Perovskite-Derivative Ni-Based Catalysts for Hydrogen Production via Steam Reforming of Long-Chain Hydrocarbon Fuel
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of Catalysts
2.3. Characterization
2.4. Catalytic Performance Evaluation
3. Results and Discussion
3.1. Structural and Morphological Characteristics of Catalysts
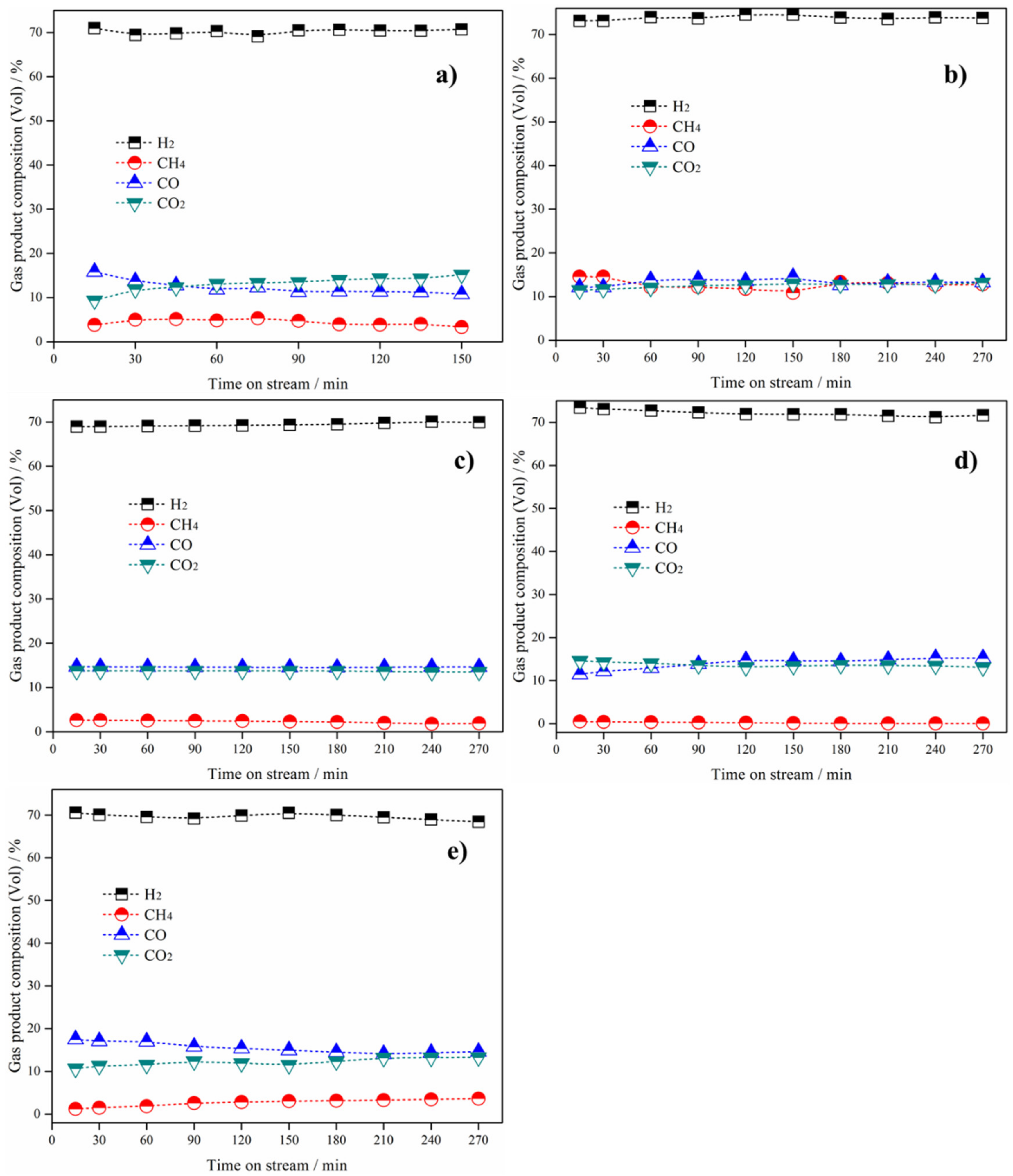
3.2. Catalytic Performance for H2 Production
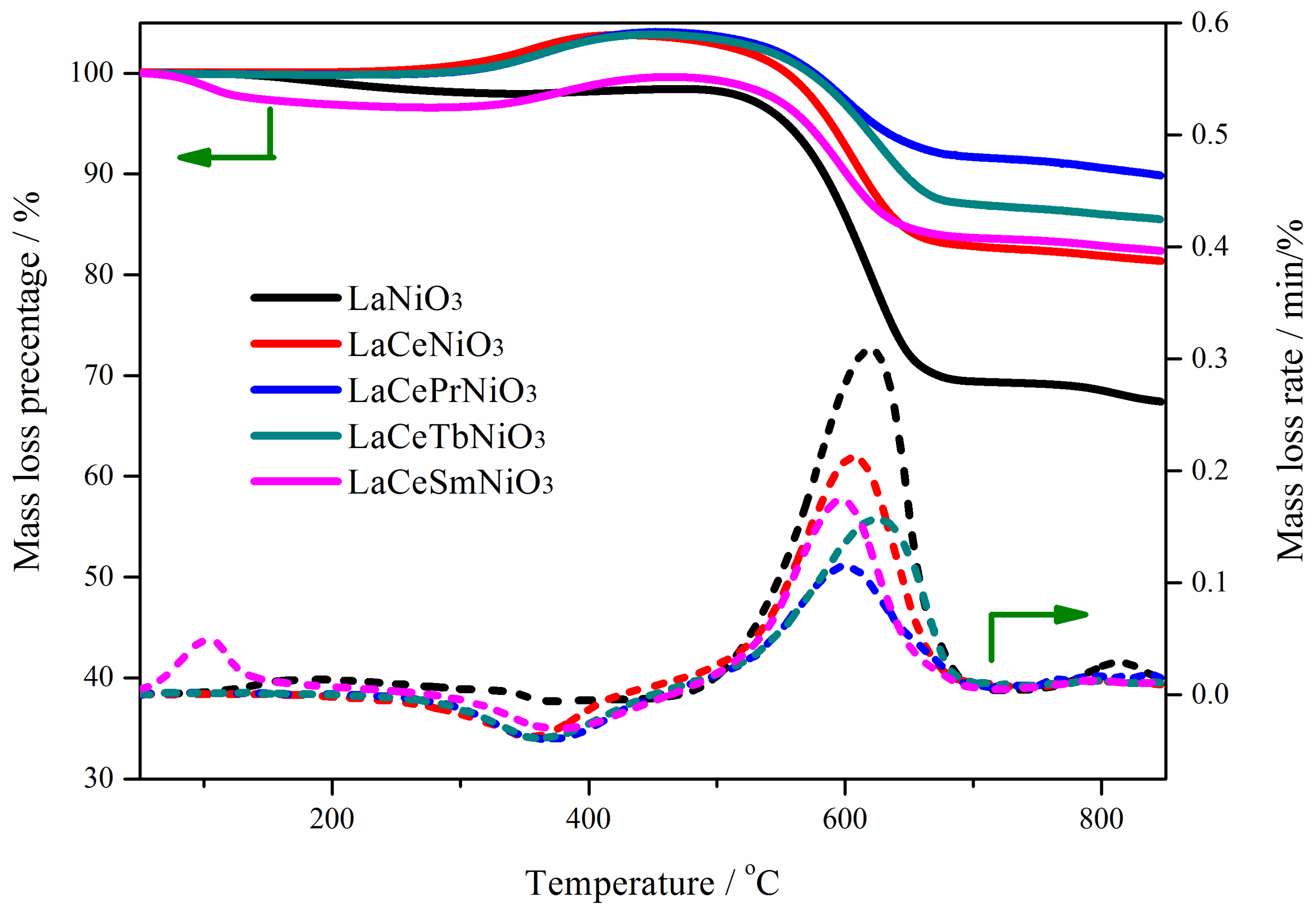
3.3. Analysis of Carbon Deposition on Catalyst after Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Su, H.R.; Hu, Y.H. Progress in low-temperature solid oxide fuel cells with hydrocarbon fuels. Chem. Eng. J. 2020, 402, 126235. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Park, H.G.; Han, S.Y.; Jun, K.W.; Woo, Y.; Park, M.J.; Kim, S.K. Bench-scale steam reforming of methane for hydrogen production. Catalysts 2019, 9, 615. [Google Scholar] [CrossRef]
- Zhan, Z.; Barnett, S.A. An octane-fueled solid oxide fuel cell. Science 2005, 308, 844–848. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, C.; Li, P.; Wang, D.; Zhang, X.; Wang, L.; Zou, J.-J.; Li, G. Engineering oxygen vacancies on Tb-doped ceria supported Pt catalyst for hydrogen production through steam reforming of long-chain hydrocarbon fuels. Chin. J. Chem. Eng. 2024, in press. [Google Scholar] [CrossRef]
- Do, L.T.; Nguyen-Phu, H.; Pham, N.N.; Jeong, D.H.; Shin, E.W. Highly dispersed nickel nanoparticles on hierarchically ordered macroporous Al2O3 and its catalytic performance for steam reforming of 1-methyl naphthalene. Catalysts 2022, 12, 1542. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Y.S.; Engelhard, M.H.; Song, C.S. Comparative study on the sulfur tolerance and carbon resistance of supported noble metal catalysts in steam reforming of liquid hydrocarbon fuel. ACS Catal. 2012, 2, 1127–1137. [Google Scholar] [CrossRef]
- Zheng, Q.C.; Xiao, Z.R.; Xu, J.S.; Pan, L.; Zhang, X.W.; Zou, J.J. Catalytic steam reforming and heat sink of high-energy-density fuels: Correlation of reaction behaviors with molecular structures. Fuel 2021, 286, 119371. [Google Scholar] [CrossRef]
- Xue, Q.Q.; Li, Z.W.; Chen, M.; Wang, Y.J.; Yan, B.H.; Luo, G.S. Co-precipitation continuous synthesis of the Ni-Rh-Ce0.75Zr0.25O2-δ catalyst in the membrane dispersion microreactor system for n-dodecane steam reforming to hydrogen. Fuel 2021, 297, 120785. [Google Scholar] [CrossRef]
- Li, D.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Metal catalysts for steam reforming of tar derived from the gasification of lignocellulosic biomass. Bioresour. Technol. 2015, 178, 53–64. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Shoynkhorova, T.B.; Simonov, P.A.; Potemkin, D.I.; Snytnikov, P.V.; Belyaev, V.D.; Ishchenko, A.V.; Svintsitskiy, D.A.; Sobyanin, V.A. Highly dispersed Rh-, Pt-, Ru/Ce0.75Zr0.25O2-δ catalysts prepared by sorption-hydrolytic deposition for diesel fuel reforming to syngas. Appl. Catal. B—Environ. 2018, 237, 237–244. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, J.S.; Lee, Y.J.; Park, N.K.; Kim, Y.C.; Hong, S.I.; Moon, D.J. Steam reforming of n-hexadecane over noble metal-modified Ni-based catalysts. Catal. Today 2008, 136, 228–234. [Google Scholar] [CrossRef]
- Ming, Q.; Healey, T.; Allen, L.; Irving, P. Steam reforming of hydrocarbon fuels. Catal. Today 2002, 77, 51–64. [Google Scholar] [CrossRef]
- Li, L.; Zuo, S.W.; An, P.F.; Wu, H.Y.; Hou, F.; Li, G.Z.; Liu, G.Z. Hydrogen production via steam reforming of n-dodecane over NiPt alloy catalysts. Fuel 2020, 262, 116469. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Z.; Zhang, X. Pt and ceria promoted Ni/SBA-15 for n-dodecane steam reforming for hydrogen production. Chem. Ind. Eng. 2019, 36, 1–10. [Google Scholar]
- Miyamoto, M.; Arakawa, M.; Oumi, Y.; Uemiya, S. Influence of metal cation doping on Ru/CeO2/Al2O3 catalyst for steam reforming of desulfurized kerosene. Int. J. Hydrogen Energy 2015, 40, 2657–2662. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Wang, L.; Liu, G. High-performance Rh/CeO2 catalysts prepared by L-lysine-assisted deposition precipitation method for steam reforming of toluene. Fuel 2023, 341, 127736. [Google Scholar] [CrossRef]
- Li, Z.W.; Li, M.; Ashok, J.; Sibudjing, K. NiCo@NiCo phyllosilicate@CeO2 hollow core shell catalysts for steam reforming of toluene as biomass tar model compound. Energy Convers. Manag. 2019, 180, 822–830. [Google Scholar] [CrossRef]
- Zhang, H.C.; Xiao, Z.R.; Yang, M.; Zou, J.J.; Liu, G.Z.; Zhang, X.W. Highly dispersible cerium-oxide modified Ni/SBA-15 for steam reforming of bio-mass based JP10. Chin. J. Chem. Eng. 2022, 43, 255–265. [Google Scholar] [CrossRef]
- Tribalis, A.; Panagiotou, G.D.; Bourikas, K.; Sygellou, L.; Kennou, S.; Ladas, S.; Lycourghiotis, A.; Kordulis, C. Ni catalysts supported on modified alumina for diesel steam reforming. Catalysts 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.L.; Xiao, Z.R.; Zhang, H.; Ye, F.; Gu, J.M.; Wang, J.D.; Li, G.Z.; Wang, D.S. Ni-TiO2 catalysts derived from metal-organic framework for efficient photo-thermal CO2 methanation. Fuel 2024, 357, 129817. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, P.; Zhang, H.; Zhang, S.; Tan, X.; Ye, F.; Gu, J.; Zou, J.-j.; Wang, D. A comprehensive review on photo-thermal co-catalytic reduction of CO2 to value-added chemicals. Fuel 2024, 362, 130906. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H.; Lee, H.; Seo, J.C.; Lee, K. Effect of Mg contents on catalytic activity and coke formation of mesoporous Ni/Mg-aluminate spinel catalyst for steam methane reforming. Catalysts 2020, 10, 828. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Li, Y.T.; Hou, F.; Wu, C.; Pan, L.; Zou, J.J.; Wang, L.; Zhang, X.W.; Liu, G.Z.; Li, G.Z. Engineering oxygen vacancies and nickel dispersion on CeO2 by Pr doping for highly stable ethanol steam reforming. Appl. Catal. B—Environ. 2019, 258, 117940. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, L.; Xu, J.; Zheng, Q.; Pan, L.; Zou, J.; Zhang, X.; Li, G. Boosting hydrogen production from steam reforming of ethanol over nickel by lanthanum doped ceria. Appl. Catal. B—Environ. 2021, 286, 119884. [Google Scholar] [CrossRef]
- Sugisawa, M.; Takanabe, K.; Harada, M.; Kubota, J.; Domen, K. Effects of La addition to Ni/Al2O3 catalysts on rates and carbon deposition during steam reforming of n-dodecane. Fuel Process. Technol. 2011, 92, 21–25. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Wu, C.; Li, L.; Li, G.Z.; Liu, G.Z.; Wang, L. Pursuing complete and stable steam reforming of n-dodecane over nickel catalysts at low temperature and high LHSV. Int. J. Hydrogen Energy 2017, 42, 5606–5618. [Google Scholar] [CrossRef]
- Ye, F.; Qian, J.; Xia, J.J.; Li, L.F.; Wang, S.J.; Zeng, Z.X.; Miao, J.; Ahamad, M.; Xiao, Z.R.; Zhang, Q.R. Effect photoelectrocatalytic degradation of pollutants over hydrophobic carbon felt loaded with Fe-doped porous carbon nitride via direct activation of molecular oxygen. Environ. Res. 2014, 249, 118497. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Li, L.; Wu, C.; Li, G.Z.; Liu, G.Z.; Wang, L. Ceria-promoted Ni–Co/Al2O3 catalysts for n-dodecane steam reforming. Catal. Lett. 2016, 146, 1780–1791. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Zheng, Q.C.; Zhang, X.W.; Li, L.; Wang, L.; Li, G.Z. Synthesis of Ni-Co catalysts supported on flower-like MgAl composite oxide for hydrogen production by n-dodecane steam reforming. Chin. J. Inorg. Chem. 2021, 37, 629–637. [Google Scholar]
- Xiao, Z.R.; Ji, S.; Hou, F.; Li, Y.T.; Zhang, H.C.; Wang, L.; Zhang, X.W.; Liu, G.Z.; Zou, J.J.; Li, G.Z. n-Dodecane steam reforming catalyzed by Ni-Ce-Pr catalysts. Part 1: Catalyst preparation and Pr doping. Catal. Today 2018, 316, 78–90. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Zhang, X.W.; Hou, F.; Wu, C.; Wang, L.; Li, G.Z. Tuning metal-support interaction and oxygen vacancies of ceria supported nickel catalysts by Tb doping for n-dodecane steam reforming. Appl. Surf. Sci. 2020, 503, 144319. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, X.; Wang, L.; Li, G. Optimizing the preparation of Ni-Ce-Pr catalysts for efficient hydrogen production by n-dodecane steam reforming. Int. J. Energy Res. 2019, 44, 1828–1842. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, P.S.; Hidajat, K.; Kawi, S. Promotional effect of Fe on perovskite LaNixFe1−xO3 catalyst for hydrogen production via steam reforming of toluene. Int. J. Hydrogen Energy 2013, 38, 5525–5534. [Google Scholar] [CrossRef]
- Chen, G.K.; Shen, Q.W.; Zhang, X.; Cai, Z.W.; Shao, Z.C.; Li, S.; Yang, G.G. Methanol steam reforming over La1-xSrxCeO3-δ catalysts for hydrogen production: Optimization of operating parameters. Catalysts 2023, 13, 248. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, S.; Chen, Y.; Yan, B.; Cheng, Y. Effect of the calcination temperature of LaNiO3 on the structural properties and reaction performance of catalysts in the steam reforming of methane. Catalysts 2023, 13, 356. [Google Scholar] [CrossRef]
- Das, S.; Bhattar, S.; Liu, L.N.; Wang, Z.G.; Xi, S.B.; Spivey, J.J.; Kawi, S. Effect of partial Fe substitution in La0.9Sr0.1NiO3 perovskite-derived catalysts on the reaction mechanism of methane dry reforming. ACS Catal. 2020, 10, 12466–12486. [Google Scholar] [CrossRef]
- Pereñiguez, R.; Gonzalez-delaCruz, V.M.; Caballero, A.; Holgado, J.P. LaNiO3 as a precursor of Ni/La2O3 for CO2 reforming of CH4: Effect of the presence of an amorphous NiO phase. Appl. Catal. B—Environ. 2012, 123–124, 324–332. [Google Scholar] [CrossRef]
- Yu, J.B.; Muhetaer, A.; Gao, X.W.; Zhang, Z.Z.; Yang, Y.Y.; Li, Q.; Chen, L.X.; Liu, H.C.; Xu, D.S. Highly active hydrogen-rich photothermal reverse water gas shift reaction on Ni/LaInO3 perovskite catalysts with near-unity selectivity. Angew. Chem. Int. Ed. 2023, 62, e202303135. [Google Scholar] [CrossRef]
- Wu, G.W.; Li, S.R.; Zhang, C.X.; Wang, T.; Gong, J.L. Glycerol steam reforming over perovskite-derived nickel-based catalysts. Appl. Catal. B—Environ. 2014, 144, 277–285. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Assaf, J.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Evaluation of the performance of Ni/La2O3 catalyst prepared from LaNiO3 perovskite-type oxides for the production of hydrogen through steam reforming and oxidative steam reforming of ethanol. Appl. Catal. A 2010, 377, 181–190. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J. Catalytic performance of Cu-Ni/La0.75Sr0.25Cr0.5Mn0.5O3-δ for dry methane reforming. Int. J. Energy Res. 2022, 46, 10522–10534. [Google Scholar] [CrossRef]
- Bussi, J.; Musso, M.; Quevedo, A.; Faccio, R.; Romero, M. Structural and catalytic stability assessment of Ni-La-Sn ternary mixed oxides for hydrogen production by steam reforming of ethanol. Catal. Today 2017, 296, 154–162. [Google Scholar] [CrossRef]
- Kim, T.; Song, K.H.; Yoon, H.; Chung, J.S. Steam reforming of n-dodecane over K2Ti2O5-added Ni-alumina and Ni-zirconia (YSZ) catalysts. Int. J. Hydrogen Energy 2016, 41, 17922–17932. [Google Scholar] [CrossRef]
- Li, D.L.; Koike, M.; Chen, J.H.; Nakagawa, Y.; Tomishige, K. Preparation of Ni-Cu/Mg/Al catalysts from hydrotalcite-like compounds for hydrogen production by steam reforming of biomass tar. Int. J. Hydrogen Energy 2014, 39, 10959–10970. [Google Scholar] [CrossRef]
- Jing, J.Y.; Wei, Z.H.; Zhang, Y.B.; Bai, H.C.; Li, W.Y. Carbon dioxide reforming of methane over MgO-promoted Ni/SiO2 catalysts with tunable Ni particle size. Catal. Today 2020, 356, 589–596. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Li, P.; Zhang, S.L.; Gu, J.M.; Wang, D.S. Effects of metal-support interactions and interfaces on catalytic performance over M2O3- (M = La, Al-) supported Ni catalysts. Int. J. Energy Res. 2023, 2023, 6504914. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.Y.; Wang, X.; Ma, H.Y.; Assabumrungrat, S.; Gong, J.L. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B—Environ. 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wang, Y.S.; Yang, Z.L.; Liang, T.; Liu, S.M.; Zhou, Z.S.; Li, X.J. Effect of Mg-modified mesoporous Ni/Attapulgite catalysts on catalytic performance and resistance to carbon deposition for ethanol steam reforming. Fuel 2018, 220, 32–46. [Google Scholar] [CrossRef]
- Xue, Q.Q.; Li, Z.W.; Yi, H.L.; Jiang, Z.; Yan, B.H.; Wang, Y.J.; Luo, G.S. Controlling sintering and carbon deposition with post-coated MgO confined group VIII metal-based catalysts towards durable high-temperature steam reforming. Appl. Catal. B—Environ. 2022, 318, 121874. [Google Scholar] [CrossRef]







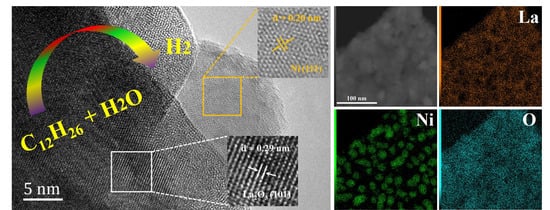
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| LaNiO3 | 2.84 | 0.65 | 14.29 |
| LaCeNiO3 | 1.15 | 0.26 | 13.00 |
| LaCePrNiO3 | 3.30 | 0.76 | 17.59 |
| LaCeSmNiO3 | 3.28 | 0.75 | 23.84 |
| LaCeTbNiO3 | 3.02 | 0.75 | 20.69 |
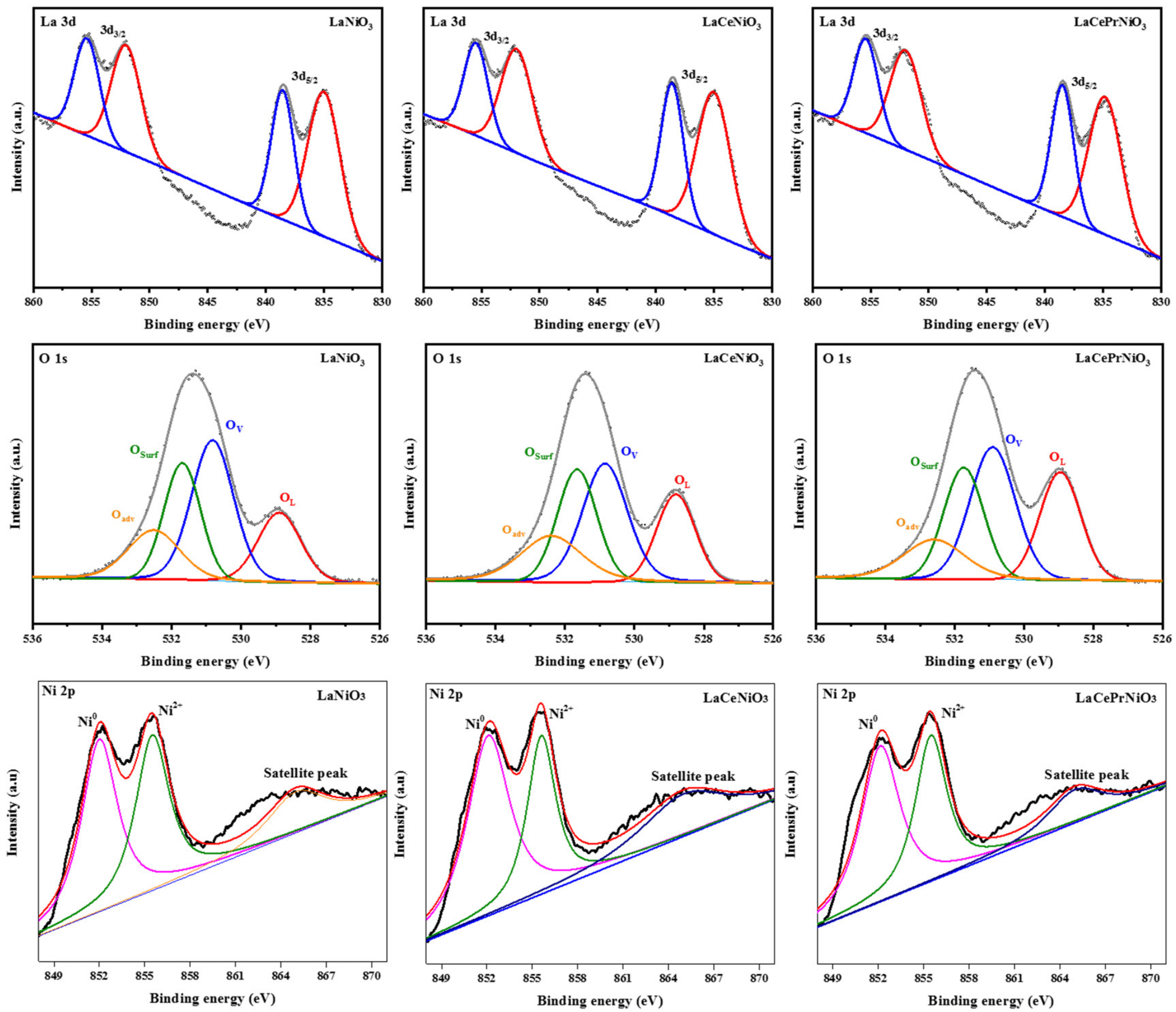
| Sample | Name | Peak Position (eV) | Area | Percentages (%) |
|---|---|---|---|---|
| LaNiO3 | La 3d5/2 | 835 | 89,853.57 | 35.4 |
| 838.55 | 56,589.05 | 22.3 | ||
| La 3d3/2 | 852.03 | 60,555.37 | 23.8 | |
| 855.39 | 46,967.88 | 18.5 | ||
| LaCeNiO3 | La 3d5/2 | 835 | 60,051.57 | 35.8 |
| 838.59 | 37,870.52 | 22.6 | ||
| La 3d3/2 | 851.97 | 41,413.96 | 24.6 | |
| 855.47 | 28,454.67 | 17.0 | ||
| LaCePrNiO3 | La 3d5/2 | 834.83 | 72,638.22 | 35.0 |
| 838.49 | 47,519.36 | 22.9 | ||
| La 3d3/2 | 851.99 | 49,820.91 | 24.0 | |
| 855.41 | 37,590.91 | 18.1 |
| Sample | Name | Peak Position (eV) | Area | Percentages (%) |
|---|---|---|---|---|
| LaNiO3 | OL | 528.9 | 13,140.8 | 19.1 |
| OV | 530.82 | 26,106.83 | 37.9 | |
| OSurf | 531.69 | 17,864.29 | 25.9 | |
| Oadv | 532.51 | 11,747.81 | 17.1 | |
| LaCeNiO3 | OL | 528.81 | 10,728.15 | 20.0 |
| OV | 530.85 | 18,226.5 | 33.9 | |
| OSurf | 531.66 | 14,346.38 | 26.7 | |
| Oadv | 532.4 | 10,391.31 | 19.4 | |
| LaCePrNiO3 | OL | 528.95 | 16,498.19 | 24.8 |
| OV | 530.91 | 23,063.9 | 34.8 | |
| OSurf | 531.74 | 16,776.45 | 25.2 | |
| Oadv | 532.6 | 10,121.5 | 15.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Zhang, H.; Zhang, C.; Guo, X.; Li, H.; Xiao, Z. Perovskite-Derivative Ni-Based Catalysts for Hydrogen Production via Steam Reforming of Long-Chain Hydrocarbon Fuel. Catalysts 2024, 14, 186. https://doi.org/10.3390/catal14030186
Guo K, Zhang H, Zhang C, Guo X, Li H, Xiao Z. Perovskite-Derivative Ni-Based Catalysts for Hydrogen Production via Steam Reforming of Long-Chain Hydrocarbon Fuel. Catalysts. 2024; 14(3):186. https://doi.org/10.3390/catal14030186
Chicago/Turabian StyleGuo, Kai, Hui Zhang, Changxuan Zhang, Xining Guo, Huiying Li, and Zhourong Xiao. 2024. "Perovskite-Derivative Ni-Based Catalysts for Hydrogen Production via Steam Reforming of Long-Chain Hydrocarbon Fuel" Catalysts 14, no. 3: 186. https://doi.org/10.3390/catal14030186