Application of Biogenic TiO2 Nanoparticles as ORR Catalysts on Cathode for Enhanced Performance of Microbial Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
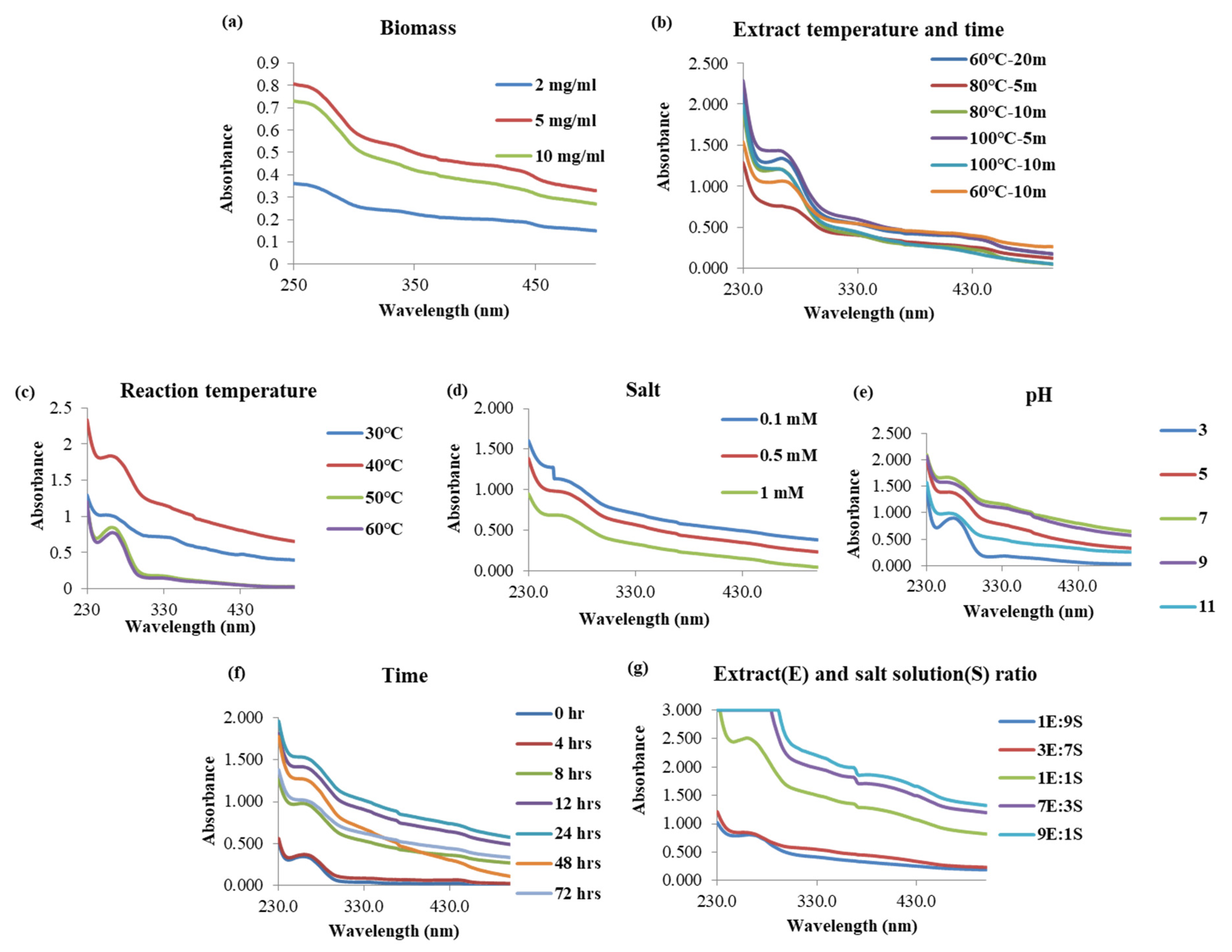
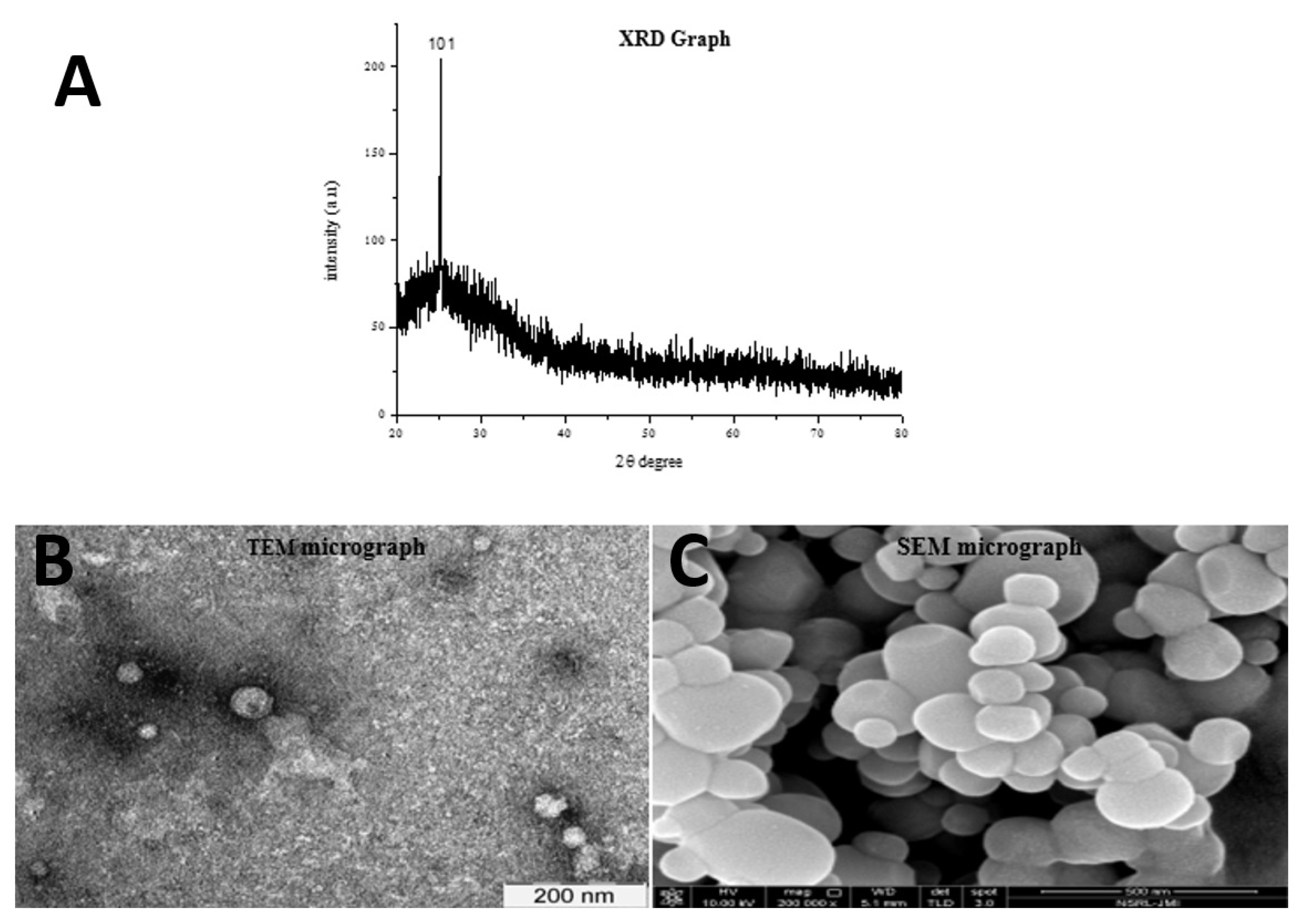
2.1. Synthesis of TiO2 Nanoparticle
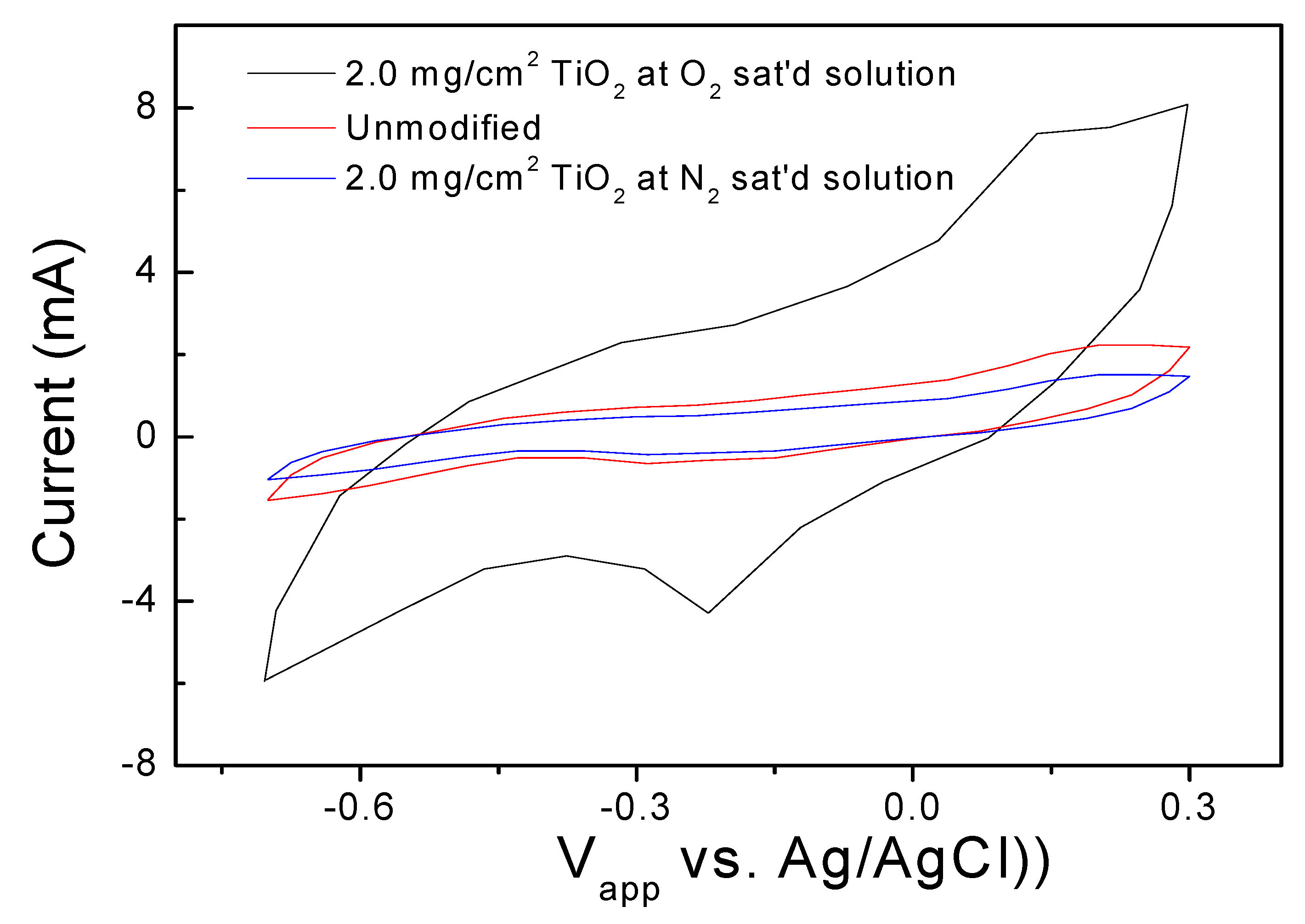
2.2. Cyclic Voltammetry of Cathode
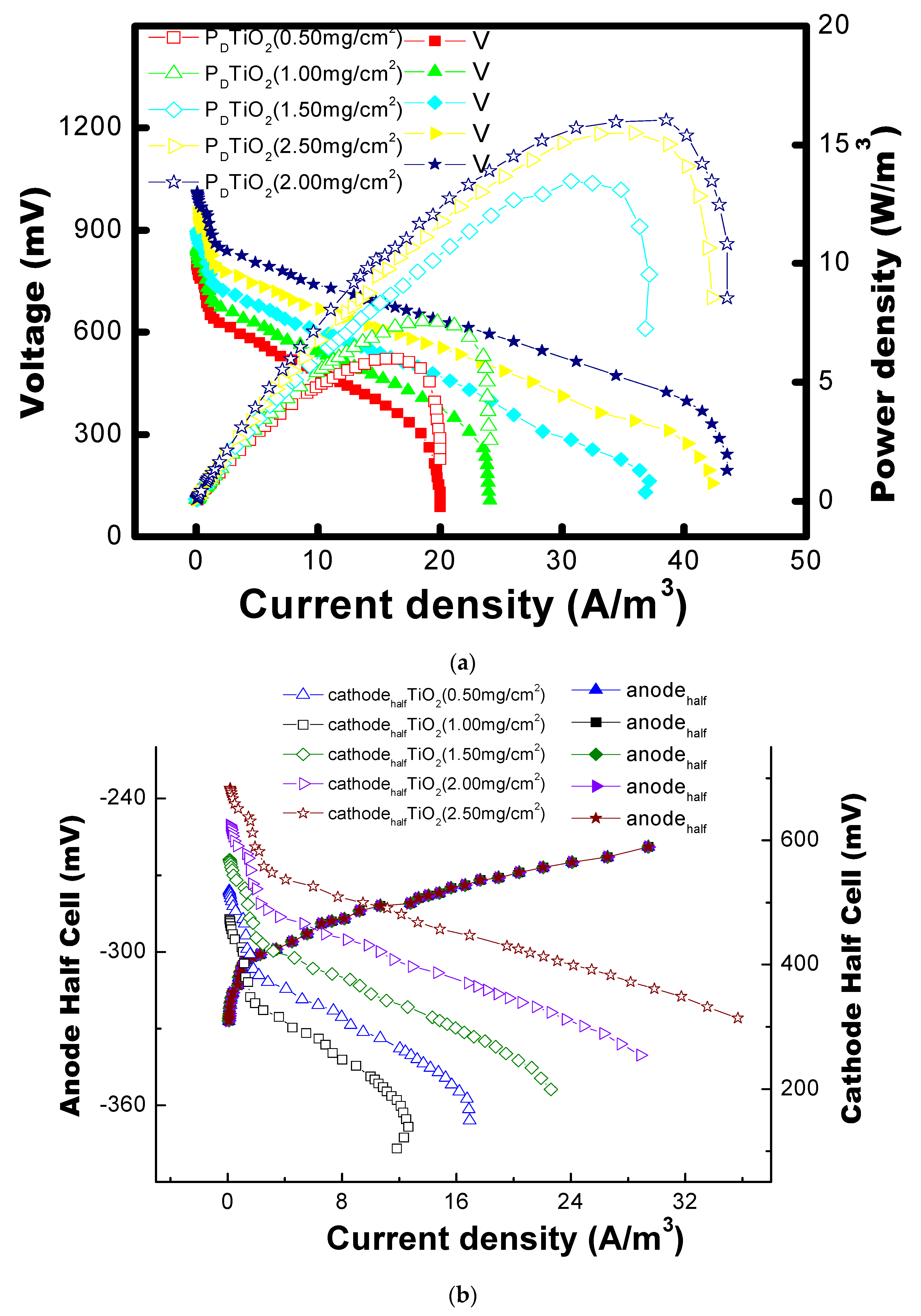
2.3. Polarization Study in MFC
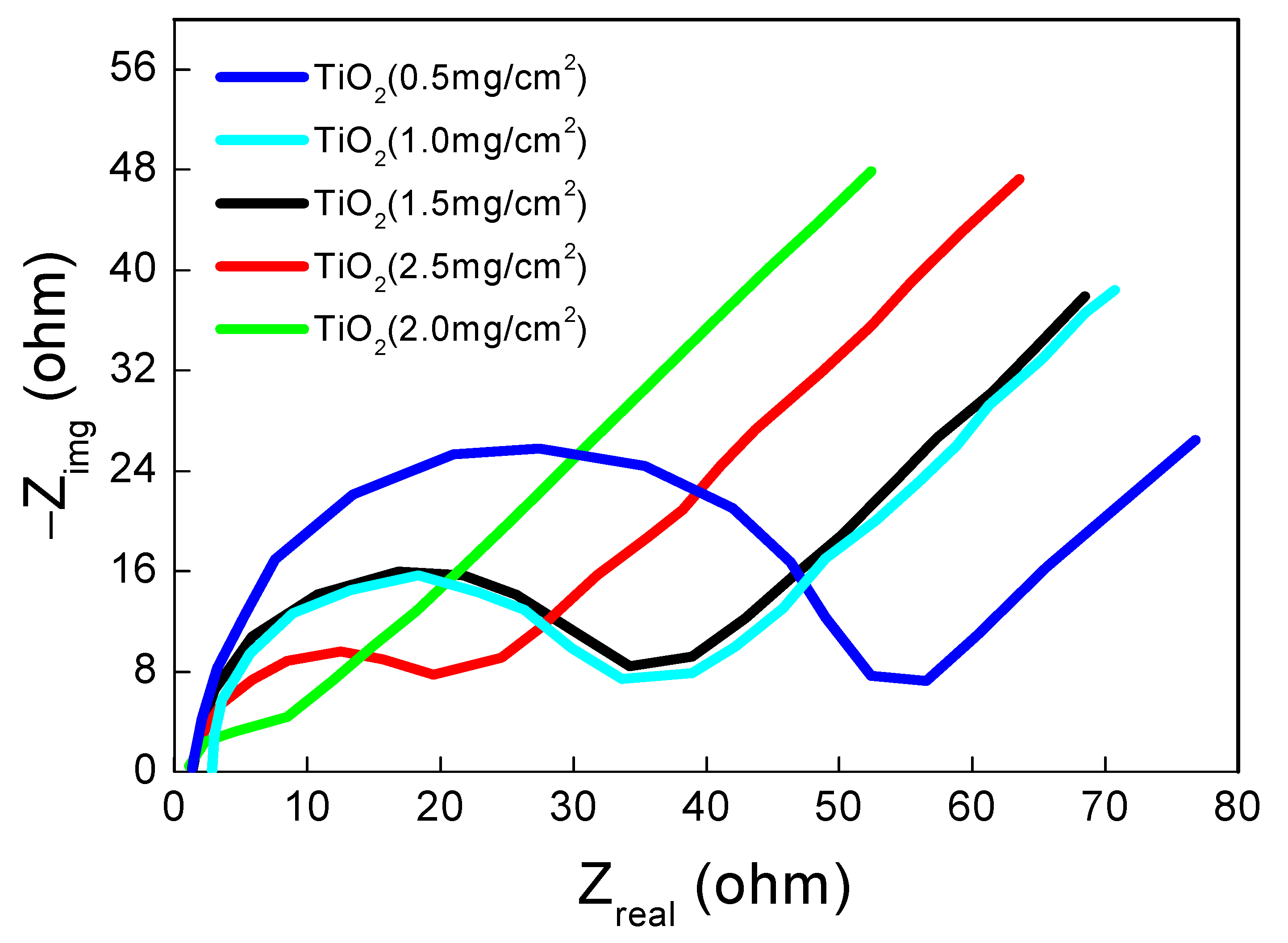
2.4. Electrochemical Impedance Studies
2.5. COD Elimination and Coulombic Efficiency
2.6. Biofouling Studies on the Cathode Surface
3. Materials and Method
3.1. Fabrication of an Impregnated Graphite Sheet Cathode Using Synthesized Biogenic TiO2 Nanoparticle
3.2. Characterization of Cyanobacteria-Derived TiO2 NPs
3.3. MFC Construction
3.4. Anodic Mixed Consortia and Anolyte
3.5. Performance Evaluation of MFCs
3.6. Biofouling Studies on TiO2-Impregnated Cathode Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Ayub, M.; Goh, H.H.; Kusworo, T.D.; Mohyuddin, A.; Chew, K.W. Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development. Sustainability 2022, 14, 16847. [Google Scholar] [CrossRef]
- Tsekouras, G.J.; Deligianni, P.M.; Kanellos, F.D.; Kontargyri, V.T.; Kontaxis, P.A.; Manousakis, N.M.; Elias, C.N. Microbial Fuel Cell for Wastewater Treatment as Power Plant in Smart Grids: Utopia or Reality? Front. Energy Res. 2022, 10, 370. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Mecheri, B.; Gokhale, R.; Santoro, C.; Costa de Oliveira, M.A.; D’Epifanio, A.; Licoccia, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Oxygen Reduction Reaction Electrocatalysts Derived from Iron Salt and Benzimidazole and Aminobenzimidazole Precursors and Their Application in Microbial Fuel Cell Cathodes. ACS Appl. Energy Mater 2018, 1, 5755–5765. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, X.; Yang, H.; Chen, S. Optimization Strategies of Preparation of Biomass-Derived Carbon Electrocatalyst for Boosting Oxygen Reduction Reaction: A Minireview. Catalysts 2020, 10, 1472. [Google Scholar] [CrossRef]
- Costa de Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. [Google Scholar] [CrossRef]
- Yin, J.; Wei, K.; Bai, Y.; Liu, Y.; Zhang, Q.; Wang, J.; Qin, Z.; Jiao, T. Integration of Amorphous CoSnO3 onto Wrinkled MXene Nanosheets as Efficient Electrocatalysts for Alkaline Hydrogen Evolution. Sep. Purif. Technol. 2023, 308, 122947. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, F.; Jiao, T.; Deng, H.; Zou, G.; Bai, Z.; Zhang, Q.; Peng, Q. Highly Efficient Catalytic Performances of Nitro Compounds via Hierarchical PdNPs-Loaded MXene/Polymer Nanocomposites Synthesized through Electrospinning Strategy for Wastewater Treatment. Chin. Chem. Lett. 2020, 31, 992–995. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, F.; Jiao, T.; Wang, W.; Zhang, G.; Jiao, J.; Jiang, G.; Zhang, Q.; Gu, J.; Peng, Q. Facile Preparation of Self-Assembled MXene@Au@CdS Nanocomposite with Enhanced Photocatalytic Hydrogen Production Activity. Sci. China Mater. 2020, 63, 2228–2238. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.; Shen, P.; Shao, Z.; Jiang, S. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef] [PubMed]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl Groups as Targets of Mercury Toxicity. Coord Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Imteyaz, S.; Murugan, B.; Lett, A.; Sridewi, N.; Kassegn, G.; Fatimah, I.; Oh, W.-C. A Comprehensive Review on Green Synthesis of Titanium Dioxide Nanoparticles and Their Diverse Biomedical Applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.-Q. Defective Titanium Dioxide Single Crystals Exposed by High-Energy {001} Facets for Efficient Oxygen Reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.-K. Green Synthesis of Titanium Dioxide Nanoparticles Using Psidium Guajava Extract and Its Antibacterial and Antioxidant Properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles: Synthesis, Characterisation and Applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-Cost and Eco-Friendly Phyto-Synthesis of Silver Nanoparticles Using Cocos Nucifera Coir Extract and Its Larvicidal Activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Siddiqui, T.; Khan, N.J.; Asif, N.; Ahamad, I.; Yasin, D.; Fatma, T. Screening, Characterisation and Bioactivities of Green Fabricated TiO2 NP via Cyanobacterial Extract. Environ. Sci. Pollut. Res. Int. 2022, 29, 39052–39066. [Google Scholar] [CrossRef]
- Siddiqui, T.; Khan, N.J.; Fatma, T. Green Synthesis of Titanium Dioxide Nanoparticles and Their Applications. In Sustainable Nanotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 135–142. ISBN 978-1-119-65029-4. [Google Scholar]
- Kumar, A.; Sharma, K.; Pandit, S.; Singh, A.; Prasad, R. Evaluation of the Algal-Derived Biochar as an Anode Modifier in Microbial Fuel Cells. Bioresour. Technol. Rep. 2023, 22, 101414. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Das, D.; Pradhan, D. Manganese Cobaltite/Polypyrrole Nanocomposite-Based Air-Cathode for Sustainable Power Generation in the Single-Chambered Microbial Fuel Cells. Biosens. Bioelectron. 2014, 54, 534–540. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Das, D.; Pradhan, D. Graphene Supported α-MnO2 Nanotubes as a Cathode Catalyst for Improved Power Generation and Wastewater Treatment in Single-Chambered Microbial Fuel Cells. RSC Adv. 2013, 3, 7902–7911. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, A.; Pandit, S.; Vempaty, A.; Kumar, M.; Thapa, B.S.; Rai, N.; Peera, G. Investigating the Performance of a Zinc Oxide Impregnated Polyvinyl Alcohol-Based Low-Cost Cation Exchange Membrane in Microbial Fuel Cells. Membranes 2023, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Improvement of Power Generation Using Shewanella Putrefaciens Mediated Bioanode in a Single Chambered Microbial Fuel Cell: Effect of Different Anodic Operating Conditions. Bioresour. Technol. 2014, 166, 451–457. [Google Scholar] [CrossRef]
- Sharma, K.; Pandit, S.; Thapa, B.S.; Pant, M. Biodegradation of Congo Red Using Co-Culture Anode Inoculum in a Microbial Fuel Cell. Catalysts 2022, 12, 1219. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, V.; Pandit, S.; Thapa, B.S.; Pant, K.; Tusher, T. Isolation of Biosurfactant-Producing Bacteria and Their Co-Culture Application in Microbial Fuel Cell for Simultaneous Hydrocarbon Degradation and Power Generation. Sustainability 2022, 14, 15638. [Google Scholar] [CrossRef]
- Kishimoto, N.; Okumura, M. Feasibility of Mercury-Free Chemical Oxygen Demand (COD) Test with Excessive Addition of Silver Sulfate. J. Water Environ. Technol. 2018, 16, 221–232. [Google Scholar] [CrossRef]
- Vempaty, A.; Kumar, A.; Pandit, S.; Gupta, M.; Mathuriya, A.S.; Lahiri, D.; Nag, M.; Kumar, Y.; Joshi, S.; Kumar, N. Evaluation of the Datura Peels Derived Biochar-Based Anode for Enhancing Power Output in Microbial Fuel Cell Application. Biocatal. Agric. Biotechnol. 2023, 47, 102560. [Google Scholar] [CrossRef]
- Sharma, K.; Pandit, S.; Singh, A.; Gupta, P.; Pant, K. Microbial Electrochemical Treatment of Methyl Red Dye Degradation Using Co-Culture Method. Water 2023, 15, 56. [Google Scholar] [CrossRef]
- Pandit, S.; Jadhav, D.; Singh, A.; Pandit, C. Blue Energy Meets Green Energy in Microbial Reverse Electrodialysis Cells: Recent Advancements and Prospective. Sustain. Energy Technol. Assess. 2023, 57, 103260. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, M.; Pundir, R.; Chatterjee, S.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Versatile Biomedical Potential of Biosynthesized Silver Nanoparticles from Acacia Nilotica Bark. J. Appl. Biomed. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Canchanya-Huaman, Y.; Mayta-Armas, A.F.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Guerra, J.A.; Ramos-Guivar, J.A. Strain and Grain Size Determination of CeO2 and TiO2 Nanoparticles: Comparing Integral Breadth Methods versus Rietveld, μ-Raman, and TEM. Nanomaterials 2021, 11, 2311. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Mohsin, M.; Ahmad, T.; Sardar, M. Alpha Amylase Assisted Synthesis of TiO2 Nanoparticles: Structural Characterization and Application as Antibacterial Agents. J. Hazard. Mater. 2015, 283, 171–177. [Google Scholar] [CrossRef]
- Sun, Y.; ter Heijne, A.; Rijnaarts, H.; Chen, W.-S. The Effect of Anode Potential on Electrogenesis, Methanogenesis and Sulfidogenesis in a Simulated Sewer Condition. Water Res. 2022, 226, 119229. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Siddiqui, T.; Pandit, S.; Roy, A.; Gacem, A.; Souwaileh, A.A.; Mathuriya, A.S.; Fatma, T.; Sharma, P.; Rustagi, S.; et al. Application of Biogenic TiO2 Nanoparticles as ORR Catalysts on Cathode for Enhanced Performance of Microbial Fuel Cell. Catalysts 2023, 13, 937. https://doi.org/10.3390/catal13060937
Kumar A, Siddiqui T, Pandit S, Roy A, Gacem A, Souwaileh AA, Mathuriya AS, Fatma T, Sharma P, Rustagi S, et al. Application of Biogenic TiO2 Nanoparticles as ORR Catalysts on Cathode for Enhanced Performance of Microbial Fuel Cell. Catalysts. 2023; 13(6):937. https://doi.org/10.3390/catal13060937
Chicago/Turabian StyleKumar, Ankit, Tabassum Siddiqui, Soumya Pandit, Arpita Roy, Amel Gacem, Abdullah Al Souwaileh, Abhilasha Singh Mathuriya, Tasneem Fatma, Promila Sharma, Sarvesh Rustagi, and et al. 2023. "Application of Biogenic TiO2 Nanoparticles as ORR Catalysts on Cathode for Enhanced Performance of Microbial Fuel Cell" Catalysts 13, no. 6: 937. https://doi.org/10.3390/catal13060937





