Ca2Fe2O5-Based WGS Catalysts to Enhance the H2 Yield of Producer Gases
Abstract
:1. Introduction
2. Results and Discussion
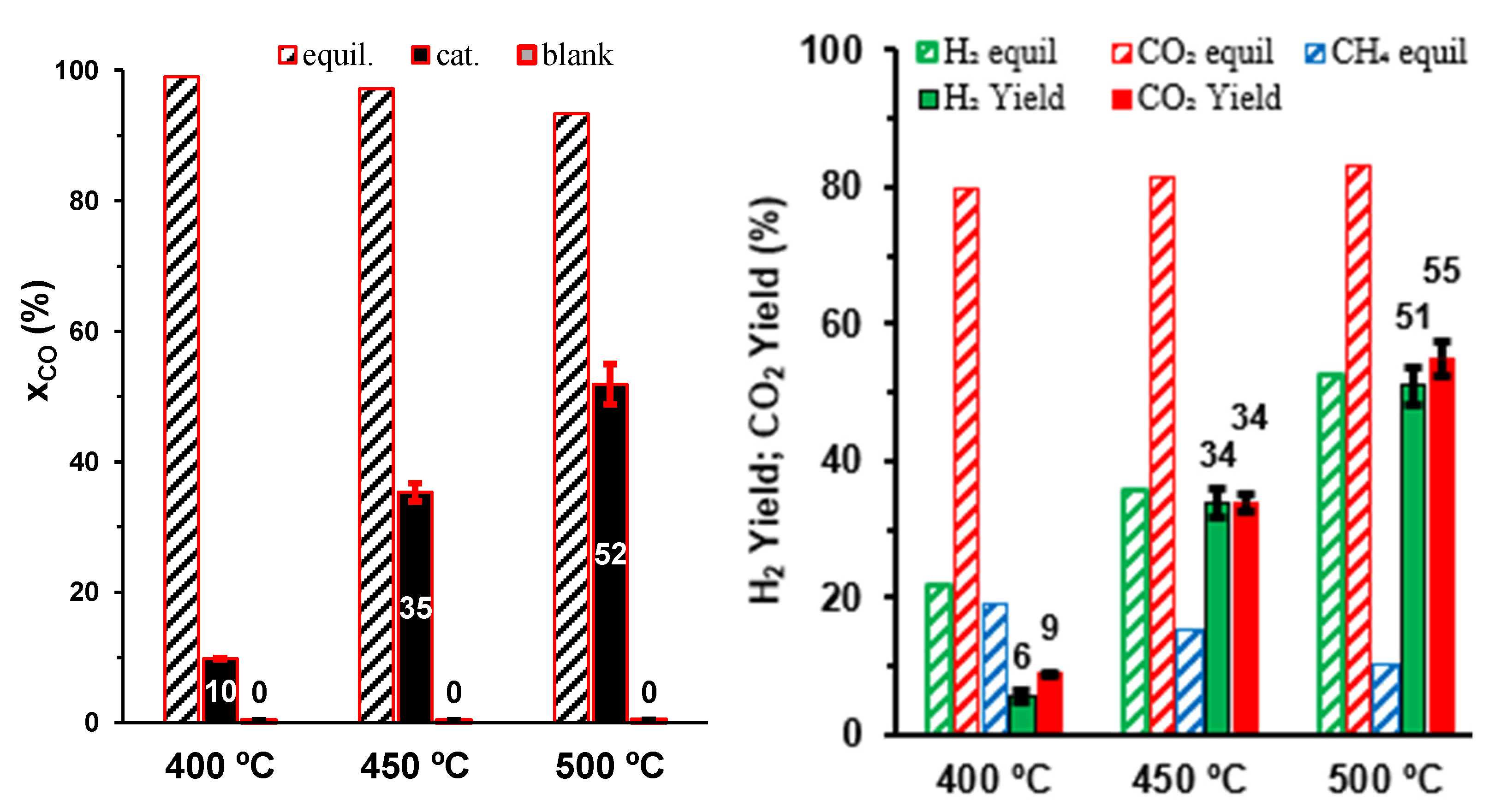
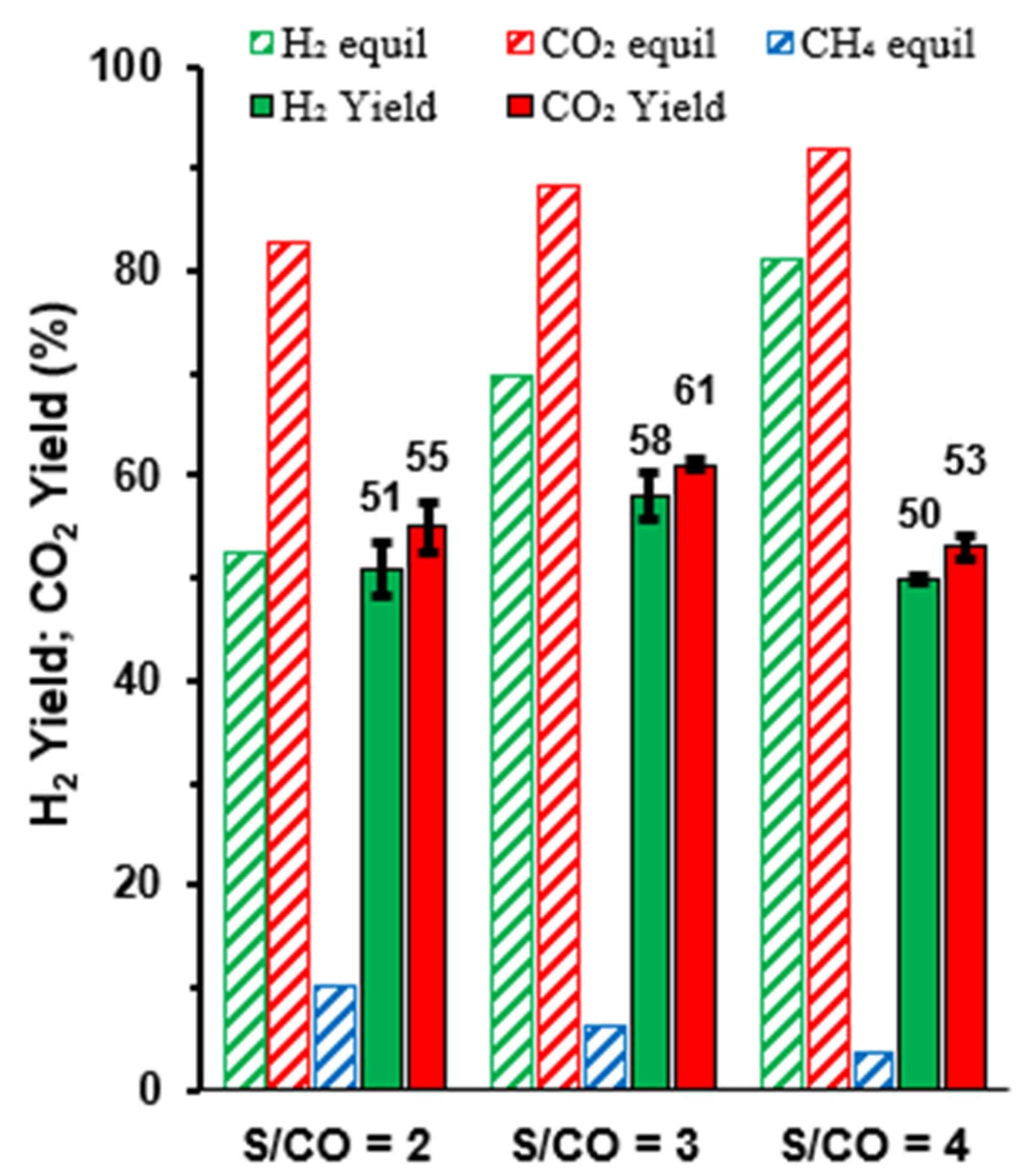
2.1. Catalytic Testing
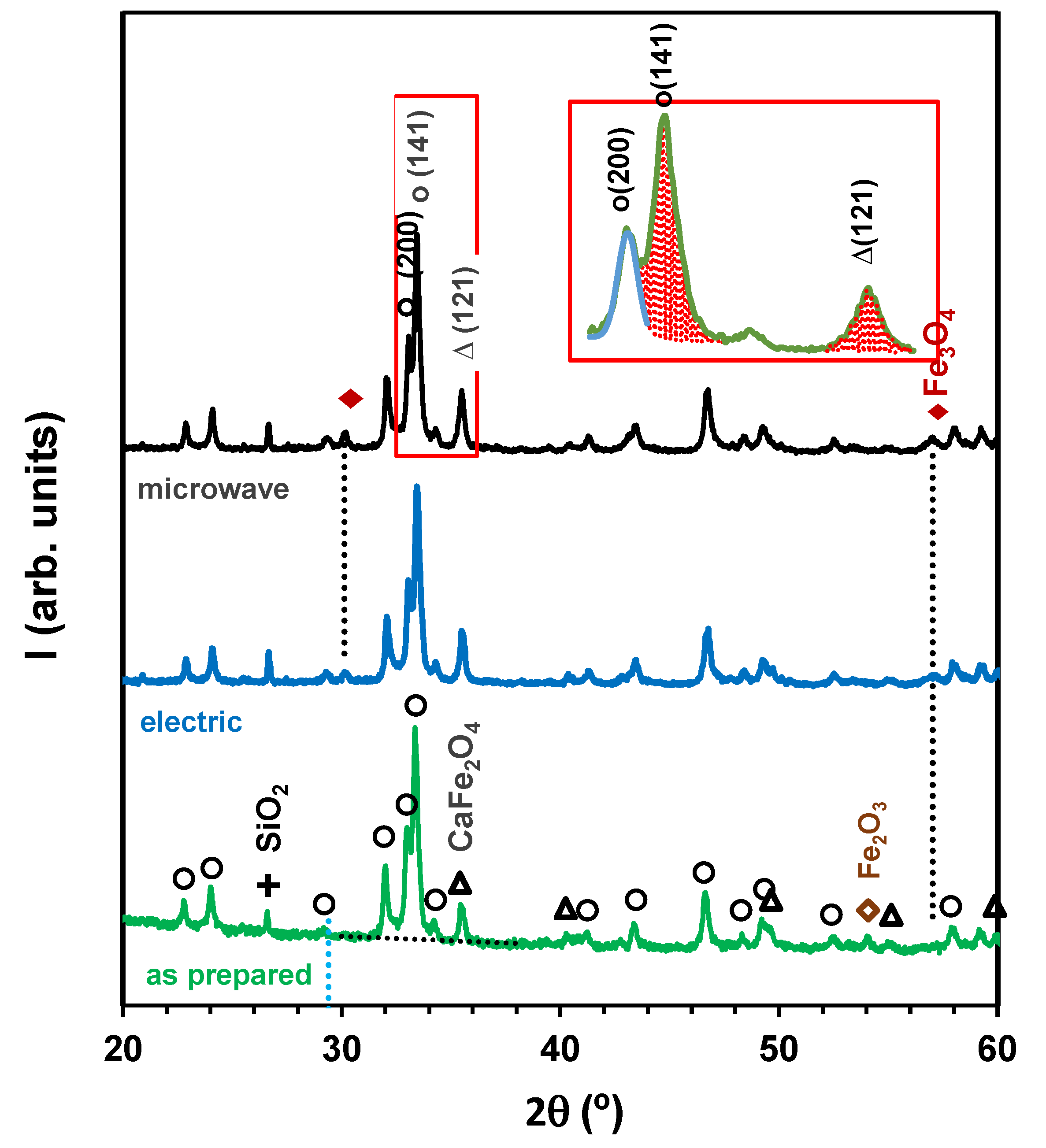
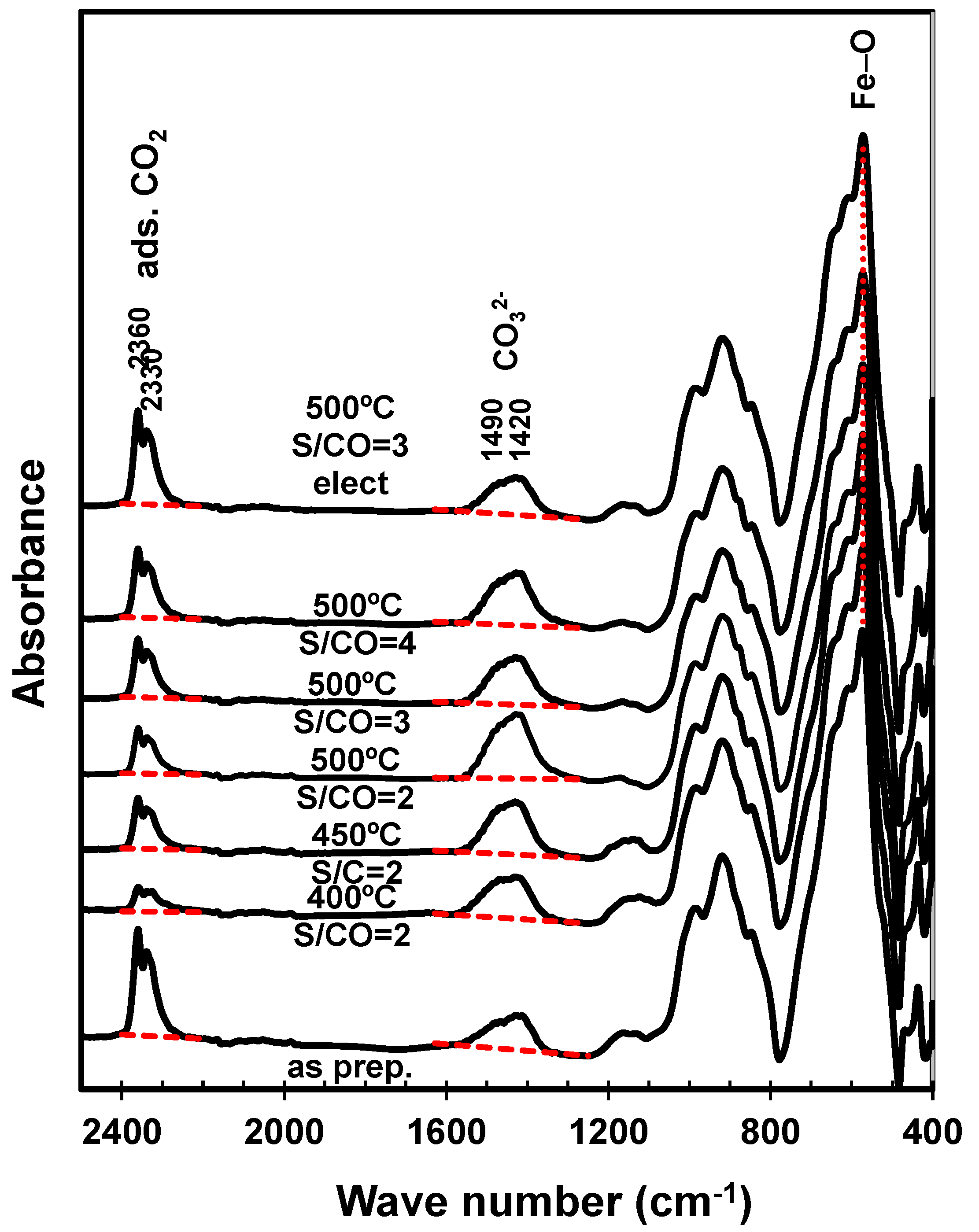
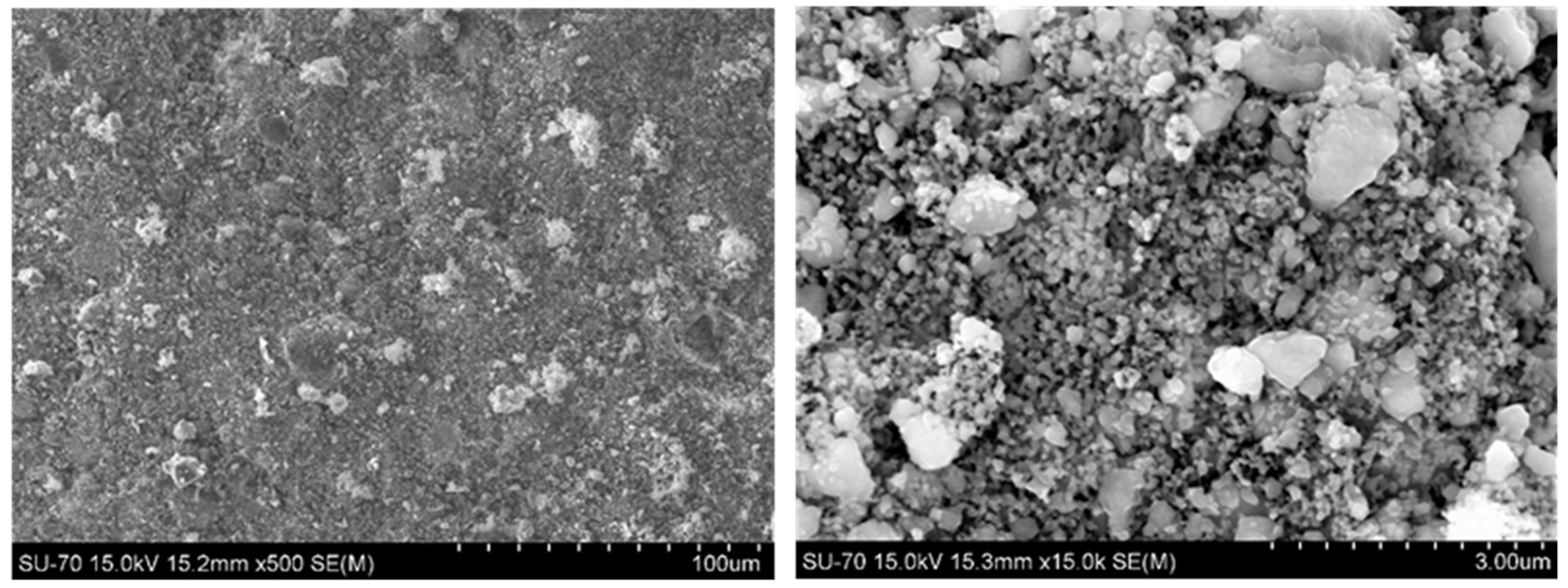
2.2. Post-Mortem Analysis
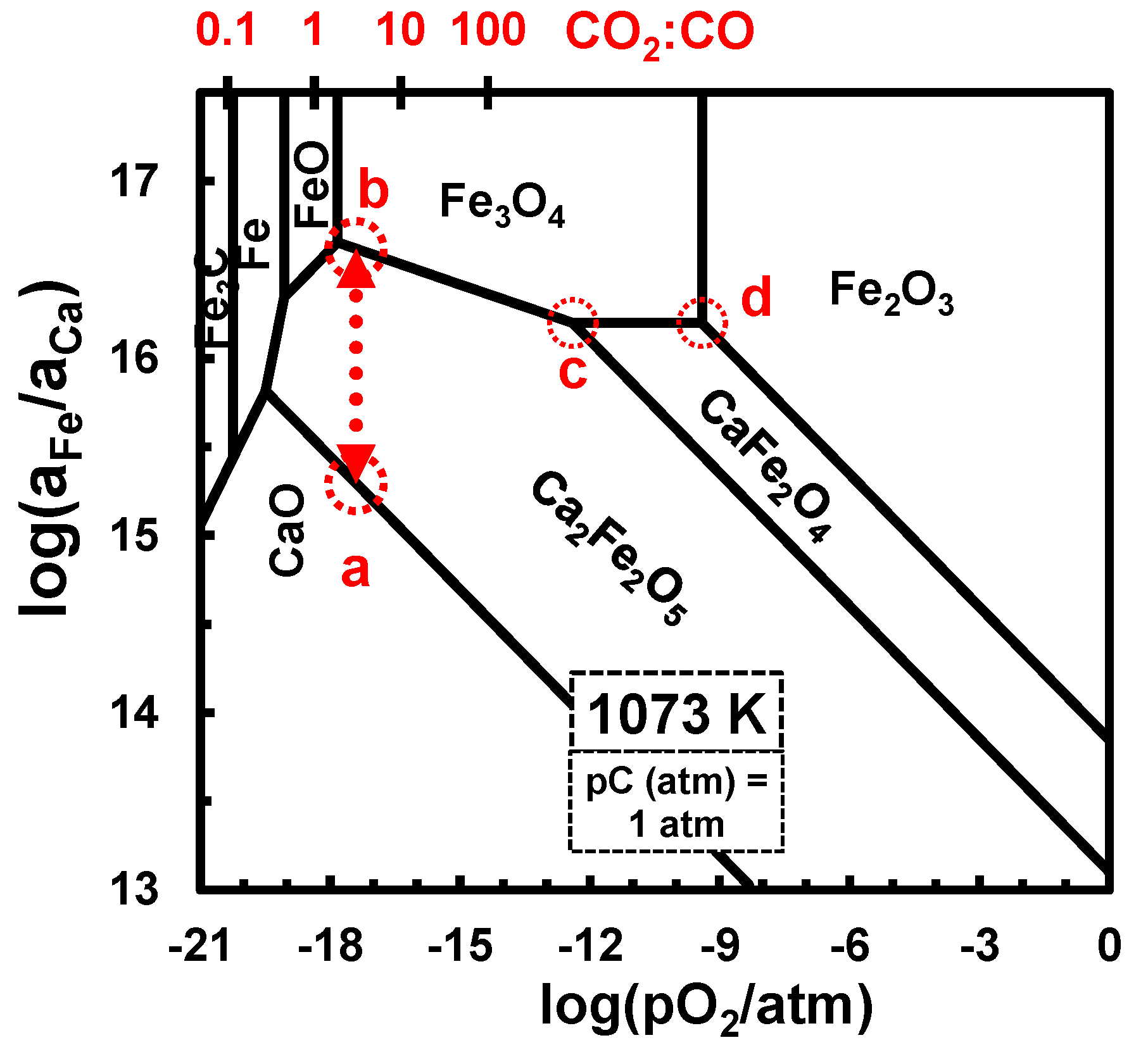
2.3. Thermodynamic Guidelines
2.4. Prospective Applicability to Upgrade Producer Gases
3. Materials and Methods
3.1. Catalyst Preparation
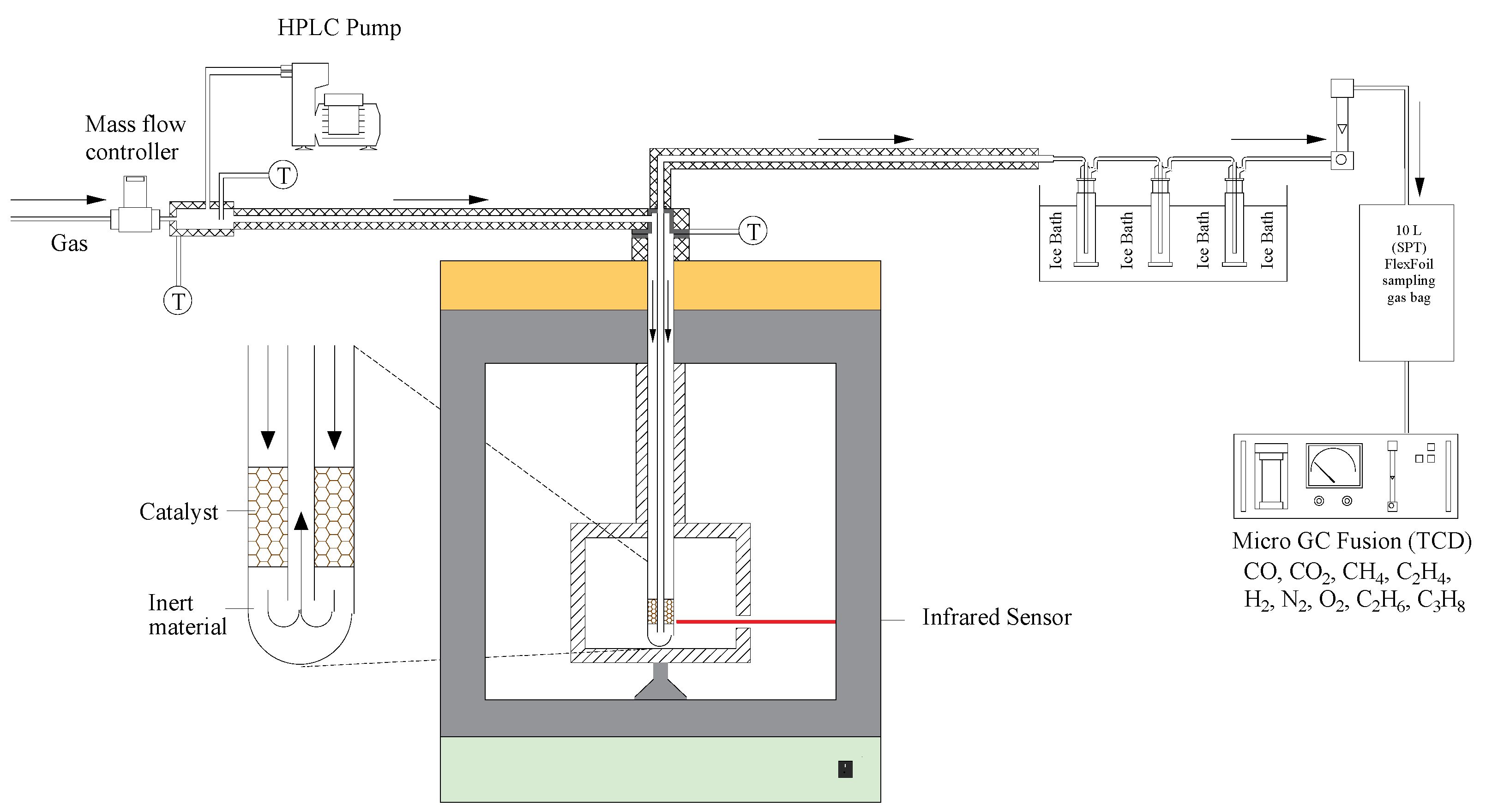
3.2. Water Gas Shift (WGS) Tests
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Reaction | Redox Conditions |
|---|---|
| |
References
- Pio, D.T.; Ruivo, L.C.M.; Tarelho, L.A.C.; Frade, J.R.; Kantarelis, E.; Engvall, K. Tar formation during eucalyptus gasification in a bubbling fluidized bed reactor: Effect of feedstock and reactor bed composition. Energy Convers. Manag. 2021, 229, 113749. [Google Scholar] [CrossRef]
- Ruivo, L.C.M.; Gomes, H.; Lopes, D.V.; Yaremchenko, A.A.; Vilas-Boas, C.; Tarelho, L.A.C.; Frade, J.R. Catalytic O2-steam gasification of biomass over Fe2−xMnxO3 oxides supported on ceramic foam filters. Fuel 2022, 324, 124566. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Chen, W.-H.; Jheng, J.-G.; Yu, A.B. Hydrogen generation from a catalytic water gas shift reaction under microwave irradiation. Int. J. Hydrogen Energy 2008, 33, 4789–4797. [Google Scholar] [CrossRef]
- Levalley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Lang, C.; Sécordel, X.; Kiennemann, A.; Courson, C. Water gas shift catalysts for hydrogen production from biomass steam gasification. Fuel Process. Technol. 2017, 156, 246–252. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M. High temperature water gas shift reaction over promoted iron based catalysts prepared by pyrolysis method. Int. J. Hydrogen Energy 2014, 39, 16318–16328. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, S.-W.; Lim, T.-H.; Lee, H.-I. Effect of pretreatment on the activity of Ni catalyst for CO removal reaction by water–gas shift and methanation. Appl. Catal. B Environ. 2008, 81, 97–104. [Google Scholar] [CrossRef]
- Kung, H.H. Deactivation of methanol synthesis catalysts—A review. Catal. Today 1992, 11, 443–453. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Influence of salts during hydrothermal biomass gasification: The role of the catalysed water-gas shift reaction. Z. Für Phys. Chem. 2005, 219, 341–366. [Google Scholar] [CrossRef]
- Huang, B.S.; Chen, H.Y.; Chuang, K.H.; Yang, R.X.; Wey, M.Y. Hydrogen production by biomass gasification in a fluidized-bed reactor promoted by an Fe/CaO catalyst. Int. J. Hydrogen Energy 2012, 37, 6511–6518. [Google Scholar] [CrossRef]
- Tang, G.; Gu, J.; Huang, Z.; Yuan, H.; Chen, Y. Cellulose gasification with Ca-Fe oxygen carrier in chemical-looping process. Energy 2022, 239, 122204. [Google Scholar] [CrossRef]
- Zamboni, I.; Courson, C.; Kiennemann, A. Fe-Ca interactions in Fe-based/CaO catalyst/sorbent for CO2 sorption and hydrogen production from toluene steam reforming. Appl. Catal. B Environ. 2017, 203, 154–165. [Google Scholar] [CrossRef]
- Chan, M.S.C.; Liu, W.; Ismail, M.; Yang, Y.; Scott, S.A.; Dennis, J.S. Improving hydrogen yields, and hydrogen:steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chem. Eng. J. 2016, 296, 406–411. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, P.; Zhao, H. Chemical looping gasification of coal using calcium ferrites as oxygen carrier. Fuel Process. Technol. 2019, 192, 75–86. [Google Scholar] [CrossRef]
- Hirabayashi, D.; Yoshikawa, T.; Mochizuki, K.; Suzuki, K.; Sakai, Y. Formation of brownmillerite type calcium ferrite (Ca2Fe2O5) and catalytic properties in propylene combustion. Catal. Lett. 2006, 110, 269–274. [Google Scholar] [CrossRef]
- Shin, S.; Hatakeyama, Y.; Ogawa, K.; Shimomura, K.Y. Catalytic decomposition of NO over brownmillerite-like compounds, Ca2Fe2O5 and Sr2Fe2O5. Mater. Res. Bull. 1979, 14, 133–136. [Google Scholar] [CrossRef]
- Penkala, B.; Gatla, S.; Aubert, D.; Ceretti, M.; Tardivat, C.; Paulus, W.; Kaper, H. In situ generated catalyst: Copper(ii) oxide and copper(i) supported on Ca2Fe2O5 for CO oxidation. Catal. Sci. Technol. 2018, 8, 5236–5243. [Google Scholar] [CrossRef]
- Antunes, I.; Ruivo, L.C.M.; Tarelho, L.A.C.; Yaremchenko, A.A.; Frade, J.R. Solid state synthesis of Ca2Fe2O5 by reactive firing of calcite and siderite. Ceram. Int. 2022, 48, 34025–34032. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Y.; Liu, G.; Ma, X. Syngas production by chemical looping gasification of biomass with steam and CaO additive. Int. J. Hydrogen Energy 2018, 43, 19375–19383. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Chan, M.S.C.; Dunstan, M.T.; Scott, S.A. Synthesis, application, and carbonation behavior of Ca2Fe2O5 for chemical looping H2 production. Energy Fuels 2016, 30, 6220–6232. [Google Scholar] [CrossRef]
- Ikenaga, N.; Ohgaito, Y.; Suzuki, T. H2S absorption behavior of calcium ferrite prepared in the presence of coal. Energy Fuels 2005, 19, 170–179. [Google Scholar] [CrossRef]
- Shaula, A.L.; Markov, A.A.; Naumovich, E.N.; Waerenborgh, J.C.; Pivak, Y.V.; Kharton, V.V. Redox behaviour and transport properties of brownmillerite Ca2(Fe,M)2O5±δ (M = Mn, Co). Solid. State Ion. 2012, 225, 206–210. [Google Scholar] [CrossRef]
- Kharton, V.V.; Tsipis, E.V.; Kolotygin, V.A.; Avdeev, M.; Viskup, A.P.; Waerenborgh, J.C.; Frade, J.R. Mixed Conductivity and Stability of CaFe2O4−δ. J. Electrochem. Soc. 2008, 155, 13–20. [Google Scholar] [CrossRef]
- Sun, S.Z.; He, S.; Wu, C.F. Ni promoted Fe-CaO dual functional materials for calcium chemical dual looping. Chem. Eng. J. 2022, 441, 135752. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S.; Russell, C.K.; Hu, J.; Rony, A.H.; Tan, G.; Chen, A.; Duan, L.; Boman, J.; Tang, J.; et al. Improvement of H2-rich gas production with tar abatement from pine wood conversion over bi-functional Ca2Fe2O5 catalyst: Investigation of inner looping redox reaction and promoting mechanisms. Appl. Energy 2018, 212, 931–943. [Google Scholar] [CrossRef]
- Smith, R.; Loganathan, M.; Shantha, M.S. A review of the water gas shift reaction kinetics. Int. J. Chem. React. Eng. 2010, 8, 4. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, T.-C.; Hung, C.-I.; Lin, B.-J. Chemical reactions and kinetics of a low-temperature water gas shift reaction heated by microwaves. Int. J. Hydrogen Energy 2012, 37, 276–289. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and limitations of microwave reactors: From chemical synthesis to the catalytic valorization of biobased chemicals, with significant improvement in H2 yield. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Li, L.; Qiao, B.; Liu, J.; Su, Y.; Wang, X. Identifying size effects of Pt as single atoms and nanoparticles supported on FeOx for the water-gas shift reaction. ACS Catal. 2018, 8, 859–868. [Google Scholar] [CrossRef]
- Saw, E.; Oemar, U.; Tan, X.; Du, Y.; Borgna, A.; Hidajat, K.; Kawit, S. Bimetallic Ni–Cu catalyst supported on CeO2 for high-temperature water–gas shift reaction: Methane suppression via enhanced CO adsorption. J. Catal. 2014, 314, 32–46. [Google Scholar] [CrossRef]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Zhang, H.; Liu, M.; Fu, Y.; Zhang, Z.S.X.; Zhong, Q. The characterization and mechanism of carbonated steel slag and its products under low CO2 pressure. Mater. Today Commun. 2023, 35, 105827. [Google Scholar] [CrossRef]
- Bekhti, H.; Boucheffa, Y.; Blal, A.H.A.; Travert, A. In situ FTIR investigation of CO2 adsorption over MgO–Impregnated NaY Zeolite. Vib. Spectrosc. 2021, 117, 103313. [Google Scholar] [CrossRef]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, S.; Zhao, L.; Yuan, X.; Wang, B.; Chen, L.; Wu, K. Synergistic and competitive effect of H2O on CO2 adsorption capture: Mechanism explanations based on molecular dynamic simulation. J. CO2 Util. 2021, 52, 101662. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Malveiro, J.; Ramos, T.; Ferreira, L.P.; Waerenborgh, J.C.; Nunes, M.R.; Godinho, M.; Carvalho, M.D. Magnesium doping on brownmillerite Ca2FeAlO5. J. Solid State Chem. 2007, 180, 1863–1874. [Google Scholar] [CrossRef]
- Phan, T.-L.; Tran, N.; Kim, D.H.; Tho, P.T.; Huy, B.T.; Dang, T.N.; Yang, D.-S.; Lee, B. Electronic structure and magnetic properties of Al-doped Ca2Fe2O5 brownmillerite compounds. J. Am. Ceram. Soc. 2018, 101, 2181–2189. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-specific enhancement of the carbon−carbon dioxide (Boudouard) reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Tanaka, M.; Kono, H.; Maruyama, K. Selective heating mechanism of magnetic metal oxides by a microwave magnetic field. Phys. Rev. B 2009, 79, 104420. [Google Scholar] [CrossRef]
- Shaula, A.L.; Pivak, Y.V.; Waerenborgh, J.C.; Gaczyñski, P.; Yaremchenko, A.A.; Kharton, V.V. Ionic conductivity of brownmillerite-type calcium ferrite under oxidizing conditions. Solid. State Ion. 2006, 177, 2923–2930. [Google Scholar] [CrossRef]
- Karbi, S.B.; Hona, R.K.; Ramezanipour, F. Effect of structure on sensor properties of oxygen-deficient perovskites, A2BB’O5 (A = Ca, Sr; B = Fe; B’ = Fe, Mn) for oxygen, carbon dioxide and carbon monoxide sensing. Electron. Mater. 2020, 49, 1557–1567. [Google Scholar] [CrossRef]
- Wang, J.; Gu, J.; Rony, A.; Fan, M.; Leszczynsky, J. Theoretical DFT study on the mechanisms of CO/CO2 conversion in chemical looping catalyzed by calcium ferrite. J. Phys. Chem. A 2021, 125, 8159–8167. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, N.; Guo, X.; Zhang, S. Reaction mechanism of Ca2Fe2O5 oxygen carrier with CO in chemical looping hydrogen production. Appl. Surf. Sci. 2020, 534, 147583. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Islam, M.S. Mixed ionic/electronic conductors Sr2Fe2O5 and Sr4Fe6O13: Atomic-scale studies of defects and ion migration. J. Mater. Chem. 2005, 15, 3200–3207. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Liang, X. Reducing gas atmosphere (H2, CO) assisted formation of Fe-Ce-Ox composite oxides with enhanced catalytic activity for water-gas shift reaction. Catal. Commun. 2020, 138, 105849. [Google Scholar] [CrossRef]
- Galeano, Y.M.; Negri, F.; Moreno, M.S.; Múnera, J.; Cornaglia, L.; Tarditi, A.M. Pt encapsulated into NaA zeolite as catalyst for the WGS reaction. Appl. Catal. A Gen. 2019, 572, 176–184. [Google Scholar] [CrossRef]
- Ruivo, L.; Oliveira, H.; Gomes, H.; Cruz, N.; Yaremchenko, A.; Tarelho, L.A.C.; Frade, J. Siderite/Concrete catalysts for H2-enriched gas production from biomass steam gasification. Energy Convers. Manag. 2022, 255, 115280. [Google Scholar] [CrossRef]
- Ruivo, L.C.M.; Pio, D.T.; Yaremchenko, A.A.; Tarelho, L.A.C.; Frade, J.R.; Kantarelis, E.; Engvall, K. Iron-based catalyst (Fe2−xNixTiO5) for tar decomposition in biomass gasification. Fuel 2021, 300, 120859. [Google Scholar] [CrossRef]
- Ruivo, L.; Silva, T.; Neves, D.; Tarelho, L.; Frade, J. Thermodynamic guidelines for improved operation of iron-based catalysts in gasification of biomass. Energy 2023, 268, 126641. [Google Scholar] [CrossRef]
- Yokokawa, H.; Kawada, T.; Dokiya, M. Construction of chemical potential diagrams for metal-metal-nonmetal systems: Applications to the decomposition of double oxides. J. Am. Ceram. Soc. 1989, 72, 2104–2110. [Google Scholar] [CrossRef]
- Yokokawa, H.; Sakai, N.; Kawada, T.; Dokiya, M. Thermodynamic stabilities of perovskite oxides for electrodes and other electrochemical materials. Solid. State Ion. 1992, 52, 43–56. [Google Scholar] [CrossRef]




| Heating Type | Yield (%) | Blank | Catalyst |
|---|---|---|---|
| Conventional | CO2 | 0.2 | 17 |
| Microwave | 0.4 | 61 | |
| Conventional | H2 | 0.1 | 16 |
| Microwave | 0.2 | 58 |
| T (°C) | H2O:CO | GHSV (h−1) | Catalyst | Heating | XCO (%) | H2 Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 400 | 1:1 | ≈28,000 | (Fe-Cr)-based | electric | ≈49 | [4] | |
| 500 | ≈50 | ||||||
| 400 | microwave | ≈44 | |||||
| 500 | ≈58 | ||||||
| 400 | 2:1 | ≈49 | |||||
| 400 | 4:1 | ≈67 | |||||
| 500 | 2:1 | ≈71 | |||||
| 200 | 2:1 | ≈28,000 | (Cu-Zn)-based | microwave | ≈42 | [30] | |
| 300 | 2:1 | ≈62 | |||||
| 300 | 4:1 | ≈93 | |||||
| 400 | 1.25:1 | ≈6000 | Ce-Fe-Ox | electric | ≈36–46 | [49] | |
| 500 | ≈71–84 | ||||||
| 300 | 2:1 | ≈9000 | Pt-NaA zeolite | electric | ≈15 | [50] | |
| 400 | ≈68 | ||||||
| 500 | 2:1 | 14,500–18,250 | 75%sid. + 25%conc. | electric | 19 | [51] | |
| 600 | 3:1 | 14,500–18,250 | 75%sid. + 25%conc. | 47 | |||
| 500 | 3:1 | 14,500–18,250 | 50%sid. + 50%conc. | 48 | |||
| 600 | 1:1 | 14,500–18,250 | 50%sid. + 50%conc. | 40 | |||
| 400 | 2:1 | ≈6000 | Ca2Fe2O5 | microwave | 10 | 6 | present work |
| 500 | 2:1 | 52 | 51 | ||||
| 500 | 3:1 | 66 | 58 | ||||
| 500 | 3:1 | electric | 24 | 16 | |||
| 500 | 3:1 | blank | microwave | 9 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, I.; Ruivo, L.C.M.; Tarelho, L.A.C.; Frade, J.R. Ca2Fe2O5-Based WGS Catalysts to Enhance the H2 Yield of Producer Gases. Catalysts 2024, 14, 12. https://doi.org/10.3390/catal14010012
Antunes I, Ruivo LCM, Tarelho LAC, Frade JR. Ca2Fe2O5-Based WGS Catalysts to Enhance the H2 Yield of Producer Gases. Catalysts. 2024; 14(1):12. https://doi.org/10.3390/catal14010012
Chicago/Turabian StyleAntunes, Isabel, Luís C. M. Ruivo, Luís A. C. Tarelho, and Jorge R. Frade. 2024. "Ca2Fe2O5-Based WGS Catalysts to Enhance the H2 Yield of Producer Gases" Catalysts 14, no. 1: 12. https://doi.org/10.3390/catal14010012






