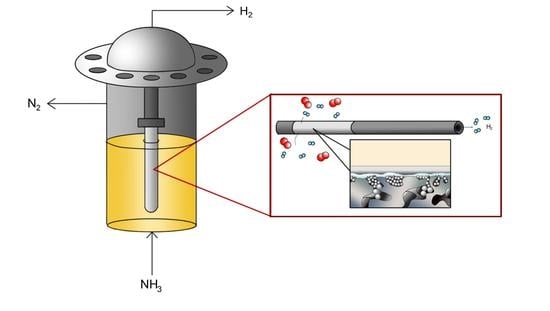
Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept
Abstract
:1. Introduction
2. Results and Discussion
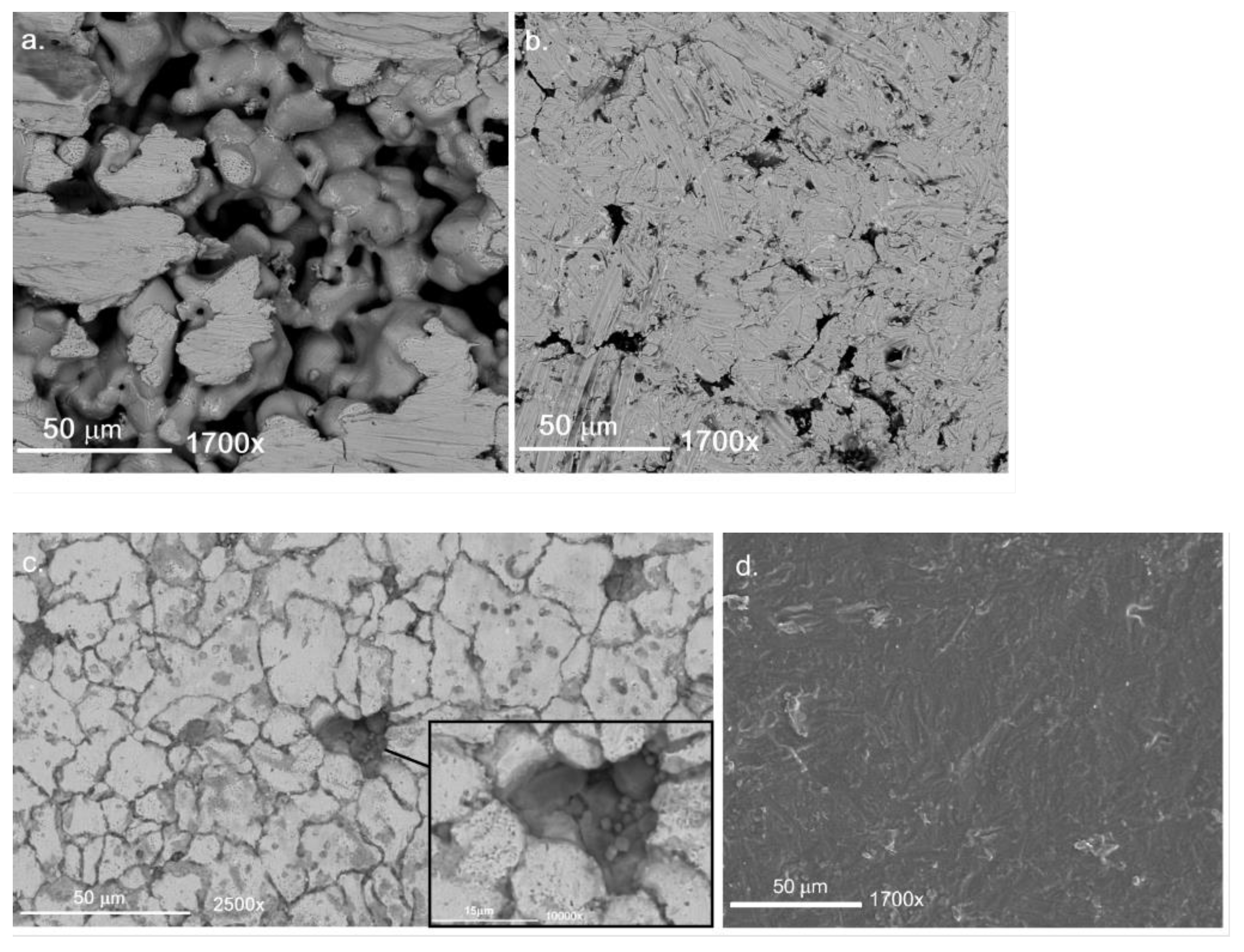
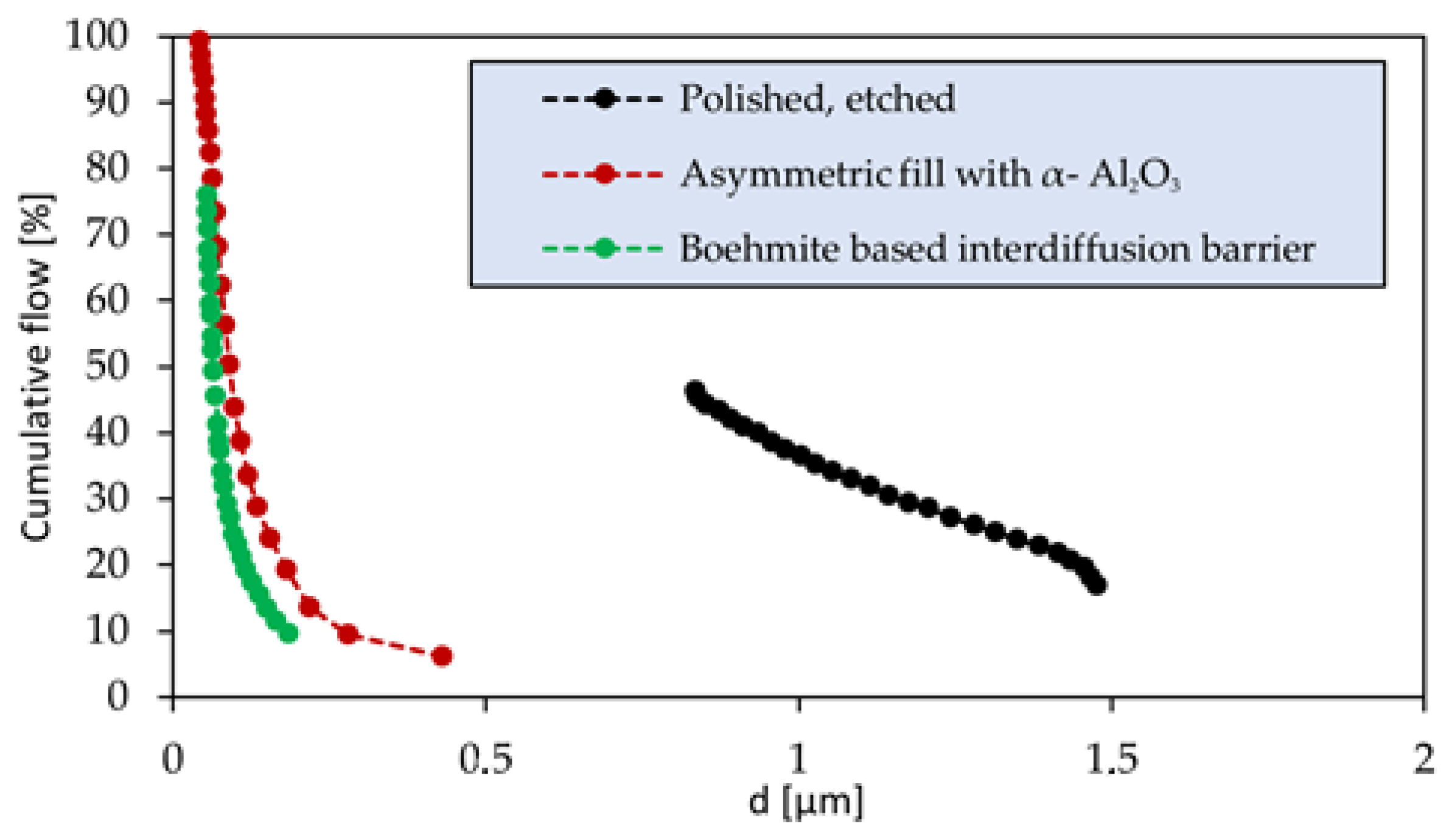
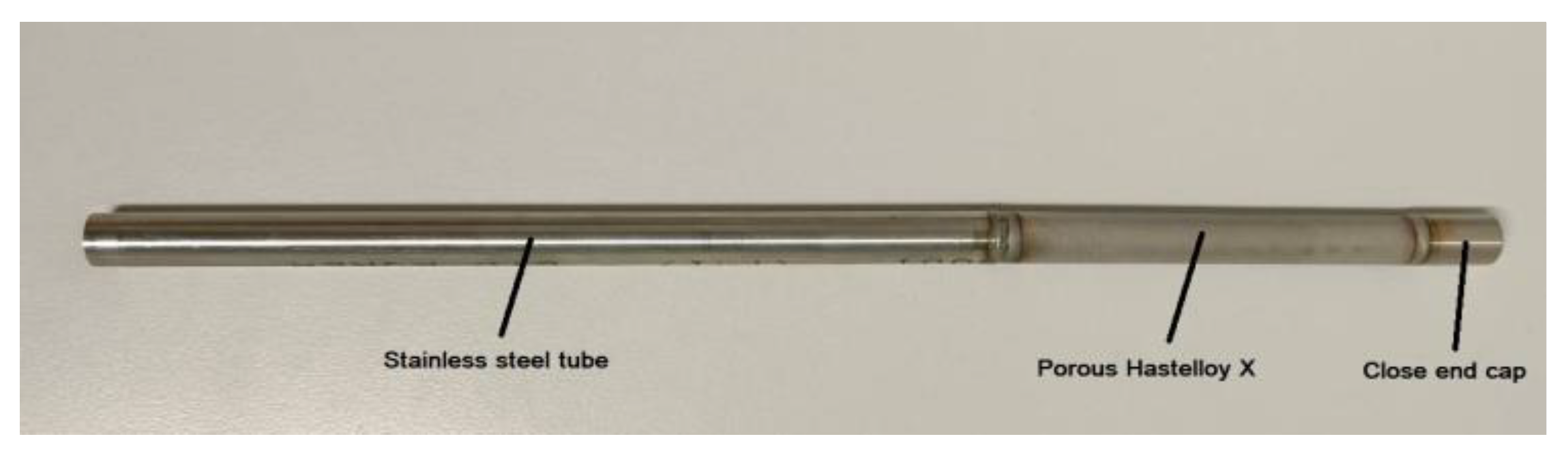
2.1. Membrane Preparation
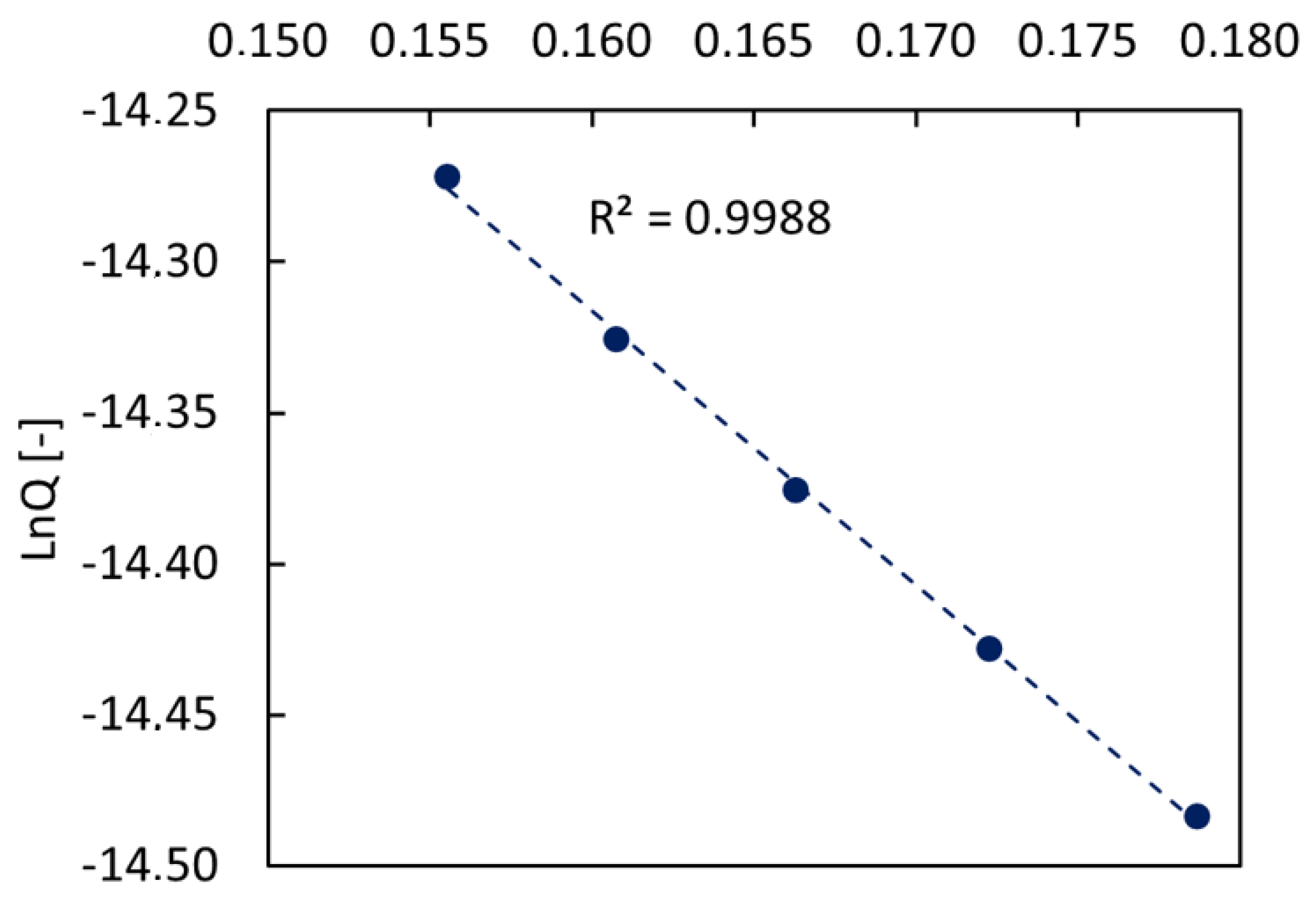
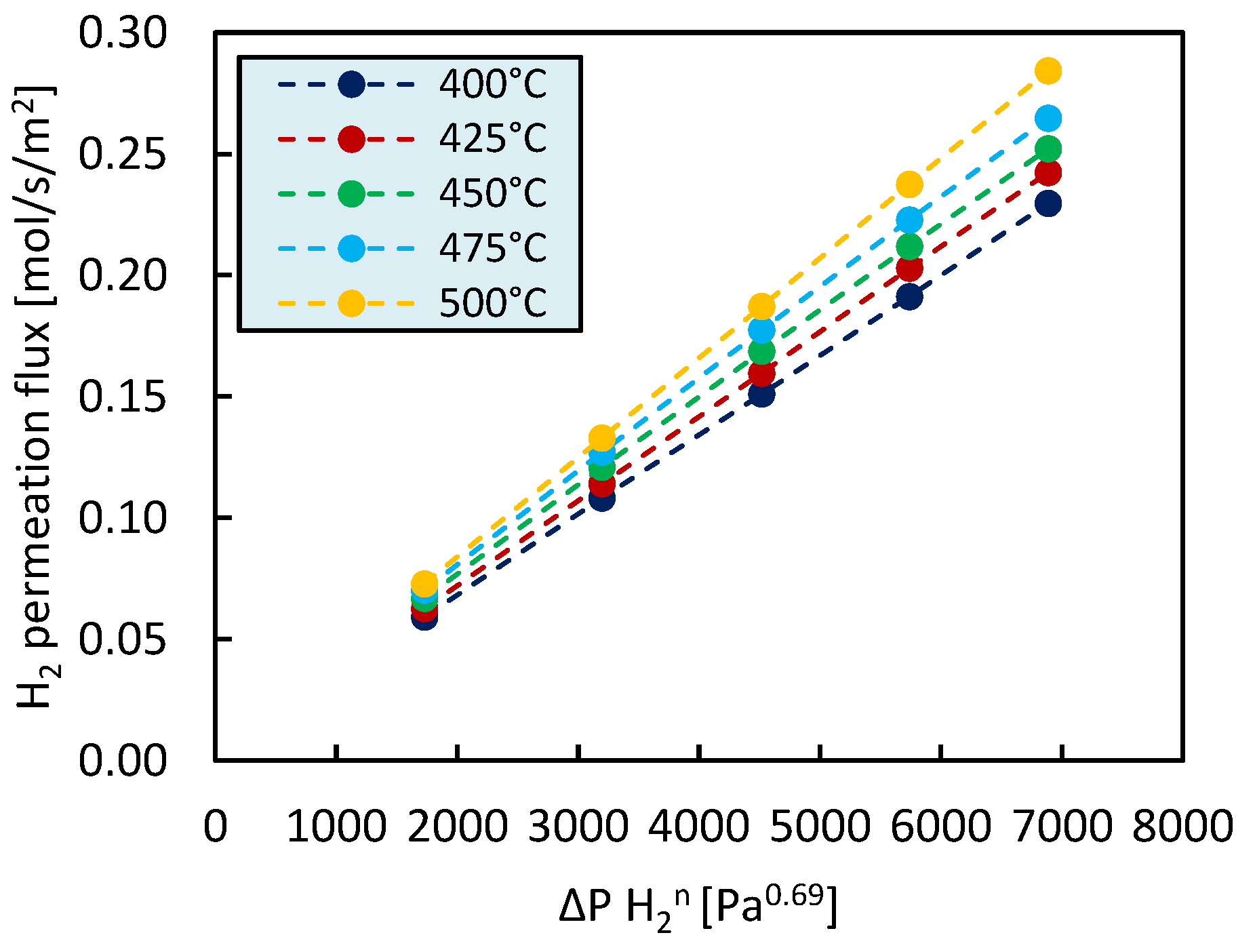
2.2. Single Gas Permeation Tests
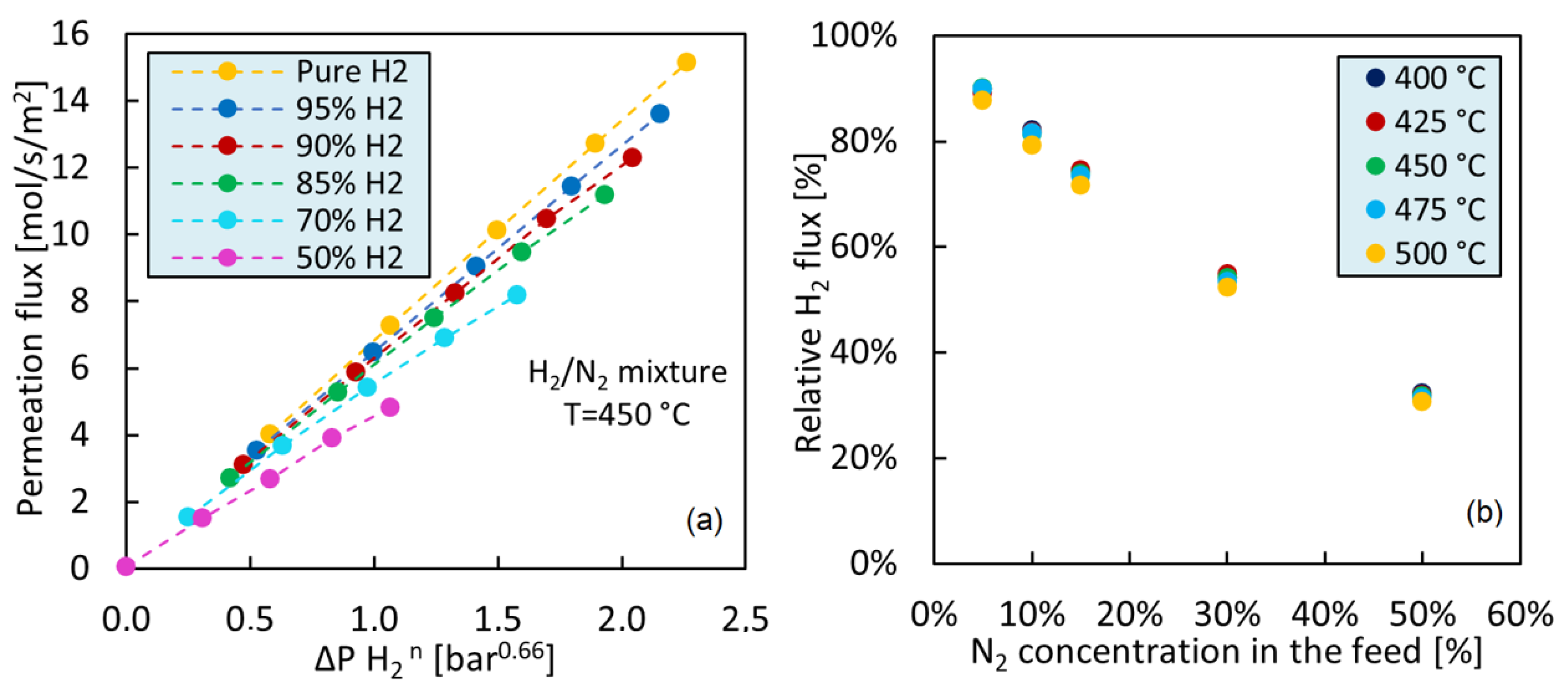
2.3. Binary Mixture (H2/N2) Permeation Tests
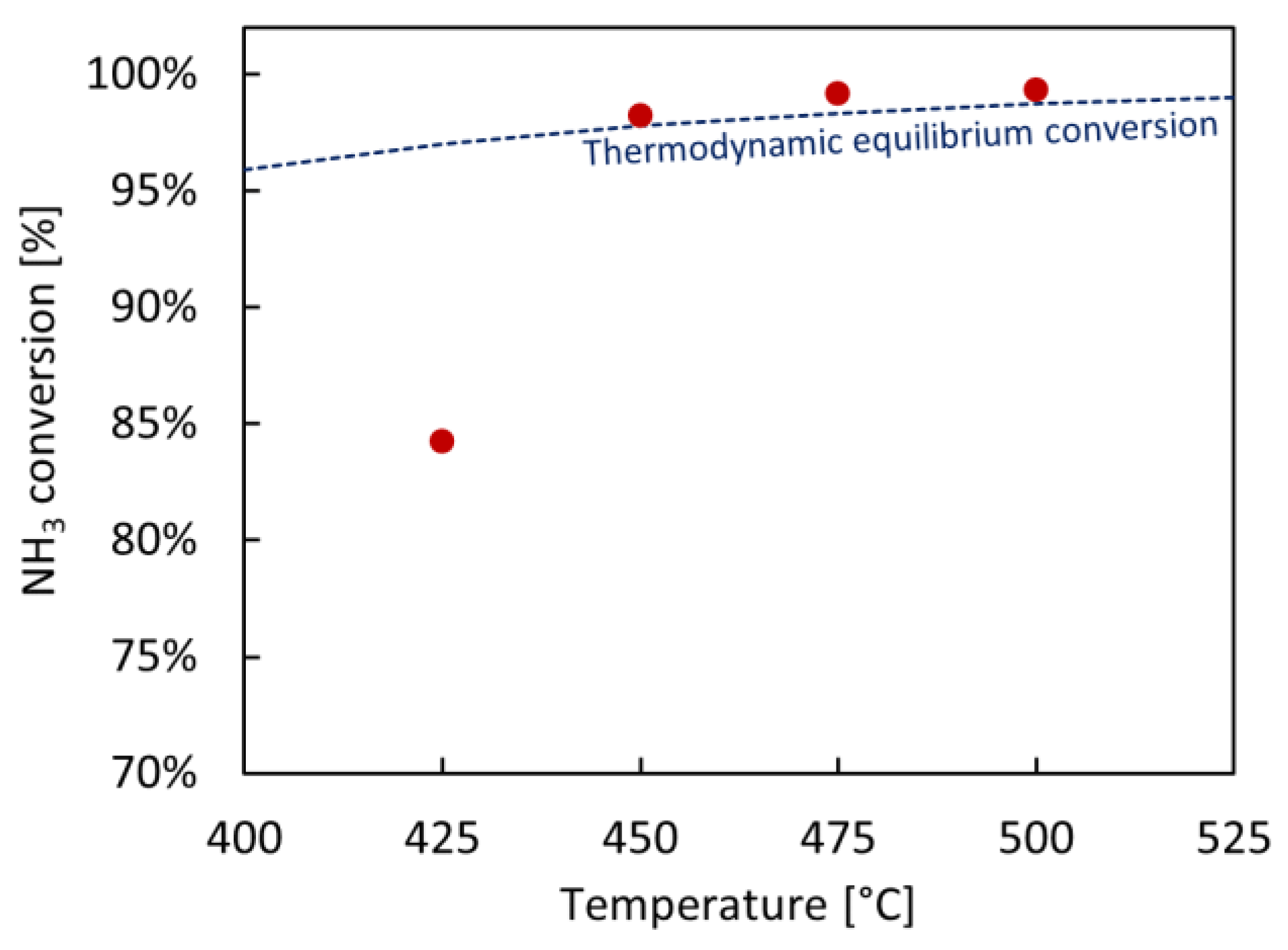
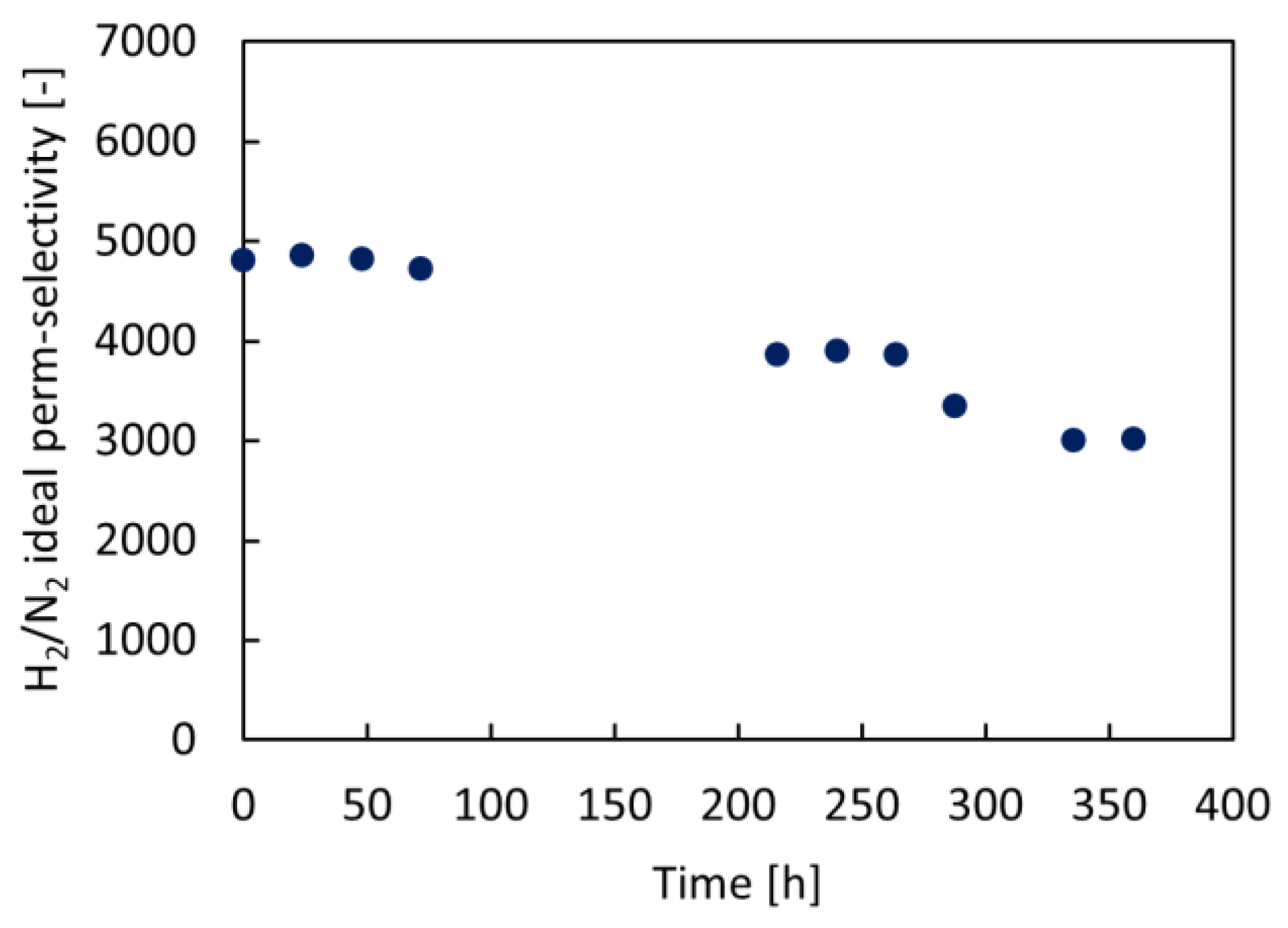
2.4. Permeation Tests under Reactive Conditions
3. Materials and Methods
3.1. Membrane Preparation
- The rough filter was polished in an industrial surface finishing machine via wet-polishing mechanism (ERBA EVT-170) for 6 h, delivering a suitable trade-off between surface roughness reduction and gas permeation preservation [42]. The polished support was then vertically submerged in aqua regia for 30 s, to recover the lost superficial porosity. After the acid attack, the filter was thoroughly rinsed with deionized water to remove all mordant residuals. An oxidation in static air atmosphere was then performed in a furnace for 1 h at 750 °C, with heating rate 2 °C/min.
- The support’s superficial pore size was improved by filling asymmetrically with α-Al2O3 of decreasing particle size via immersion in a magnetically stirred α-Al2O3-H2O suspension, improved by addition of HNO3 (67 vol.%) dropwise. The filler was pulled through the superficial pores via vacuum-assisted dip coating, with a lower wait time of 60 s per immersion cycle. Between each cycle the support was gently rinsed with distilled water. 20 aspiration cycles were performed with alumina 18 µm (AA-18, Sumitomo), 10 aspiration cycles with alumina 5 µm (AA-5, Sumitomo) and 10 with alumina 1.5 µm (AA-1, Sumitomo).
- Finally, a mesoporous smoothening interdiffusion barrier was deposited to complete the improvement of support’s surface uniformity. A solution with boehmite loading 0.9 wt.% was prepared in distilled water, incorporating a water-based solution of organic additives, namely 3.5 wt.% polyvinyl alcohol (PVA) (MW 130,000) and 1 wt.% polyethylene glycol (PEG) (MW 400). The deposited layer was dried under rotation in a climate chamber at 40 °C and 60% relative humidity for 1 h and sintered for 1 h at 550 °C in a static air furnace.
3.2. Membrane Characterizations
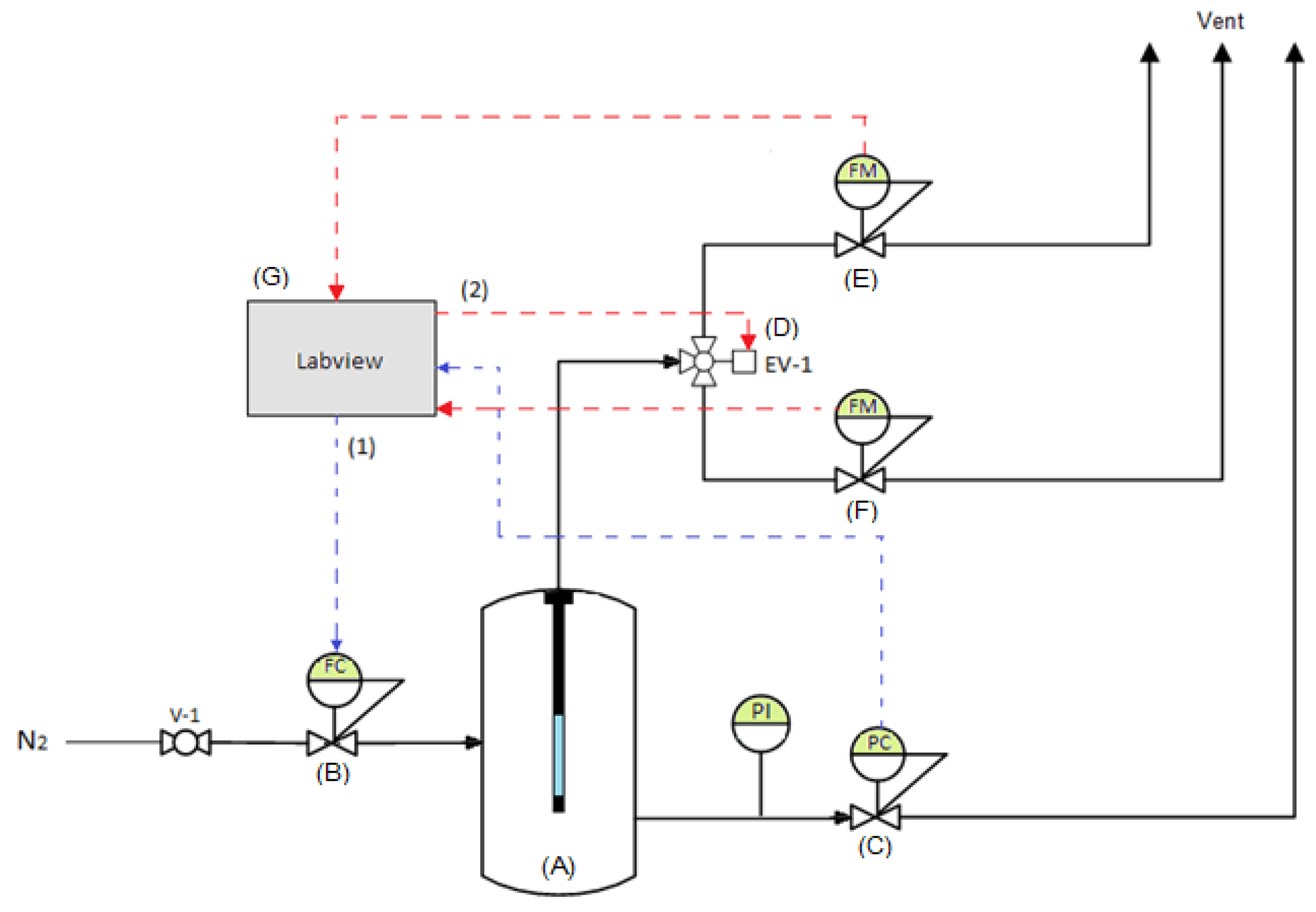
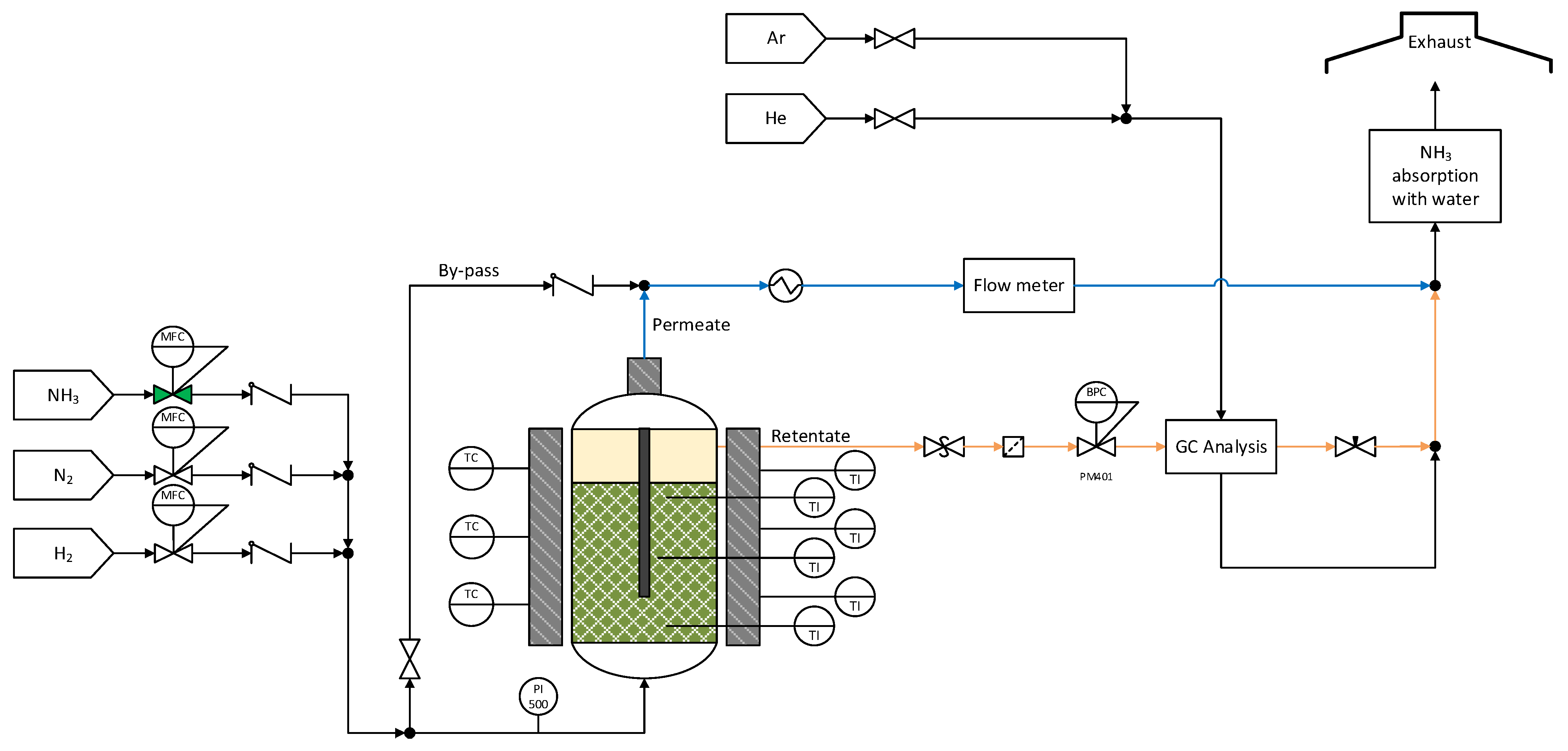
3.3. Experimental Setup for Permeation and Ammonia Decomposition
3.4. Experimental Methods
3.4.1. Single Gas (H2 and N2) Permeation Tests
3.4.2. Binary Mixture (H2/N2) Permeation Tests
3.4.3. Permeation Tests under Reactive Conditions
4. Conclusions
Author Contributions
Funding

Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
References
- Dell, R.M.; Bridger, N.J. Hydrogen—The ultimate fuel. Appl. Energy 1975, 1, 279–292. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Bockris, J.O.M. The hydrogen economy: Its history. Int. J. Hydrog. Energy 2013, 38, 2579–2588. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The energy source for the 21st century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar]
- Lan, R.; Tao, S. Ammonia as a Suitable Fuel for Fuel Cells. Front. Energy Res. 2014, 2, 35. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrog. Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.; Nørskov, J.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; De Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Cha, J.; Jo, Y.S.; Jeong, H.; Han, J.; Nam, S.W.; Song, K.H.; Yoon, C.W. Ammonia as an efficient COX-free hydrogen carrier: Fundamentals and feasibility analyses for fuel cell applications. Appl. Energy 2018, 224, 194–204. [Google Scholar] [CrossRef]
- Sørensen, R.Z.; Nielsen, L.J.; Jensen, S.; Hansen, O.; Johannessen, T.; Quaade, U.; Christensen, C.H. Catalytic ammonia decomposition: Miniaturized production of COx-free hydrogen for fuel cells. Catal. Commun. 2005, 6, 229–232. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Highly enhanced ammonia decomposition in a bimodal catalytic membrane reactor for COx-free hydrogen production. Catal. Commun. 2011, 15, 60–63. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Li, W. High-purity COx-free H2 generation from NH3 via the ultra permeable and highly selective Pd membranes. J. Memb. Sci. 2006, 277, 85–93. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia decomposition kinetics over Ni-Pt/Al2O3 for PEM fuel cell applications. Appl. Catal. A Gen. 2002, 227, 231–240. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, Y.; Kim, H.W.; Lee, S.U.; Kim, J.R.; Kim, T.W.; Lee, Y.J.; Chae, H.J. Ru-supported lanthania-ceria composite as an efficient catalyst for COx-free H2 production from ammonia decomposition. Appl. Catal. B Environ. 2021, 285, 119831. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Liguori, S.; Fuerst, T.F.; Way, J.D.; Wolden, C.A. Efficient Ammonia Decomposition in a Catalytic Membrane Reactor to Enable Hydrogen Storage and Utilization. ACS Sustain. Chem. Eng. 2019, 7, 5975–5985. [Google Scholar] [CrossRef]
- Itoh, N.; Oshima, A.; Suga, E.; Sato, T. Kinetic enhancement of ammonia decomposition as a chemical hydrogen carrier in palladium membrane reactor. Catal. Today 2014, 236, 70–76. [Google Scholar] [CrossRef]
- Itoh, N.; Kikuchi, Y.; Furusawa, T.; Sato, T. Tube-wall catalytic membrane reactor for hydrogen production by low-temperature ammonia decomposition. Int. J. Hydrog. Energy 2020, 46, 20257–20265. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Medrano, J.A.; Makhloufi, C.; Zuniga, J.; Gallucci, F. H2 production via ammonia decomposition in a catalytic membrane reactor. Fuel Process. Technol. 2021, 216, 106772. [Google Scholar] [CrossRef]
- Rizzuto, E.; Palange, P.; Del Prete, Z. Characterization of an ammonia decomposition process by means of a multifunctional catalytic membrane reactor. Int. J. Hydrog. Energy 2014, 39, 11403–11410. [Google Scholar] [CrossRef]
- Israni, S.H.; Nair, B.K.R.; Harold, M.P. Hydrogen generation and purification in a composite Pd hollow fiber membrane reactor: Experiments and modeling. Catal. Today 2009, 139, 299–311. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Lee, H.R.; Maeda, M.; Yoshioka, T.; Tsuru, T. Preparation of a novel bimodal catalytic membrane reactor and its application to ammonia decomposition for COx-free hydrogen production. Int. J. Hydrog. Energy 2012, 37, 12105–12113. [Google Scholar] [CrossRef]
- Park, Y.; Cha, J.; Oh, H.T.; Lee, T.; Lee, S.H.; Park, M.G.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; et al. A catalytic composite membrane reactor system for hydrogen production from ammonia using steam as a sweep gas. J. Memb. Sci. 2020, 614, 118483. [Google Scholar] [CrossRef]
- Jo, Y.S.; Cha, J.; Lee, C.H.; Jeong, H.; Yoon, C.W.; Nam, S.W.; Han, J. A viable membrane reactor option for sustainable hydrogen production from ammonia. J. Power Sources 2018, 400, 518–526. [Google Scholar] [CrossRef]
- Liu, J.; Ju, X.; Tang, C.; Liu, L.; Li, H.; Chen, P. High performance stainless-steel supported Pd membranes with a finger-like and gap structure and its application in NH3 decomposition membrane reactor. Chem. Eng. J. 2020, 388, 124245. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; McCullough, K.; Lauterbach, J.; Li, S.; Yu, M. Novel hollow fiber membrane reactor for high purity H2 generation from thermal catalytic NH3 decomposition. J. Memb. Sci. 2021, 629, 119281. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Martinez, R.G.; Plazaola, A.A.; Gallucci, F. Ultra-pure hydrogen production via ammonia decomposition in a catalytic membrane reactor. Int. J. Hydrog. Energy 2022, 47, 21220–21230. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Morlanés, N.; Kulkarni, S.R.; Realpe, N.; Ramírez, A.; Katikaneni, S.P.; Paglieri, S.N.; Lee, K.; Harale, A.; Solami, B.; et al. High purity, self-sustained, pressurized hydrogen production from ammonia in a catalytic membrane reactor. Chem. Eng. J. 2022, 431, 134310. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, E.H.; Byun, S.; Seo, D.W.; Hwang, H.J.; Yoon, H.C.; Kim, H.; Ryi, S.K. Highly selective Pd composite membrane on porous metal support for high-purity hydrogen production through effective ammonia decomposition. Energy 2022, 260, 125209. [Google Scholar] [CrossRef]
- Sitar, R.; Shah, J.; Zhang, Z.; Wikoff, H.; Way, J.D.; Wolden, C.A. Compact ammonia reforming at low temperature using catalytic membrane reactors. J. Memb. Sci. 2022, 644, 120147. [Google Scholar] [CrossRef]
- Omata, K.; Sato, K.; Nagaoka, K.; Yukawa, H.; Matsumoto, Y.; Nambu, T. Direct high-purity hydrogen production from ammonia by using a membrane reactor combining V-10mol%Fe hydrogen permeable alloy membrane with Ru/Cs2O/Pr6O11 ammonia decomposition catalyst. Int. J. Hydrog. Energy 2022, 47, 8372–8381. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of supported pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef]
- Nooijer, N.D.; Arratibel Plazaola, A.; Meléndez Rey, J.; Fernandez, E.; Pacheco Tanaka, D.A.; Sint Annaland, M.V.; Gallucci, F. Long-term stability of thin-film Pd-based supported membranes. Processes 2019, 7, 106. [Google Scholar] [CrossRef]
- Straczewski, G.; Völler-Blumenroth, J.; Beyer, H.; Pfeifer, P.; Steffen, M.; Felden, I.; Heinzel, A.; Wessling, M.; Dittmeyer, R. Development of thin palladium membranes supported on large porous 310L tubes for a steam reformer operated with gas-to-liquid fuel. Chem. Eng. Process. Process Intensif. 2014, 81, 13–23. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Soria, M.A.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Modifications of Porous Stainless Steel Previous to the Synthesis of Pd Membranes; Elsevier Masson SAS: Issy-les-Moulineaux, France, 2010; Volume 175. [Google Scholar]
- Do, H.Y.; Kim, C.H.; Han, J.Y.; Kim, H.S.; Ryi, S.K. Low-temperature proton-exchange membrane fuel cell-grade hydrogen production by membrane reformer equipped with Pd-composite membrane and methanation catalyst on permeation stream. J. Memb. Sci. 2021, 634, 119373. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Fletcher, S.; Speight, J.D.; Book, D. Hydrogen permeation through porous stainless steel for palladium-based composite porous membranes. J. Memb. Sci. 2016, 515, 22–28. [Google Scholar] [CrossRef]
- Agnolin, S.; Melendez, J.; Di Felice, L.; Gallucci, F. Surface roughness improvement of Hastelloy X tubular filters for H2 selective supported Pd–Ag alloy membranes preparation. Int. J. Hydrog. Energy 2022, 47, 28505–28517. [Google Scholar] [CrossRef]
- Nam, S.E.; Lee, K.H. Hydrogen separation by Pd alloy composite membranes: Introduction of diffusion barrier. J. Memb. Sci. 2001, 192, 177–185. [Google Scholar] [CrossRef]
- Medrano, J.A.; Fernandez, E.; Melendez, J.; Parco, M.; Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. Pd-based metallic supported membranes: High-temperature stability and fluidized bed reactor testing. Int. J. Hydrog. Energy 2016, 41, 8706–8718. [Google Scholar] [CrossRef]
- Bottino, A.; Broglia, M.; Capannelli, G.; Comite, A.; Pinacci, P.; Scrignari, M.; Azzurri, F. Sol-gel synthesis of thin alumina layers on porous stainless steel supports for high temperature palladium membranes. Int. J. Hydrog. Energy 2014, 39, 4717–4724. [Google Scholar] [CrossRef]
- Xu, N.; Ryi, S.; Li, A.; Grace, J.R.; Lim, J.; Boyd, T. Improved pre-treatment of porous stainless steel substrate for preparation of Pd-based composite membrane. Can. J. Chem. Eng. 2013, 91, 1695–1701. [Google Scholar] [CrossRef]
- Tong, J.; Matsumura, Y.; Suda, H.; Haraya, K. Thin and dense Pd/CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane. Sep. Purif. Technol. 2005, 46, 1–10. [Google Scholar] [CrossRef]
- Qiao, A.; Zhang, K.; Tian, Y.; Xie, L.; Luo, H.; Lin, Y.S.; Li, Y. Hydrogen separation through palladium–copper membranes on porous stainless steel with sol–gel derived ceria as diffusion barrier. Fuel 2010, 89, 1274–1279. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; van Sint Annaland, M.; Gallucci, F.; Tanaka, D.P. Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Tarditi, A.; Gerboni, C.; Cornaglia, L. PdAu membranes supported on top of vacuum-assisted ZrO2-modified porous stainless steel substrates. J. Memb. Sci. 2013, 428, 1–10. [Google Scholar] [CrossRef]
- Chotirach, M.; Tantayanon, S.; Tungasmita, S.; Kriausakul, K. Zr-based intermetallic diffusion barriers for stainless steel supported palladium membranes. J. Memb. Sci. 2012, 405–406, 92–103. [Google Scholar] [CrossRef]
- Yepes, D.; Cornaglia, L.M.; Irusta, S.; Lombardo, E.A. Different oxides used as diffusion barriers in composite hydrogen permeable membranes. J. Memb. Sci. 2006, 274, 92–101. [Google Scholar] [CrossRef]
- Chen, C.H.; Ma, Y.H. The effect of H2S on the performance of Pd and Pd/Au composite membrane. J. Memb. Sci. 2010, 362, 535–544. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Fernandez, E.; Sanchez-Garcia, J.A.; Melendez, J.; Spallina, V.; van Sint Annaland, M.; Gallucci, F.; Tanaka, D.P.; Prema, R. Development of highly permeable ultra-thin Pd-based supported membranes. Chem. Eng. J. 2016, 305, 149–155. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Park, J.-S.; Kim, S.-H.; Cho, S.-H.; Kim, D.-W. The effect of support resistance on the hydrogen permeation behavior in Pd–Cu–Ni ternary alloy membrane deposited on a porous nickel support. J. Memb. Sci. 2006, 280, 883–888. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. On the high pressure performance of thin supported Pd–23%Ag membranes—Evidence of ultrahigh hydrogen flux after air treatment. J. Memb. Sci. 2011, 378, 28–34. [Google Scholar] [CrossRef]
- Agnolin, S.; Apostolo, F.; Di Felice, L.; Rey, J.M.; Tanaka, A.P.; Tanco, M.L.; Gallucci, F. Development of selective Pd–Ag membranes on porous metal filters. Int. J. Hydrog. Energy 2023. [Google Scholar] [CrossRef]
- ISO 14687:2019; Hydrogen Fuel Quality—Product Specification. ISO: Geneva, Switzerland, 2019.
- Choudhary, T.; Sivadinarayana, C.; Goodman, D.W. Catalytic ammonia decomposition: COx-free hydrogen production for fuel cell applications. Catal. Lett. 2001, 72, 197–201. [Google Scholar] [CrossRef]
- AS, C. Compact fuel processors for PEM fuel cells. Fuel 2002, 10000, 12000. [Google Scholar]
- Alagharu, V.; Palanki, S.; West, K.N. Analysis of ammonia decomposition reactor to generate hydrogen for fuel cell applications. J. Power Sources 2010, 195, 829–833. [Google Scholar] [CrossRef]
- Luna, B.; Somi, G.; Winchester, J.; Grose, J.; Mulloth, L.; Perry, J. Evaluation of Commercial Off-the-Shelf Sorbents & Catalysts for Control of Ammonia and Carbon Monoxide. In Proceedings of the 40th International Conference on Environmental Systems, Barcelona, Spain, 11–15 July 2010. [Google Scholar]
- Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Ichikawa, T.; Kojima, Y. Highly purified hydrogen production from ammonia for PEM fuel cell. Int. J. Hydrog. Energy 2018, 43, 14486–14492. [Google Scholar] [CrossRef]
- Ouyang, W.; Zheng, S.; Wu, C.; Hu, X.; Chen, R.; Zhuo, L.; Wang, Z. Dynamic ammonia adsorption by FAU zeolites to below 0.1 ppm for hydrogen energy applications. Int. J. Hydrog. Energy 2021, 46, 32559–32569. [Google Scholar] [CrossRef]
- Cechetto, V.; Struijk, C.L.; Di Felice, L.; de Leeuw den Bouter, A.W.N.; Gallucci, F. Adsorbents development for hydrogen cleanup from ammonia decomposition in a catalytic membrane reactor. Chem. Eng. J. 2022, 455, 140762. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Preparation of ‘pore-fill’ type Pd-YSZ-γ-Al2O3 composite membrane supported on α-Al2O3 tube for hydrogen separation. J. Memb. Sci. 2008, 320, 436–441. [Google Scholar] [CrossRef]
- Tanaka, D.A.; Tanco, M.A.; Niwa, S.I.; Wakui, Y.; Mizukami, F.; Namba, T.; Suzuki, T.M. Preparation of palladium and silver alloy membrane on a porous α-alumina tube via simultaneous electroless plating. J. Memb. Sci. 2005, 247, 21–27. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Bredesen, R. Effects of thermal activation on hydrogen permeation properties of thin, self-supported Pd/Ag membranes. Sep. Purif. Technol. 2009, 68, 403–410. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. The effect of air exposure on palladium–copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]


| Temperature [°C] | H2 Permeance [mol/s/m2/Pa] | N2 Permeance [mol/s/m2/Pa] | H2/N2 Ideal Perm-Selectivity [-] |
|---|---|---|---|
| 400 | 5.8 × 10−7 | 1.1 × 10−10 | 5287 |
| 450 | 6.6 × 10−7 | 1.1 × 10−10 | 5892 |
| 500 | 7.3 × 10−7 | 1.9 × 10−11 | 38,839 |
| Conventional System | Membrane Reactor | ||
|---|---|---|---|
| Temperature [°C] | Thermodynamic Equilibrium Conversion [%] | NH3 Conversion [%] | H2 Recovery [%] |
| 425 | 97.0 | 84.2 | 37.6 |
| 450 | 97.8 | 98.2 | 55.5 |
| 475 | 98.3 | 99.2 | 60.7 |
| 500 | 98.7 | 99.3 | 62.9 |
| Reaction pressure = 5 bar, NH3 feed flow rate = 0.5 LN/min | |||
| Conventional System | Membrane Reactor | ||
|---|---|---|---|
| Pressure [bar] | Thermodynamic Equilibrium Conversion [%] | NH3 Conversion [%] | H2 Recovery [%] |
| 3 | 99.0 | 99.2 | 42.7 |
| 4 | 98.7 | 99.2 | 51.9 |
| 5 | 98.3 | 99.2 | 60.7 |
| 6 | 98.0 | 99.1 | 66.1 |
| Reaction temperature = 475 °C, NH3 feed flow rate = 0.5 LN/min | |||
| This Work | Cechetto et al. [23] | |
|---|---|---|
| Membrane | ||
| Configuration | Supported tubular Pd-based membrane | Supported tubular Pd-based membrane with a porous Al2O3-YSZ protective layer |
| Support | Metallic (Hastelloy X) | Ceramic (Al2O3) |
| Selective layer composition | Pd-Ag | Pd-Ag |
| Selective layer thickness [μm] | 6–8 | 6–8 |
| Length [mm] | 90 | 195 |
| H2 permeance at 450 °C and 1 bar [mol/s/m2/Pa] | 6.6 × 10−7 | 1.2 × 10−6 |
| H2/N2 ideal perm-selectivity at 450 °C and 1 bar [-] | 5890 | 68,960 |
| NH3 conversion [%] * | ||
| T = 425 °C | 84.2 | 96.5 |
| T = 450 °C | 98.2 | 99.7 |
| T = 475 °C | 99.2 | 99.8 |
| T = 500 °C | 99.3 | 99.8 |
| H2 recovery [%] * | ||
| T = 425 °C | 37.6 | 79.5 |
| T = 450 °C | 55.5 | 87.5 |
| T = 475 °C | 60.7 | 88.9 |
| T = 500 °C | 62.9 | 88.9 |
| MEMBRANE | REACTOR OPERATING CONDITONS | CATALYST | PERFORMANCE MR | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selective Layer Composition | Selective Layer Thickness | Length [mm] | Type of Support | Support Material (Thickness) | Temperature [°C] | Reaction Pressure [bar] | Permeate Pressure [bar] | GHSV [mL/(gcat h)] | NH3 Conversion [%] | H2 Recovery [%] | ||
| Pd | 6.2 | N/A | Ceramic | YSZ (130 mm) | 400 | 5 | 1 | N/A | Ru (impregnated in the membrane support) | 98 | 87.5 | [20] |
| Pd | 3 | N/A | Ceramic | Al2O3 (N/A) | 500 | 5 | 1 | 2000 | Ni/La-Al2O3—6 g | >99 * | 92 * | [15] |
| Pd-Ag | 6–8 | 195 | Ceramic | α-Al2O3 (3.5 mm) | 500 | 6 | 1 | 120 ** | (2 wt.%) Ru/Al2O3—250 g | 99.8 | 91.6 | [31] |
| Pd-Ag | 4.61 | 202 | Ceramic | α-Al2O3 (2 mm) | 400 | 4 | Vacuum | 120 ** | (2 wt.%) Ru/Al2O3—250 g | 99.3 | 93.5 | [23] |
| Pd-Au | 8 | 186 (*) | Ceramic | N/A | 485 | 5 | 1 | 1200 ** | (0.5 wt.%) Ba-CoCe—10 g | >99 * | 92 * | [32] |
| Pd | 6.5 | N/A | Metallic | Stainless steel (+MnCO3) | 400 | 3 | 1 | 1880 ** | (5 wt.%) Ru/MgO—1.5 g | 99.8 | N/A | [29] |
| Pd-Ag | 1.8 | 100 (*) | Ceramic | N/A | 450 | 7 | 1 | 5000 | (3 wt.%) Ru/Y/K/Al2O3—3 g | 99.11 | 90.6 | [30] |
| Pd | 200 | 65 | NS | N/A | 450 | 1 | Vacuum | 1164 ** | (5 wt.%) Ru/SiO2—0.5 g | 87 * | 59 * | [21] |
| Pd | 2 | 90 | Ceramic | α-Al2O3 (N/A) | 375 | 1 | Vacuum | 680 ** | (2 wt.%) Ru/Al2O3—0.88 g | >99 * | - | [22] |
| Pd | 5 | 450 | Metallic | Inconel 600 (N/A) | 430 | 5 | Vacuum | N/A | (2 wt.%) Ru/Al2O3—200 ml | 99.4 | 97.5 | [33] |
| Pd-Ag/V-Fe | ~0.2 µm Pd-Ag ~100 µm V-Fe | N/A | Metallic | V-10 mol.%-Fe alloy (~100 µm V-Fe) | 350 | 3 | 1 | 3000 ** | (5 wt.%) Ru/Cs2O/Pr6O11—0.2 g | 89 * | 89 * | [35] |
| Pd/Ta | ~ 0.4 µm Pd ~250 µm Ta | N/A | Metallic | Tantalum (~250 µm) | 450 | 6.5 | 1 | 30,000 | (1.6 wt.%) Ru/La-Al2O3—6 g | >99.5 | N/A | [28] |
| Pd/Ta/Pd | 1–2 µm Pd ~250 µm Ta | N/A | Metallic | Tantalum (250 µm) | 500 | 5 | 1 | 6000 | (0.65 wt.%) Ru/La-Al2O3—1 g | 95 * | 86 * | [27] |
| Pd | 13 | 156 | Ceramic | α-Al2O3 (0.5 mm) | 500 | 3 | 1 | 135 ** | (70 wt.% ) Ni/γ-Al2O3—29 g | 99 * | 80 * | [25] |
| Pd | 4.23 | 73 | Ceramic | YSZ (130 mm) | 450 | 5 | 1 | 1200 ** | (0.5 wt.%) Ru/Al2O3—5 g in the catalyst bed (1.9 wt.%) Ru/YSZ—Ru impregnated in the membrane support | >99 | >90 | [34] |
| Pd | N/A | N/A | Metallic | N/A | 450 | 5 | 1 | N/A | (-) Ru/Al2O3—N/A | >99 * | 91 * | [24] |
| Pd-Ag | 6–8 | 90 | Metallic | Hastelloy X | 500 | 5 | 1 | 1200 | (2 wt.%) Ru/Al2O3—250 g | 99.1 | 66.1 | This study |
| Single gas permeation tests | |
| Single gases investigated | H2, N2 |
| Temperature [°C] | 400, 425, 450, 475, 500 |
| Retentate pressure [bar] | 2, 3, 4, 5, 6 |
| Permeate pressure [bar] | 1 |
| Binary mixture permeation tests | |
| Binary mixture | H2/N2 |
| Temperature [°C] | 400, 425, 450, 475, 500 |
| Retentate pressure [bar] | 2, 3, 4, 5, 6 |
| Permeate pressure [bar] | 1 |
| Ammonia decomposition | |
| Temperature [°C] | 425, 450, 475, 500 |
| Retentate pressure [bar] | 3, 4, 5, 6 |
| Permeate pressure [bar] | 1 |
| NH3 feed flow rate [LN/min] | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cechetto, V.; Agnolin, S.; Di Felice, L.; Pacheco Tanaka, A.; Llosa Tanco, M.; Gallucci, F. Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept. Catalysts 2023, 13, 920. https://doi.org/10.3390/catal13060920
Cechetto V, Agnolin S, Di Felice L, Pacheco Tanaka A, Llosa Tanco M, Gallucci F. Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept. Catalysts. 2023; 13(6):920. https://doi.org/10.3390/catal13060920
Chicago/Turabian StyleCechetto, Valentina, Serena Agnolin, Luca Di Felice, Alfredo Pacheco Tanaka, Margot Llosa Tanco, and Fausto Gallucci. 2023. "Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept" Catalysts 13, no. 6: 920. https://doi.org/10.3390/catal13060920