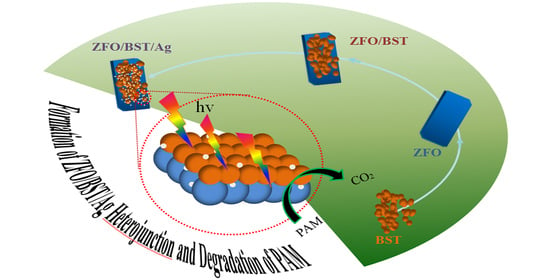
Construction of Spinel/Perovskite Heterojunction for Boosting Photocatalytic Performance for Polyacrylamide
Abstract
:1. Introduction
2. Results
2.1. Phase Structure Analysis of ZnFe2O4/Ba0.7Sr0.3TiO3
2.2. Composition Structure and Surface State of ZnFe2O4/Ba0.7Sr0.3TiO3
2.3. Analysis of Specific Surface Area of ZnFe2O4/Ba0.7Sr0.3TiO3
2.4. Analysis of Electron–Hole Pair Separation Efficiency in ZnFe2O4/Ba0.7Sr0.3TiO3
2.5. Degradation Mechanism and Products of PAM
3. Discussion
3.1. Study on the Photocatalytic Performance of ZnFe2O4/Ba0.7Sr0.3TiO3
3.2. Study on the Photocatalytic Performance of ZnFe2O4/Ba0.7Sr0.3TiO3/Ag
4. Materials and Methods
4.1. Preparation of ZnFe2O4
4.2. Preparation of Ba0.7Sr0.3TiO3
4.3. Preparation of ZnFe2O4/Ba0.7Sr0.3TiO3
4.4. Preparation of ZnFe2O4/Ba0.7Sr0.3TiO3/Ag
4.5. Performance Testing of Photocatalytic Degradation of PAM
4.5.1. Evaluation of Catalytic Performance of ZnFe2O4/Ba0.7Sr0.3TiO3
4.5.2. Evaluation of Catalytic Performance of ZnFe2O4/Ba0.7Sr0.3TiO3/Ag
4.6. Sample Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, H.; Liu, J.F.; Li, C.Y.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerobic biodegradation of partially hydrolyzed polyacrylamide in long-term methanogenic enrichment cultures from production water of oil reservoirs. Biodegradation 2018, 29, 233–243. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Wang, T. Progress of research work on physical and chemical degradation of polyacrylamide. Coal Prep. Technol. 2020, 2, 1–5. [Google Scholar] [CrossRef]
- Moussa, T.; Tiu, C. Factors affecting polymer degradation in turbulent pipe flow. Chem. Eng. Sci. 1994, 49, 1681–1692. [Google Scholar] [CrossRef]
- van Wijngaarden, L. Mechanics of collapsing cavitation bubbles. Ultrason. Sonochem. 2016, 29, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.P.; Madras, G. Effect of temperature on the ultrasonic degradation of polyacrylamide and poly(ethylene oxide). Polym. Degrad. Stab. 2004, 84, 341–344. [Google Scholar] [CrossRef]
- Yen, H.Y.; Yang, M.H. The ultrasonic degradation of polyacrylamide solution. Polym. Test. 2003, 22, 129–131. [Google Scholar] [CrossRef]
- Tsay, D.K.; Yang, M.H.; Wang, J.H. On the thermal degradation of polysulfones IX. The early stages of thermal degradation of poly(1-butene sulfone) and poly(2-methyl-1-pentene sulfone). Polym. Degrad. Stab. 2002, 76, 251–257. [Google Scholar] [CrossRef]
- Silva, M.; Dutra, E.R.; Mano, V.; Machado, J.C. Preparation and thermal study of polymers derived from acrylamide. Polym. Degrad. Stab. 2000, 67, 491–495. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides.1. Effects of artificial environmental conditions: Temperature, light, and pH. Ecotoxicol. Environ. Saf. 1996, 35, 121–135. [Google Scholar] [CrossRef]
- Keshipour, S.; Hadidi, M.; Gholipour, O. A Review on Hydrogen Generation by Photo-, Electro-, and Photoelectro-Catalysts Based on Chitosan, Chitin, Cellulose, and Carbon Materials Obtained from These Biopolymers. Adv. Polym. Technol. 2023, 2023, 8835940. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.P.; Senapati, D.; Madras, G. Pulsed laser degradation of polyethylene oxide and polyacrylamide in aqueous solution. Polym. Degrad. Stab. 2005, 87, 521–526. [Google Scholar] [CrossRef]
- Wang, X.B.; Guan, F.W.; Huang, Z.G.; He, H.; Wang, L.; Li, K.F. Study on low temperature plasma combined with AC/Mn + TiO2-Al2O3 catalytic treatment of sewage-containing polyacrylamide. Water Sci. Technol. 2023, 87, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Feng, C.; Wang, L.; Liu, J.Q.; Jin, A.; Zhu, C.Y.; Guan, F.W.; Huang, Z.G.; He, H. Experimental study on reducing the viscosity of sewage containing PAM catalyzed by low temperature plasma synergistic AC/Mn + TiO2. Environ. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Xiong, B.Y.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. Npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Du, J.; Lv, C.H.; Lan, X.T.; Song, J.F.; Liu, P.L.; Chen, X.; Wang, Q.; Liu, J.M.; Guo, G.X. A review on viscosity retention of PAM solution for polymer flooding technology. Pet. Sci. Technol. 2022, 1–34. [Google Scholar] [CrossRef]
- Zhu, B.J.; Jiang, G.F.; Lv, Y.; Liu, F.; Sun, J. Photocatalytic degradation of polyacrylamide by rGO@Fe3O4/Cu2O@ZnO magnetic recyclable composites. Mater. Sci. Semicond. Process. 2021, 131, 105841. [Google Scholar] [CrossRef]
- Rong, X.S.; Qiu, F.X.; Zhang, C.; Fu, L.; Wang, Y.Y.; Yang, D.Y. Preparation of Ag-AgBr/TiO2-graphene and its visible light photocatalytic activity enhancement for the degradation of polyacrylamide. J. Alloys Compd. 2015, 639, 153–161. [Google Scholar] [CrossRef]
- Khordadpour Siahkal Mahalleh, M.; Ahour, F.; Keshipour, S. Development of copper electrochemical sensor using D-penicillamine functionalized graphene oxide modified electrode. Appl. Chem. 2023, 18, 71–90. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Naiyun, L.; Yixian, L.; Yunliang, L.; Yaxi, L.; Yuanyuan, C.; Haitao, L. Modulation of photogenerated holes for enhanced photoelectrocatalytic performance. Microstructures 2023, 3, 2023001. [Google Scholar] [CrossRef]
- Peng, C.; Jingwei, H.; Lianzhou, W. Metal-organic framework-tailored perovskite solar cells. Microstructures 2022, 2, 2022014. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhang, B.P. SPR propelled visible-active photocatalysis on Au-dispersed Co3O4 films. Appl. Catal. A Gen. 2013, 467, 585–592. [Google Scholar] [CrossRef]
- Cho, Y.J.; Moon, G.H.; Kanazawa, T.; Maeda, K.; Choi, W. Selective dual-purpose photocatalysis for simultaneous H-2 evolution and mineralization of organic compounds enabled by a Cr2O3 barrier layer coated on Rh/SrTiO3. Chem. Commun. 2016, 52, 9636–9639. [Google Scholar] [CrossRef]
- Chandel, N.; Sharma, K.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Singh, P. Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arab. J. Chem. 2020, 13, 4324–4340. [Google Scholar] [CrossRef]
- Mousavi-Salehi, S.; Keshipour, S.; Ahour, F. Gold supported on graphene oxide/silica photocatalyst for hydrogen generation from formic acid. J. Phys. Chem. Solids 2023, 176, 111239. [Google Scholar] [CrossRef]
- Tang, H.X.; Sodano, H.A. Ultra High Energy Density Nanocomposite Capacitors with Fast Discharge Using Ba0.2Sr0.8TiO3 Nanowires. Nano Lett. 2013, 13, 1373–1379. [Google Scholar] [CrossRef]
- Asghari, A.; Keshipour, S. Green and one-pot synthesis of Co(II) citrate complex nanoparticles/graphene oxide nanocomposites towards photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 34750–34765. [Google Scholar] [CrossRef]
- Li, M.X.; Li, B.C.; Chen, J.S.; Shen, X.Y.; Cui, S.; He, X.C.; Liu, K.R.; Han, Q. Analysis of thermal decomposition of acidified sediments in gold plants and harmless disposal of it. J. Hazard. Mater. 2022, 431, 128472. [Google Scholar] [CrossRef]
- Cigeroglu, Z.; Kazan-Kaya, E.S.; El Messaoudi, N.; Fernine, Y.; Americo-Pinheiro, J.H.P.; Jada, A. Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: Optimization, modeling, and theoretical calculation. J. Mol. Liq. 2023, 369, 120866. [Google Scholar] [CrossRef]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Ghorpade, S.P.; Krishna, R.H.; Melavanki, R.M.; Dubey, V.; Patil, N. Effect of Eu3+ on optical and energy bandgap of SrY2O4 nanophosphors for FED applications. Optik 2020, 208, 164533. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Zhang, B. Mechanism of Enhancement Ozonation by Ultrasound for Degradation of Polyacrylamide in ASP Produced. CIESC J. 2015, 6, 2242–2247. [Google Scholar]
- Su, J.; Shang, Q.K.; Guo, T.T.; Yang, S.; Wang, X.Y.; Ma, Q.; Guan, H.Y.; Xu, F.; Tsang, S.C. Construction of heterojunction ZnFe2O4/ZnO/Ag by using ZnO and Ag nanoparticles to modify ZnFe2O4 and its photocatalytic properties under visible light. Mater. Chem. Phys. 2018, 219, 22–29. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Wu, Z.; Jia, Y.; Xiao, L.; Liu, Y. Strong pyro-electro-chemical coupling of Ba0.7Sr0.3TiO3@Ag pyroelectric nanoparticles for room-temperature pyrocatalysis. Nano Energy 2018, 50, 581–588. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Y.; Zhou, Y.; Dong, F. Photoelectrocatalytic carbon dioxide reduction: Fundamental, advances and challenges. Nano Mater. Sci. 2021, 3, 344–367. [Google Scholar] [CrossRef]
- Abu Tariq, M.; Faisal, M.; Saquib, M.; Muneer, M. Heterogeneous photocatalytic degradation of an anthraquinone and a triphenylmethane dye derivative in aqueous suspensions of semiconductor. Dye. Pigment. 2008, 76, 358–365. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Luo, Y.; Yang, K.; Che, G.; Wang, H.; Qi, J. Construction of Spinel/Perovskite Heterojunction for Boosting Photocatalytic Performance for Polyacrylamide. Catalysts 2023, 13, 1424. https://doi.org/10.3390/catal13111424
Zhu Q, Luo Y, Yang K, Che G, Wang H, Qi J. Construction of Spinel/Perovskite Heterojunction for Boosting Photocatalytic Performance for Polyacrylamide. Catalysts. 2023; 13(11):1424. https://doi.org/10.3390/catal13111424
Chicago/Turabian StyleZhu, Qinghan, Yuxue Luo, Ke Yang, Guangbo Che, Haiwang Wang, and Jian Qi. 2023. "Construction of Spinel/Perovskite Heterojunction for Boosting Photocatalytic Performance for Polyacrylamide" Catalysts 13, no. 11: 1424. https://doi.org/10.3390/catal13111424