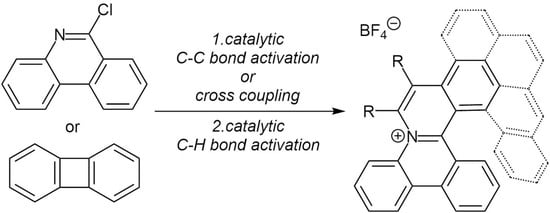
2.1. Formation of 9-Substituted Phenanthridine Derivatives by C–C Bond Activation in Biphenylene and Insertion of Nitriles
Biphenylene (
1) was synthesized according to the previously reported procedure in four steps [
19]. Our previous results demonstrated that the nitrile insertion proceeded successfully with various benzonitriles and cyanopyridines by using two Rh-based catalytic systems ([Rh(COD)
2BF
4]/dppe or [Rh(COD)Cl]
2/dppe, microwave irradiation 190 °C) [
18]. The former turned out to give better yields of the corresponding 9-aryl or 9-heteroarylphenanthridines; therefore, it was used for the subsequent C–C bond activation studies. Since our later results indicated that lowering the reaction temperature to 180 °C led to improved product yields, all C–C bond activation reactions were run by using [Rh(COD)
2BF
4] (10 mol%)/dppe (10 mol%) and microwave irradiation at 180 °C. Based on these facts, we initially studied the insertion reactions of three benzonitriles: benzonitrile (
3a), 4-trifluorobenzonitrile (
3b), and 4-methoxybenzonitrile (
3c). In all cases, we obtained the corresponding products in generally good yields (
Table 1). 6-Phenylphenanthridine (
4a), 6-(4-methoxyphenyl)phenanthridine (
4b), and 6-(4-(trifluoromethyl)phenyl)phenanthridine (
4c) were obtained in 88, 47, and 85% isolated yields, respectively. Gratifyingly, the yield of
4c was higher than the one reported by us previously [
18] and demonstrated the beneficial effect of a lower reaction temperature.
Then, we decided to explore the scope of the reaction with respect to nitriles bearing larger aromatic systems. For that purpose, we applied the above-mentioned conditions in reactions with 1-cyanonaphthalene (
3d) (
Table 2). Surprisingly, the desired product 6-(naphthalen-1-yl)phenanthridine (
4d) was isolated in 3% yield only (Entry 1). In order to improve the yield of the reaction, catalytic systems using other ligands were tested as well. When dppp was used as a ligand (Entry 2), formation of product
4d was not observed (according to a TLC analysis of the reaction mixture). The use of dppb (Entry 3) or PPh
3 (Entry 4) did not improve the reaction outcome either:
4d was isolated in only 2% yields in both cases. The change from a Rh(I) catalyst for an Ir(I) one also did not lead to an improvement in the reaction yield and
4d was isolated in only 1% yield (entry 5). According to the respective TLC analyses, the starting material remained unreacted and formation of side products in significant amounts was not detected.
Since the insertion of 1-cyanonaphthalene (
3d) into the C–C bond in biphenylene (
1) did not proceed as expected, we attempted the insertion of 2-cyanonaphthalene (
3e), which has a less sterically hindered nitrile group (
Scheme 2). Running the reaction under the same reaction conditions as with
3e provided the desired product 6-(naphthalen-2-yl)phenanthridine (
4e) in 36% isolated yield. Carrying out the reaction at a higher temperature of 200 °C allowed obtaining product
3e in 62% isolated yield. Its structure was unequivocally confirmed with single-crystal X-ray diffraction analysis (
Figure 1a).
A comparison of results obtained for the insertions of nitriles 3d and 3e indicate that the course of insertion of a nitrile after the C–C bond activation step is probably hampered by the steric hindrance of the nitrile group. Since the synthesis of larger helical cationic aza-aromatics would require the use of even more sterically hindered aromatic nitriles, we decided to change our strategy and to prepare the required phenanthridines by using cross-coupling routes.
2.3. C–H Activation/Annulation Sequence
With a series of 6-arylphenanthridines in hand, we proceeded with the C–H activation/annulation sequence with various internal alkynes. Initially, we focused on the reactions of
4a–
4c with alkynes
6a–
6c by using a catalytic system comprising [Cp*RhCl
2]
2 (5 mol%), Cu(BF
4)
2 (1.5 eq.), and O
2 at 100 °C for 24 h that was shown to be suitable for the reactions of various arylated heterocycles with sp
2-
N [
22]. However, the isolated yields of the desired products were in the range of 0–44% only (
Table S3). Further steps led to tuning the reaction conditions for a reaction of
4c with
6c (
Table S4), which otherwise did not provide any product under the standard conditions. It turned out that it was necessary to increase not only the catalyst amount but also to change both the source of tetrafluoroborate anion as well as an oxidant and a solvent. Thus, a catalytic system composed of [Cp*RhCl
2]
2 (10 mol%), AgBF
4 (1 eq.), and Cu(OAc)
2 (1 eq.) at 100 °C for 24 h in DCE (
Table S4) [
23] gave the best results. Hence, further experiments were carried out under such conditions.
Thus, the reactions of 6-phenylphenanthridine (
4a) with 1,2-diphenylethyne (
6a) and 1,2-bis(4-methoxyphenyl)ethyne (
6b) proceeded uneventfully, giving rise to 6,7-diphenylisoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate (
7aa) and 6,7-bis(4-methoxyphenyl)isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate (
7ab) in 88 and 85% isolated yields, respectively (
Table 3, Entries 1 and 2). Interestingly, a reaction with 1,2-bis(4-(trifluoromethyl)phenyl)ethyne (
6c) took place as well; however, the expected product
7ac turned out to be unstable and resisted full characterization.
The reactions of 6-(4-methoxyphenyl)phenanthridine (4b) with 6a and 6b also proceeded well and furnished 9-methoxy-6,7-diphenylisochinolino[2,1-f]phenanthridin-5-ium tetrafluoroborate (7ba) and 9-methoxy-6,7-bis(4-methoxyphenyl)isochinolino[2,1-f]phenanthridin-5-ium tetrafluoroborate (7bb) in 85 and 90% isolated yields, respectively (Entries 3 and 4). As in the previous case, a reaction with 6c provided an unstable product 7cc that defied full characterization.
Surprisingly, all reactions of 6-(4-trifluoromethylphenyl)phenanthridine (
4c) with the above-mentioned alkynes provided products
7ca–
7cc that were not stable and underwent uncontrollable degradation immediately after isolation (for details, see comments in
Supplementary Materials). In this context, it is interesting that all compounds possessing 4-trifluoromethylphenyl moieties (
7ac,
7bc, and
7ca–
7cc) were not stable under ambient conditions. The only evidence for their formation is the presence of their respective molecular peaks in MS.
Then, we turned our attention to the reactions of
4d and explored its reactivity in the C–H activation/annulation reaction with various alkynes. Initially, a reaction with diphenylethyne (
6a) under the previously used conditions ([Cp*RhCl
2]
2 (10 mol%), AgBF
4 (1 eq.), and Cu(OAc)
2 (1 eq.) in DCE at 100 °C for 24 h) was tested (
Table 4). The reaction furnished the respective product
7da (6,7-diphenylbenzo[7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate) in a rather mediocre 27% yield (Entry 1). To increase the product yield, the amounts of additives were varied. In general, these attempts led to only a marginal improvement in yields (
Table S5). Despite that, it turned out that using a catalytic system composed of [Cp*RhCl
2]
2 (10 mol%), AgBF
4 (1 eq.), Cu(OAc)
2 (1.1 eq.), and two equivalents of
6a in DCE at 100 °C for 24 h gave the product in 29% isolated yield (Entry 2). Based on the above-mentioned results, the latter conditions were used for the subsequent reactions of
4d with alkynes
6b–
6c. A reaction of with 1,2-bis(4-methoxyphenyl)ethyne (
6b) led to the formation of
7db (6,7-bis(4-methoxyphenyl)benzo[7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate), which was isolated in 31% yield (Entry 3). A reaction with 1,2-bis(4-(trifluoromethyl)phenyl)ethyne (
6c) yielded
7dc (6,7-bis(4-(trifluoromethyl)phenyl)benzo[7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate) in 21% isolated yield (Entry 4). Then, we decided to attempt a reaction with a representative of alkyl-substituted alkynes. For that purpose, we chose oct-4-yne (
6d) and its reaction gave rise to
7dd (6,7-dipropylbenzo[7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate) in 68% isolated yield (Entry 5). Perhaps, a smaller steric hindrance imparted by the
n-propyl group in the annulation step can rationalize a higher reaction yield in comparison with that obtained in reactions with diarylethynes. The structure of
7da was unequivocally confirmed with single-crystal X-ray diffraction analysis (
Figure 2a).
Next, phenanthridine
4e was tested in a C–H bond activation/annulation sequence with alkynes
6a–
6d under the previously optimized reaction conditions (
Scheme 4). The respective reactions proceeded in all cases, as can be judged by the disappearance of the starting material; however, the expected benzo[6,7]isoquinolino[2,1-
f]phenanthridin-5-ium salts
7ea–
7ed turned out to be unstable and underwent continuous decomposition during column chromatography on silica gel (or with any other isolation techniquessuch as crystallization, etc.) and they could not be isolated as analytically pure substances. Currently, it is not clear how and why the decomposition process takes place and what kind of products are formed. According to
1H NMR measurements, salts
7ea–
7ed decompose to a complex mixture of various substances. Therefore, the structures of the salts
7ea–
7ed were confirmed only through HRMS analyses.
C–H bond activation 6-(phenanthren-9-yl)phenanthridine (
4f) proved to be more challenging than in phenanthridines
4d and
4e. A reaction of
4f with diphenylethyne (
6a) under the previously optimized reaction conditions (method B,
Table 4) did not proceed as expected and the respective product was obtained only in trace amounts (according to TLC analyses) and both starting materials were reisolated in almost quantitative amounts. Attempts to modify the composition of the catalytic system were not successful and the respective product was not formed in reasonable isolable amounts (
Table S6).
Since the C–H bond activation in
4f followed by annulation with
6a was not successful, we attempted to carry out the reaction using a less sterically demanding alkyne, oct-4-yne (
6d) (
Table 5). A reaction under the optimized reaction conditions (method B,
Table 4) provided the desired product
7fd (18,19-dipropyldibenzo[5,6:7,8]isoquinolino[2,1-
f]phenanthridin-17-ium tetrafluoroborate) in a rather low 10% isolated yield (Entry 1). Attempts to improve the yield by using [Cp*IrCl
2]
2 (10 mol%) as a catalyst (Entry 2) did not fare better and
7fd was isolated in only 7% yield (Entry 2). When [Cp*Co(CO)I
2] (10 mol%) was used as a catalyst, the reaction did not proceed at all (Entry 3). The starting material was fully recovered from the reaction mixture.
Finally, 6-(phenanthren-4-yl)phenanthridine (
4g) was subjected to a catalytic C–H bond activation/annulation reaction sequence with alkynes
6a–
6d (
Table 6) under the previously optimized reaction conditions (method B,
Table 4). First, a reaction with diphenylacetylene (
6a) furnished
7ga (6,7-diphenylnaphtho[2′,1′:7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate) in a reasonable 51% isolated yield (Entry 1). Its structure possessing the [
6] helical scaffold was unequivocally confirmed with single-crystal X-ray analysis (
Figure 2b). Subsequent reactions under the same conditions with alkynes
6b–
6d provided products
7gb (6,7-bis(4-methoxyphenyl)naphtho[2′,1′:7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate),
7gc (6,7-bis(4-(trifluoromethyl)phenyl)naphtho[2′,1′:7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate), and
7gd (6,7-dipropylnaphtho[2′,1′:7,8]isoquinolino[2,1-
f]phenanthridin-5-ium tetrafluoroborate) in isolated yields of 31, 21, and 20%, respectively (Entries 2–4).