.3 | 0.4 | 1.0 | n.d. |
| Aromatics | n.d. | 1.4 | 1.2 | 0.5 | n.d. | 0.8 | 1.7 | 0.7 |
| Raw Oil | Catalyst | H2 | CH4 | CO2 | CO | Others | Pcold (bar) |
|---|---|---|---|---|---|---|---|
| C200 | Mo2C(10)/CNF | 88.5 | 2.4 | 7.4 | 0 | 1.7 | 23.7 |
| Mo2C(20)/CNF | 87.9 | 2.1 | 7.2 | 0 | 2.8 | 24.3 | |
| Mo2C(30)/CNF | 87.7 | 2.8 | 8.0 | 0 | 1.5 | 25.6 | |
| A250 | Mo2C(10)/CNF | 88.2 | 6.7 | 5.0 | 0 | 0.1 | 21.5 |
| Mo2C(20)/CNF | 81.1 | 6.6 | 6.7 | 0 | 5.6 | 20.4 | |
| Mo2C(30)/CNF | 88.7 | 4.1 | 6.1 | 0 | 1.1 | 26 |
| Ref. | Biomass | T (°C) | Catalyst | Mo (wt.%) | τ (min) | H2 Pressure (bar) | Yoil (wt.%) | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|
| This work | Corncob (C200) | 350 | Mo2C/CNF | 10% | 120 | 10 | 55 | 34 |
| This work | Agave (A250) | 350 | Mo2C/CNF | 10% | 120 | 10 | 48 | 34 |
| [24] | Acacia sawdust | 350 | Mo2C | - | 240 | 50 | - | 41 |
| [25] | Rubber seed | 350 | Mo/γ-Al2O3 | 12% | - | 35 | 44 | - |
| [25] | Rubber seed | 350 | Mo/γ-Al2O3 | 15% | - | 35 | 62 | - |
| [39] | Maize oil | 260 | Mo2C/CNS | <20% | 180 | 25 | 67 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Cortés, A.; Torres, D.; Frecha, E.; Arcelus-Arrillaga, P.; Villafán-Vidales, H.I.; Longoria, A.; Pinilla, J.L.; Suelves, I. Catalytic Hydrodeoxygenation of Solar Energy Produced Bio-Oil in Supercritical Ethanol with Mo2C/CNF Catalysts: Effect of Mo Concentration. Catalysts 2023, 13, 1500. https://doi.org/10.3390/catal13121500
Ayala-Cortés A, Torres D, Frecha E, Arcelus-Arrillaga P, Villafán-Vidales HI, Longoria A, Pinilla JL, Suelves I. Catalytic Hydrodeoxygenation of Solar Energy Produced Bio-Oil in Supercritical Ethanol with Mo2C/CNF Catalysts: Effect of Mo Concentration. Catalysts. 2023; 13(12):1500. https://doi.org/10.3390/catal13121500
Chicago/Turabian StyleAyala-Cortés, Alejandro, Daniel Torres, Esther Frecha, Pedro Arcelus-Arrillaga, Heidi Isabel Villafán-Vidales, Adriana Longoria, José Luis Pinilla, and Isabel Suelves. 2023. "Catalytic Hydrodeoxygenation of Solar Energy Produced Bio-Oil in Supercritical Ethanol with Mo2C/CNF Catalysts: Effect of Mo Concentration" Catalysts 13, no. 12: 1500. https://doi.org/10.3390/catal13121500