Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
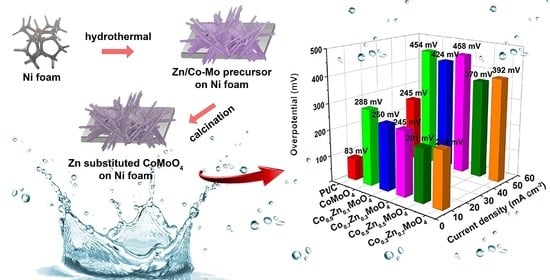
2.2. Electrocatalytic Hydrogen Evolution
2.3. Post Electrolysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co1-xZnxMoO4@NF
3.3. Preparation of Pt/C@NF Electrocatalyst
3.4. Materials Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Chamani, S.; Sadeghi, E.; Peighambardoust, N.S.; Doganay, F.; Yanalak, G.; Eroglu, Z.; Aslan, E.; Asghari, E.; Metin, O.; Patir, I.H.; et al. Photocatalytic hydrogen evolution performance of metal ferrites/polypyrrole nanocomposites. Int. J. Hydrogen Energy 2022, 47, 32940–32954. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.; Li, C.; Li, Y. Fe-based electrocatalysts for oxygen evolution reaction: Progress and perspectives. ACS Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Ramírez, A.M.R.; Heidari, S.; Vergara, A.; Aguilera, M.V.; Preuss, P.; Camarada, M.B.; Fischer, A. Rhenium-Based Electrocatalysts for Water Splitting. ACS Mater. Au 2023. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Cho, J.; Kim, S.Y.; Jang, H.W. Toward multicomponent single-atom catalysis for efficient electrochemical energy conversion. ACS Mater. Au 2021, 2, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Mater. 2022, e12441. [Google Scholar] [CrossRef]
- Yang, W.-D.; Xiang, J.; Zhao, R.-D.; Loy, S.; Li, M.-T.; Ma, D.-M.; Li, J.; Wu, F.-F. Nanoengineering of ZnCo2O4@CoMoO4 heterogeneous structures for supercapacitor and water splitting applications. Ceram. Int. 2023, 49, 4422–4434. [Google Scholar] [CrossRef]
- Sadeghi, E.; Peighambardoust, N.S.; Chamani, S.; Aydemir, U. Designing In Situ Grown Ternary Oxide/2D Ni-BDC MOF Nanocomposites on Nickel Foam as Efficient Electrocatalysts for Electrochemical Water Splitting. ACS Mater. Au 2023, 3, 143–163. [Google Scholar] [CrossRef]
- Chamani, S.; Khatamian, M.; Peighambardoust, N.S.; Aydemir, U. Microwave-Assisted Auto-Combustion Synthesis of Binary/Ternary Co x Ni1− x Ferrite for Electrochemical Hydrogen and Oxygen Evolution. ACS Omega 2021, 6, 33024–33032. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Liu, C.-H.; Huang, W.; Wang, X.-L.; Dong, L.-Z.; Li, S.-L.; Lan, Y.-Q. Bimetallic carbides-based nanocomposite as superior electrocatalyst for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 16977–16985. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, L.; Li, D.; Yang, R.; Jiang, D.; Chen, M. Engineering Ni(OH)2 nanosheet on CoMoO4 nanoplate array as efficient electrocatalyst for oxygen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 16086–16095. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.; Xia, G.; Chen, J.; Zhang, R.; Chen, Q. Tuning electronic structures of nonprecious ternary alloys encapsulated in graphene layers for optimizing overall water splitting activity. ACS Catal. 2017, 7, 469–479. [Google Scholar] [CrossRef]
- Wen, Y.; Li, C.-X.; Wang, J.; Qian, Y.-Y.; Shen, F.-C. Hierarchically Self-Supporting Phosphorus-Doped CoMoO4 Nanoflowers Arrays toward Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 6814–6822. [Google Scholar] [CrossRef]
- Suryanto, B.H.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Shang, X.; Dong, B.; Chai, Y.-M. Triple Ni-Co-Mo metal sulfides with one-dimensional and hierarchical nanostructures towards highly efficient hydrogen evolution reaction. J. Catal. 2018, 361, 204–213. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Zhang, L.; Zhou, F.; Liang, Y.; Wang, R. Molybdenum phosphide/carbon nanotube hybrids as pH-universal electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2018, 28, 1706523. [Google Scholar] [CrossRef]
- Yan, X.; Tian, L.; Atkins, S.; Liu, Y.; Murowchick, J.; Chen, X. Converting CoMoO4 into CoO/MoOx for overall water splitting by hydrogenation. ACS Sustain. Chem. Eng. 2016, 4, 3743–3749. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, M.; Pan, Y.; Zhang, J. Porous Co–Mo phosphide nanotubes: An efficient electrocatalyst for hydrogen evolution. J. Mater. Sci. 2017, 52, 10406–10417. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Z.; Lin, Y.; Wang, J.; Pan, H.; Xu, Z. Hierarchical heterostructure NiCo2O4@CoMoO4/NF as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 16950–16958. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, P.; Ye, Z.; Cen, T.; Peng, X.; Yuan, D.; Wu, H. P doped CoMoO4/RGO as an efficient hybrid catalyst for hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 15157–15165. [Google Scholar] [CrossRef]
- Qian, J.; Wang, T.; Xia, B.; Xi, P.; Gao, D. Zn-doped MoSe2 nanosheets as high-performance electrocatalysts for hydrogen evolution reaction in acid media. Electrochim. Acta 2019, 296, 701–708. [Google Scholar] [CrossRef]
- Qu, Y.; Medina, H.; Wang, S.W.; Wang, Y.C.; Chen, C.W.; Su, T.Y.; Manikandan, A.; Wang, K.; Shih, Y.C.; Chang, J.W.; et al. Wafer scale phase-engineered 1T-and 2H-MoSe2/Mo core–shell 3D-hierarchical nanostructures toward efficient electrocatalytic hydrogen evolution reaction. Adv. Mater. 2016, 28, 9831–9838. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, T.; Ge, B.; Han, L.; Zheng, L.; Lin, F.; Xu, Z.; Hu, W.B.; Du, X.W.; Davey, K.; et al. Well-dispersed nickel-and zinc-tailored electronic structure of a transition metal oxide for highly active alkaline hydrogen evolution reaction. Adv. Mater. 2019, 31, 1807771. [Google Scholar] [CrossRef]
- Chen, Z.; Kronawitter, C.X.; Koel, B.E. Facet-dependent activity and stability of Co3O4 nanocrystals towards the oxygen evolution reaction. Phys. Chem. Chem. Phys. 2015, 17, 29387–29393. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.-Y.; Wang, H.; Jiao, Y.; Hu, Z.; Zheng, Y.; Zheng, L.; Mao, J.; Liu, H.; Du, X.-W.; et al. Activating cobalt (II) oxide nanorods for efficient electrocatalysis by strain engineering. Nat. Commun. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. 2016, 128, 5363–5367. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Li, Y.; Jiang, H.; Li, C. Continuous oxygen vacancy engineering of the Co3O4 layer for an enhanced alkaline electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 13506–13510. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, X.; Wang, S. Phosphorus-doped CoS2 nanosheet arrays as ultra-efficient electrocatalysts for the hydrogen evolution reaction. Chem. Commun. 2015, 51, 14160–14163. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, L.; Luo, L.; Lu, J.; Yin, S.; Shen, P.K.; Tsiakaras, P. N-doped porous molybdenum carbide nanobelts as efficient catalysts for hydrogen evolution reaction. Appl. Catal. B Environ. 2018, 224, 533–540. [Google Scholar] [CrossRef]
- Ren, M.; Guo, X.; Huang, S. Transition metal atoms (Fe, Co, Ni, Cu, Zn) doped RuIr surface for the hydrogen evolution reaction: A first-principles study. Appl. Surf. Sci. 2021, 556, 149801. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Wang, J.; Lin, Y.; Gong, Y.; Jing, X. Regulating the electronic structure of CoMoO4 microrod by phosphorus doping: An efficient electrocatalyst for the hydrogen evolution reaction. Dalton Trans. 2020, 49, 13152–13159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn metal atom doping on the surface plane of one-dimesional NiMoO4 nanorods with improved redox chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Costa, R.K.; Teles, S.C.; Siqueira, K.P. The relationship between crystal structures and thermochromism in CoMoO4. Chem. Pap. 2021, 75, 237–248. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. Facile fabrication of novel ZnO/CoMoO4 nanocomposites: Highly efficient visible-light-responsive photocatalysts in degradations of different contaminants. J. Photochem. Photobiol. A Chem. 2018, 363, 31–43. [Google Scholar] [CrossRef]
- Mandal, M.; Ghosh, D.; Giri, S.; Shakir, I.; Das, C.K. Polyaniline-wrapped 1D CoMoO4·0.75H2O nanorods as electrode materials for supercapacitor energy storage applications. RSC Adv. 2014, 4, 30832–30839. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, S.; Zeng, Y. Facile fabrication of a novel visible light active g-C3N4-CoMoO4 heterojunction with largely improved photocatalytic performance. Mater. Lett. 2020, 281, 128661. [Google Scholar] [CrossRef]
- Rafieezadeh, M.; Kianfar, A.H. Fabrication of heterojunction ternary Fe3O4/TiO2/CoMoO4 as a magnetic photocatalyst for organic dyes degradation under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2022, 423, 113596. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Abinaya, M.; Chen, S.-M. Ultrasonic assisted preparation of CoMoO4 nanoparticles modified electrochemical sensor for chloramphenicol determination. J. Solid State Chem. 2021, 302, 122392. [Google Scholar] [CrossRef]
- Ahmed, J.; Ubaidullah, M.; Ahmad, T.; Alhokbany, N.; Alshehri, S.M. Synthesis of graphite oxide/cobalt molybdenum oxide hybrid nanosheets for enhanced electrochemical performance in supercapacitors and the oxygen evolution reaction. ChemElectroChem 2019, 6, 2524–2530. [Google Scholar] [CrossRef]
- Badawi, A.; Althobaiti, M.; Alharthi, S.S.; Alharbi, A.N.; Alkathiri, A.A.; Alomairy, S.E. Effect of zinc doping on the structure and optical properties of iron oxide nanostructured films prepared by spray pyrolysis technique. Appl. Phys. A 2022, 128, 123. [Google Scholar] [CrossRef]
- Kumar, L.; Kumar, P.; Kar, M. Cation distribution by Rietveld technique and magnetocrystalline anisotropy of Zn substituted nanocrystalline cobalt ferrite. J. Alloys Compd. 2013, 551, 72–81. [Google Scholar] [CrossRef]
- Fei, B.; Chen, Z.; Ha, Y.; Wang, R.; Yang, H.; Xu, H.; Wu, R. Anion-cation co-substitution activation of spinel CoMoO4 for efficient oxygen evolution reaction. Chem. Eng. J. 2020, 394, 124926. [Google Scholar] [CrossRef]
- Liu, Z.; Zhan, C.; Peng, L.; Cao, Y.; Chen, Y.; Ding, S.; Xiao, C.; Lai, X.; Li, J.; Wei, S. A CoMoO4–Co2Mo3O8 heterostructure with valence-rich molybdenum for a high-performance hydrogen evolution reaction in alkaline solution. J. Mater. Chem. A 2019, 7, 16761–16769. [Google Scholar] [CrossRef]
- Sadeghi, E.; Peighambardoust, N.S.; Aydemir, U. Tailoring the Morphology of Cost-Effective Vanadium Diboride Through Cobalt Substitution for Highly Efficient Alkaline Water Oxidation. Inorg. Chem. 2021, 60, 19457–19466. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhang, Z.-J.; Yuan, X.-Q.; Liu, H.-J.; Zhou, Y.-L.; Lv, R.-Q.; Liu, Y.-B.; Chai, Y.-M.; Dong, B. Molten salt assisted Co1−xAgxMoO4 with lattice Ag doping and oxygen vacancy for stable water oxidation. Appl. Surf. Sci. 2023, 614, 156075. [Google Scholar] [CrossRef]
- Sun, H.; Tung, C.-W.; Qiu, Y.; Zhang, W.; Wang, Q.; Li, Z.; Tang, J.; Chen, H.-C.; Wang, C.; Chen, H.M. Atomic metal–support interaction enables reconstruction-free dual-site electrocatalyst. J. Am. Chem. Soc. 2021, 144, 1174–1186. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Yu, Y.; Shi, Y.; Yu, Y.; Niu, Z.; Zhang, B. Hydrogen evolution activity enhancement by tuning the oxygen vacancies in self-supported mesoporous spinel oxide nanowire arrays. Nano Res. 2018, 11, 603–613. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wu, X.; Zhang, H.; Zhang, J. A hybrid zeolitic imidazolate framework-derived ZnO/ZnMoO4 heterostructure for electrochemical hydrogen production. Dalton Trans. 2021, 50, 11365–11369. [Google Scholar] [CrossRef]
- Brijesh, K.; Bindu, K.; Shanbhag, D.; Nagaraja, H. Chemically prepared Polypyrrole/ZnWO4 nanocomposite electrodes for electrocatalytic water splitting. Int. J. Hydrogen Energy 2019, 44, 757–767. [Google Scholar] [CrossRef]
- Selvan, R.K.; Gedanken, A. The sonochemical synthesis and characterization of Cu1−xNixWO4 nanoparticles/nanorods and their application in electrocatalytic hydrogen evolution. Nanotechnology 2009, 20, 105602. [Google Scholar] [CrossRef] [PubMed]
- Padmanathan, N.; Shao, H.; Razeeb, K.M. Honeycomb micro/nano-architecture of stable β-NiMoO4 electrode/catalyst for sustainable energy storage and conversion devices. Int. J. Hydrogen Energy 2020, 45, 30911–30923. [Google Scholar] [CrossRef]
- Zhu, J.; Lv, W.; Yang, Y.; Huang, L.; Yu, W.; Wang, X.; Han, Q.; Dong, X. Hexagonal NiMoO4-MoS2 nanosheet heterostructure as a bifunctional electrocatalyst for urea oxidation assisted overall water electrolysis. New J. Chem. 2022, 46, 10280–10288. [Google Scholar] [CrossRef]
- Huang, S.; Meng, Y.; Cao, Y.; Yao, F.; He, Z.; Wang, X.; Pan, H.; Wu, M. Amorphous NiWO4 nanoparticles boosting the alkaline hydrogen evolution performance of Ni3S2 electrocatalysts. Appl. Catal. B Environ. 2020, 274, 119120. [Google Scholar] [CrossRef]
- Du, X.; Huang, C.; Zhang, X. Synthesis of CoMoO4/Co9S8 network arrays on nickel foam as efficient urea oxidation and hydrogen evolution catalyst. Int. J. Hydrogen Energy 2019, 44, 19595–19602. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Song, Y.; Zhou, W.; Shao, Z. Designing high-valence metal sites for electrochemical water splitting. Adv. Funct. Mater. 2021, 31, 2009779. [Google Scholar] [CrossRef]
- Sadeghi, E.; Peighambardoust, N.S.; Khatamian, M.; Unal, U.; Aydemir, U. Metal doped layered MgB2 nanoparticles as novel electrocatalysts for water splitting. Sci. Rep. 2021, 11, 3337. [Google Scholar] [CrossRef]
- Rani, B.J.; Swathi, S.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Babu, E.S.; Alfarraj, S.; Alharbi, S.A.; Velauthapillai, D. Solvothermal synthesis of CoMoO4 nanostructures for electrochemical applications. J. Mater. Sci. Mater. Electron. 2021, 32, 5989–6000. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.-G.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Xue, X.; Hong, G.; Wang, C. Engineering hierarchical CoSe/NiFe layered-double-hydroxide nanoarrays as high efficient bifunctional electrocatalyst for overall water splitting. J. Power Source 2019, 425, 138–146. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamani, S.; Sadeghi, E.; Unal, U.; Peighambardoust, N.S.; Aydemir, U. Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation. Catalysts 2023, 13, 798. https://doi.org/10.3390/catal13050798
Chamani S, Sadeghi E, Unal U, Peighambardoust NS, Aydemir U. Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation. Catalysts. 2023; 13(5):798. https://doi.org/10.3390/catal13050798
Chicago/Turabian StyleChamani, Sanaz, Ebrahim Sadeghi, Ugur Unal, Naeimeh Sadat Peighambardoust, and Umut Aydemir. 2023. "Tuning Electrochemical Hydrogen-Evolution Activity of CoMoO4 through Zn Incorporation" Catalysts 13, no. 5: 798. https://doi.org/10.3390/catal13050798