Dual-Doping Strategy for Enhancing Hydrogen Evolution on Molybdenum Carbide Catalysts
Abstract
:1. Introduction
2. Results and Discussion
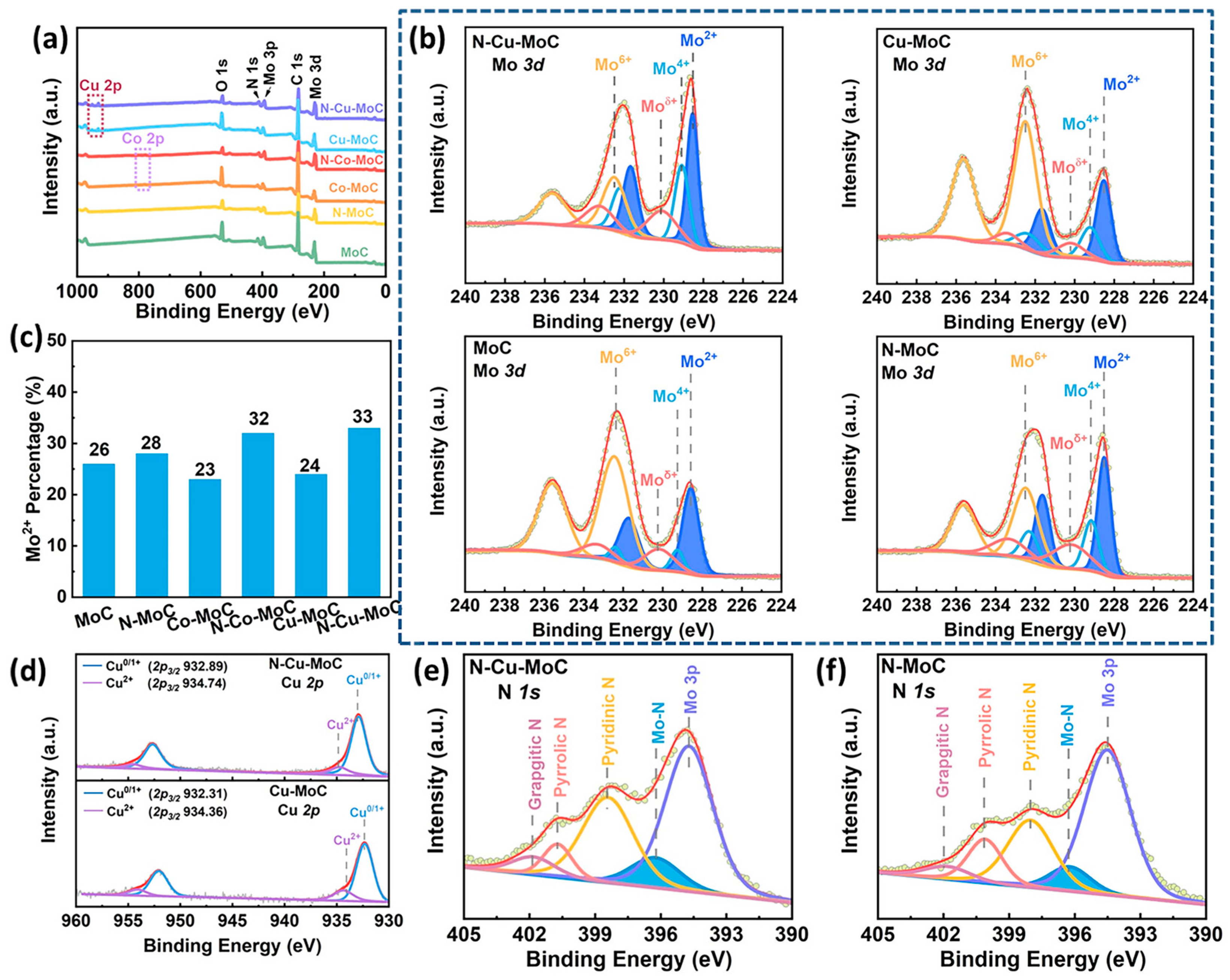
2.1. Catalyst Structure Investigation
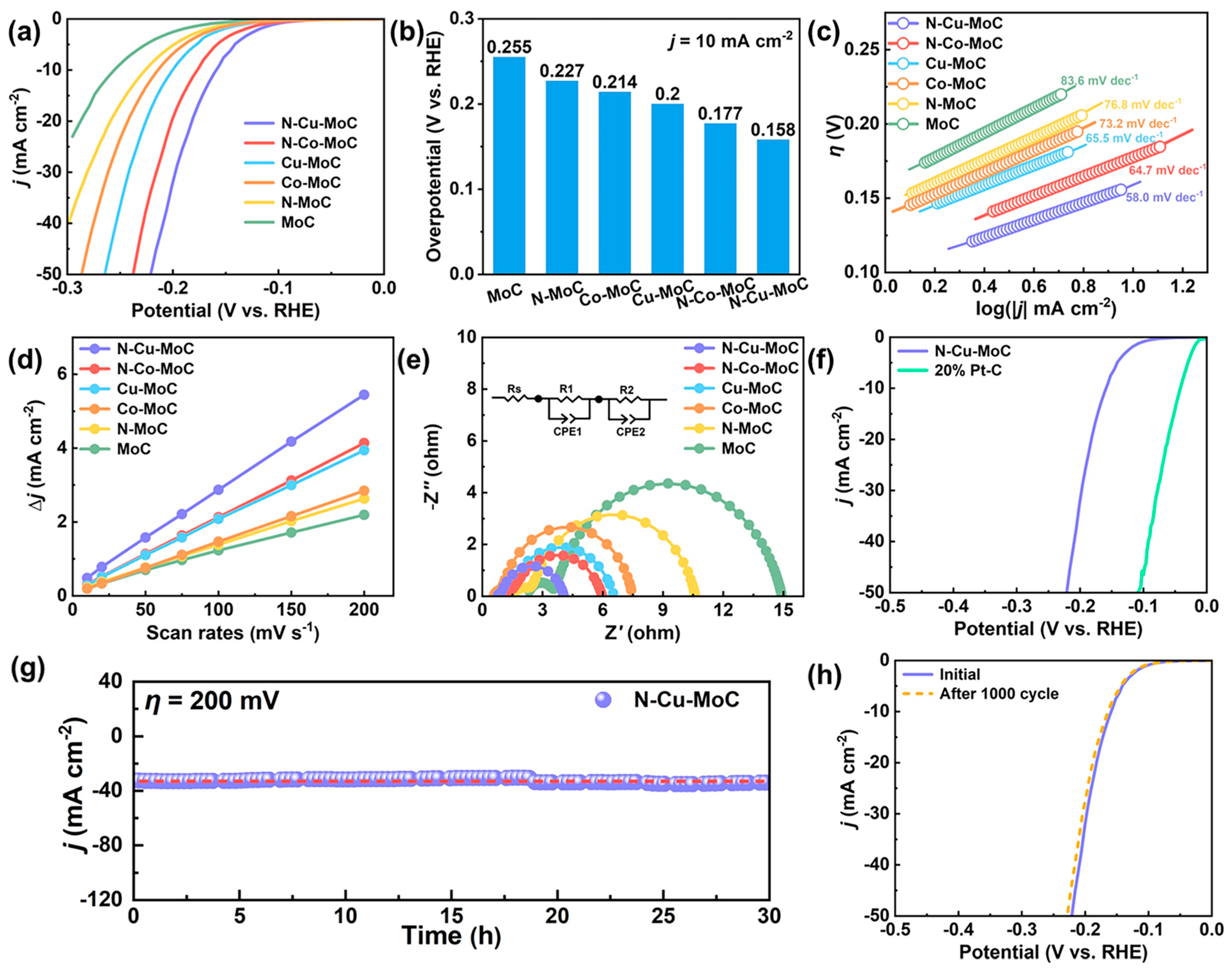
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Materials Synthesis
3.2. Catalyst Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, X.; Tan, H.; Ren, H.; Chen, S.; Yang, W.; Smith, S.; Zhao, C. Phosphine vapor−assisted construction of heterostructured Ni2P/NiTe2 catalysts for efficient hydrogen evolution. Energy Environ. Sci. 2020, 13, 1799–1807. [Google Scholar] [CrossRef]
- Li, R.; Wang, D. Understanding the structure−performance relationship of active sites at atomic scale. Nano Res. 2022, 15, 6888–6923. [Google Scholar] [CrossRef]
- Wismann, S.; Engbaek, J.; Vendelbo, S.; Bendixen, F.; Eriksen, W.; Aasberg−Petersen, K.; Frandsen, C.; Chorkendorff, I.; Mortensen, P. Electrified methane reforming: A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef]
- Herdem, M.; Sinaki, M.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. A review. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Li, H.; Zhang, Z.; Mu, Y.; Do, D.; Zhou, B.; Dong, J.; Yan, W.; Qin, Y.; et al. O−coordinated W−Mo dual−atom catalyst for pH−universal electrocatalytic hydrogen evolution. Sci. Adv. 2020, 6, eaba6586. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.H.; Tan, S.; Xu, K.; Wang, Y.; Wang, D.; Li, Y. The electronic metal−support interaction directing the design of single atomic site catalysts: Achieving high efficiency towards hydrogen evolution. Angew. Chem. Int. Ed. 2021, 60, 19085–19091. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Kitiphatpiboon, N.; Li, X.; Wang, J.; Hao, X.; Abudula, A.; Ma, Y.; Guan, G. Zn−VOx−Co nanosheets with amorphous/crystalline heterostructure for highly efficient hydrogen evolution reaction. Chem. Eng. J. 2022, 432, 134329. [Google Scholar] [CrossRef]
- Chen, M.; Su, Q.; Kitiphatpiboon, N.; Zhang, J.; Feng, C.; Li, S.; Zhao, Q.; Abudula, A.; Ma, Y.; Guan, G. Heterojunction engineering of Ni3S2/NiS nanowire for electrochemical hydrogen evolution. Fuel 2023, 331, 125794. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Zhao, Q.; Abudula, A.; Ma, Y.; Yan, K.; Guan, G. Tuning octahedron sites in MnFe2O4 spinel by boron doping for highly efficient seawater splitting. Appl. Catal. B 2023, 330, 122577. [Google Scholar] [CrossRef]
- Zhuang, L.; Jia, Y.; Liu, H.; Li, Z.; Li, M.; Zhang, L.; Wang, X.; Yang, D.; Zhu, Z.; Yao, X. Sulfur−modified oxygen vacancies in iron−cobalt oxide nanosheets: Enabling extremely high activity of the oxygen evolution reaction to achieve the industrial water splitting benchmark. Angew. Chem. Int. Ed. 2020, 59, 14664–14670. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Driess, M.; Menezes, P. Self−supported electrocatalysts for practical water electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Abudula, A.; Ma, Y.; Guan, G. Recent progress in transition−metal−oxide−based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review. eScience 2023, 3, 100111. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self−supported transition−metal−based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, e1806326. [Google Scholar] [CrossRef]
- Xu, K.; Sun, Y.; Li, X.; Zhao, Z.; Zhang, Y.; Li, C.; Fan, H. Fluorine−induced dual defects in cobalt phosphide nanosheets enhance hydrogen evolution reaction activity. ACS Mater. Lett. 2020, 2, 736–743. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.; Leonard, B. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef]
- Tian, D.; Denny, S.; Li, K.; Wang, H.; Kattel, S.; Chen, J. Density functional theory studies of transition metal carbides and nitrides as electrocatalysts. Chem. Soc. Rev. 2021, 50, 12338–12376. [Google Scholar] [CrossRef]
- Lin, F.; Qin, H.; Wang, T.; Yang, L.; Cao, X.; Jiao, L. Few−layered MoN−MnO heterostructures with interfacial−O synergistic active centers boosting electrocatalytic hydrogen evolution. J. Mater. Chem. A 2021, 9, 8325–8331. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, W.; Zhang, Y.; Han, F.; Yang, X. Nanocarbon encapsulating Ni−doped MoP/graphene composites for highly improved electrocatalytic hydrogen evolution reaction. Compos. Commun. 2021, 26, 100792. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, M.; Geng, H.; Dong, H.; Wu, P.; Li, X.; Guan, G.; Wang, T. Synergistically tuning electronic structure of porous β-Mo2C spheres by Co doping and Mo−vacancies defect engineering for optimizing hydrogen evolution reaction activity. Adv. Funct. Mater. 2020, 30, 2000561. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.; Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Ouyang, T.; Ye, Y.; Wu, C.; Xiao, K.; Liu, Z. Heterostructures composed of N−doped carbon nanotubes encapsulating cobalt and beta−Mo2C nanoparticles as bifunctional electrodes for water splitting. Angew. Chem. Int. Ed. 2019, 58, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sung, M.; Yoon, H.; Ju, B.; Kim, D. Ultrafine alpha−Phase molybdenum carbide decorated with platinum nanoparticles for efficient hydrogen production in acidic and alkaline media. Adv. Sci. 2019, 6, 1802135. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen−evolution electrocatalyst. Angew. Chem. Int. Ed. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, W.; Jian, C.; He, X.; Cai, Q.; Liu, W. The intrinsic hydrogen evolution performance of 2D molybdenum carbide. J. Mater. Chem. A 2020, 8, 24204–24211. [Google Scholar] [CrossRef]
- Anjum, M.; Lee, M.; Lee, J. BCN network−encapsulated multiple phases of molybdenum carbide for efficient hydrogen evolution reactions in acidic and alkaline media. J. Mater. Chem. A 2017, 5, 13122–13129. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C nanoparticles dispersed on hierarchical carbon microflowers for efficient electrocatalytic hydrogen evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef]
- Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two−dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678. [Google Scholar] [CrossRef]
- Zubair, M.; Ul Hassan, M.; Mehran, M.; Baig, M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Du, Q.; Zhao, R.; Du, J. Engineering micro/nanostructures of Mo2C/porous nanocarbon for enhanced hydrogen production. New J. Chem. 2023, 47, 3417–3424. [Google Scholar] [CrossRef]
- Zhao, Y.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. Hydrogen evolution by tungsten carbonitride nanoelectrocatalysts synthesized by the formation of a tungsten acid/polymer hybrid in situ. Angew. Chem. Int. Ed. 2013, 52, 13638–13641. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, K.; Lu, Z.; Li, Y.; Xu, W.; Gao, T.; Cai, Z.; Zhang, Y.; Batista, V.; Liu, W.; et al. Nitrogen−doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, G.; Li, G.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen−rich nanocarbon leads to efficient hydrogen−evolution electrocatalytic sites. Angew. Chem. Int. Ed. 2015, 54, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Jiang, W.; Zhang, X.; Dai, Z.; Wan, L.; Hu, J. Pomegranate—like N,P—doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N−doped Mo2C nanosheets with exposed active sites as efficient electrocatalyst for hydrogen evolution reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Li, P.; Yang, F.; Cheng, G.; Chen, S.; Luo, W. Nitrogen−doped CoP as robust electrocatalyst for high−efficiency pH universal hydrogen evolution reaction. Appl. Catal. B 2019, 253, 21–27. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.; Ji, Y.; Li, Y.; White, J.; Liu, S.; Han, X.; Lee, S.; Ma, T. Air−stable phosphorus−doped molybdenum nitride for enhanced electrocatalytic hydrogen evolution. Commun. Chem. 2018, 1, 95. [Google Scholar] [CrossRef]
- Li, F.; Han, G.; Noh, H.; Jeon, J.; Ahmad, I.; Chen, S.; Yang, C.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Balancing hydrogen adsorption/desorption by orbital modulation for efficient hydrogen evolution catalysis. Nat. Commun. 2019, 10, 4060. [Google Scholar] [CrossRef]
- Fan, H.; Yu, H.; Zhang, Y.; Zheng, Y.; Luo, Y.; Dai, Z.; Li, B.; Zong, Y.; Yan, Q. Fe−doped Ni3C nanodots in N−doped carbon nanosheets for efficient hydrogen−evolution and oxygen−evolution electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 12566–12570. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gao, Y.; Lang, Z.; Ma, Y.; Yin, L.; Du, J.; Tan, H.; Wang, Y.; Li, Y. Electrocatalytic performance of ultrasmall Mo2C affected by different transition metal dopants in hydrogen evolution reaction. Nanoscale 2018, 10, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nosheen, F.; Wang, X. Ni−decorated molybdenum carbide hollow structure derived from carbon−coated metal−organic framework for electrocatalytic hydrogen evolution reaction. Chem. Mater. 2016, 28, 6313–6320. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Tang, Y.; Gao, Q. Cobalt−Doping in molybdenum−carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 26, 5590–5598. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Chen, M.; Yang, Q.; Chen, H.; Guan, G.; Qin, Y.; Wang, T. Selective hydrogenation of naphthalene to decalin over surface-engineered α-MoC based on synergy between Pd doping and Mo vacancy generation. Adv. Funct. Mater. 2022, 32, 2112435. [Google Scholar] [CrossRef]
- Shi, J.; Hu, L.; Liu, J.; Chen, M.; Li, C.; Guan, G.; Ma, Y.; Wang, T. Phase−transition engineering induced lattice contraction of the molybdenum carbide surface for highly efficient hydrogen evolution reaction. J. Mater. Chem. A 2022, 10, 11414–11425. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.; Aslam, M.; Zhang, Y.; Wang, R.; Ouyang, Z.; Gou, Z.; Zhang, H. Recent advances in two−dimensional materials and their nanocomposites in sustainable energy conversion applications. Nanoscale 2019, 11, 21622–21678. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH−universal self−standing MoC−Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12, 6776. [Google Scholar] [CrossRef]
- Li, H.; Hu, M.; Zhang, L.; Huo, L.; Jing, P.; Liu, B.; Gao, R.; Zhang, J.; Liu, B. Hybridization of bimetallic molybdenum−tungsten carbide with nitrogen−doped carbon: A rational design of super active porous composite nanowires with tailored electronic structure for boosting hydrogen evolution catalysis. Adv. Funct. Mater. 2020, 30, 2003198. [Google Scholar] [CrossRef]
- Reynard, D.; Nagar, B.; Girault, H. Photonic flash synthesis of Mo2C/graphene electrocatalyst for the hydrogen evolution reaction. ACS Catal. 2021, 11, 5865–5872. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Liang, T.; Ang, E.; Zhang, X.; Ma, F.; Dai, Z. Nitrogen−doped carbon wrapped Co−Mo2C dual Mott−Schottky nanosheets with large porosity for efficient water electrolysis. Appl. Catal. B 2021, 284, 119738. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Wang, H.; Wang, C.; Pinna, N.; Lu, X. Ni Strongly coupled with Mo2C encapsulated in nitrogen−doped carbon nanofibers as robust bifunctional catalyst for overall water splitting. Adv. Energy Mater. 2019, 9, 1803185. [Google Scholar] [CrossRef]
- Kim, S.; Choi, C.; Hwang, J.; Park, J.; Jeong, J.; Jun, H.; Lee, S.; Kim, S.; Jang, J.; Jung, Y.; et al. Interaction Mediator Assisted Synthesis of Mesoporous Molybdenum Carbide: Mo−valence state adjustment for optimizing hydrogen evolution. ACS Nano 2020, 14, 4988–4999. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, S.; Xue, S.; Guo, Z.; Li, J.; Qu, K.; Cai, W. Heterointerface−rich Mo2C/MoO2 porous nanorod enables superior alkaline hydrogen evolution. Chem. Eng. J. 2021, 421, 127807. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel−rich RuCu nanosheets for pH−universal overall water splitting electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, K.; Cheong, W.; Zheng, L.; Zhang, C.; Liu, S.; Cao, X.; Wu, K.; Pan, Y.; Zhuang, Z.; et al. Synergistically interactive pyridinic−N−MoP sites: Identified active centers for enhanced hydrogen evolution in alkaline solution. Angew. Chem. Int. Ed. 2020, 59, 8982–8990. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co nanoparticles encapsulated in carbon−nanotubes−grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction. Adv. Mater. 2018, 30, e1802011. [Google Scholar] [CrossRef]
- Cui, S.; Li, M.; Bo, X. Co/Mo2C composites for efficient hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energ. 2020, 45, 21221–21231. [Google Scholar] [CrossRef]
- Ai, L.; Su, J.; Wang, M.; Jiang, J. Bamboo-structured nitrogen-doped carbon nanotube coencapsulating cobalt and molybdenum carbide nanoparticles: An efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 2018, 6, 9912–9920. [Google Scholar] [CrossRef]
- Ji, M.; Niu, S.; Du, Y.; Song, B.; Xu, P. Anion-induced size selection of β-Mo2C supported on nitrogen-doped carbon nanotubes for electrocatalytic hydrogen evolution. ACS Sustain. Chem. Eng. 2018, 6, 11922–11929. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, B.; Wu, P.; Liang, H.; Yu, Z.; Lin, Y.; Zheng, Y.; Li, Z.; Yu, S. Mo2C nanoparticles embedded within bacterial cellulose-derived 3D N-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Huang, H.; Yu, C.; Huang, H.; Guo, W.; Zhang, M.; Han, X.; Wei, Q.; Cui, S.; Tan, X.; Qiu, J. Microwave-assisted ultrafast synthesis of molybdenum carbide nanoparticles grown on carbon matrix for efficient hydrogen evolution reaction. Small Methods 2019, 3, 1900259. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, Y.; Gao, M.; Lang, C.; Zheng, Y.; Yu, S. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Wang, X.; Pan, Q.; Liu, L.; Su, Z. A substrate-free Mo2C-based electrocatalyst by facile glucose-blowing for efficient hydrogen production. New J. Chem. 2019, 43, 18970–18974. [Google Scholar] [CrossRef]
- Li, S.; Dong, B.; Yuan, y.; Xu, P.; Xu, P. Synthesis of porous Mo2C/nitrogen-doped carbon nanocomposites for efficient hydrogen evolution reaction. Chemistryselect 2020, 5, 14307–14311. [Google Scholar] [CrossRef]
- Wang, D.; Guo, T.; Wu, Z. Hierarchical Mo2C/C scaffolds organized by nanosheets as highly efficient electrocatalysts for hydrogen production. ACS Sustain. Chem. Eng. 2018, 6, 13995–14003. [Google Scholar] [CrossRef]
- Wang, Q.; Mi, F.; Li, J.; Wu, Y.; Zhou, X.; Ma, G.; Ren, S. Tungsten doping generated Mo2C-MoC heterostructure to improve HER performance in alkaline solution. Electrochimica Acta 2021, 370, 137796. [Google Scholar] [CrossRef]
- Ying, L.; Sun, S.; Liu, W.; Zhu, H.; Zhu, Z.; Liu, A.; Yang, L.; Lu, S.; Duan, F.; Yang, C.; et al. Heterointerface engineering in bimetal alloy/metal carbide for superior hydrogen evolution reaction. Renew. Energ. 2020, 161, 1036–1045. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energy Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhu, Y.; Tian, D.; Wang, C.; Lu, X. Mo/Mo2C encapsulated in nitrogen-doped carbon nanofibers as efficiently integrated heterojunction electrocatalysts for hydrogen evolution reaction in wide pH range. Appl. Surf. Sci. 2019, 496, 143672. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Ji, L.; Czioska, S.; Guo, L.; Chen, Z. Highly dispersed Mo2C nanoparticles embedded in ordered mesoporous carbon for efficient hydrogen evolution. ACS Appl. Energ. Mater. 2018, 1, 736–743. [Google Scholar] [CrossRef]
- Wan, J.; Wu, J.; Gao, X.; Li, T.; Hu, Z.; Yu, H.; Huang, L. Structure confined porous Mo2C for efficient hydrogen evolution. Adv. Funct. Mater. 2017, 27, 1703933. [Google Scholar] [CrossRef]
- Ma, L.; Ting, L.; Molinari, V.; Giordano, C.; Yeo, B. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A. 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, G.; Chou, K. Controllable synthesis of Mo2C with different morphology and application to electrocatalytic hydrogen evolution reaction. Nanotechnology 2021, 33, 105402. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J.; Liu, R.; Xia, K.; Xuan, C.; Guo, J.; Lei, W.; Wang, D. Facile preparation of carbon sphere supported molybdenum compounds (P, C and S) as hydrogen evolution electrocatalysts in acid and alkaline electrolytes. Nano Energy 2017, 32, 511–519. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Xu, L.; Hu, L.; Wang, T.; Ma, Y. Dual-Doping Strategy for Enhancing Hydrogen Evolution on Molybdenum Carbide Catalysts. Catalysts 2023, 13, 931. https://doi.org/10.3390/catal13060931
Wei J, Xu L, Hu L, Wang T, Ma Y. Dual-Doping Strategy for Enhancing Hydrogen Evolution on Molybdenum Carbide Catalysts. Catalysts. 2023; 13(6):931. https://doi.org/10.3390/catal13060931
Chicago/Turabian StyleWei, Junling, Li Xu, Lihua Hu, Tiejun Wang, and Yufei Ma. 2023. "Dual-Doping Strategy for Enhancing Hydrogen Evolution on Molybdenum Carbide Catalysts" Catalysts 13, no. 6: 931. https://doi.org/10.3390/catal13060931





