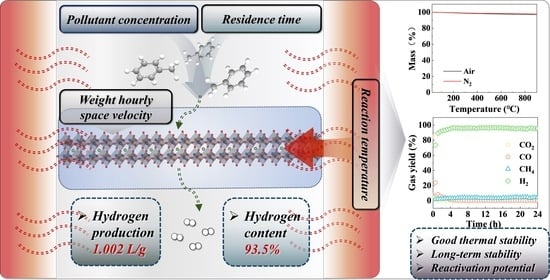
Deciphering the Pivotal Reaction Conditions for Hydrogen Production from Tar Catalytic Cracking by Perovskite
Abstract
:1. Introduction
2. Results and Discussion
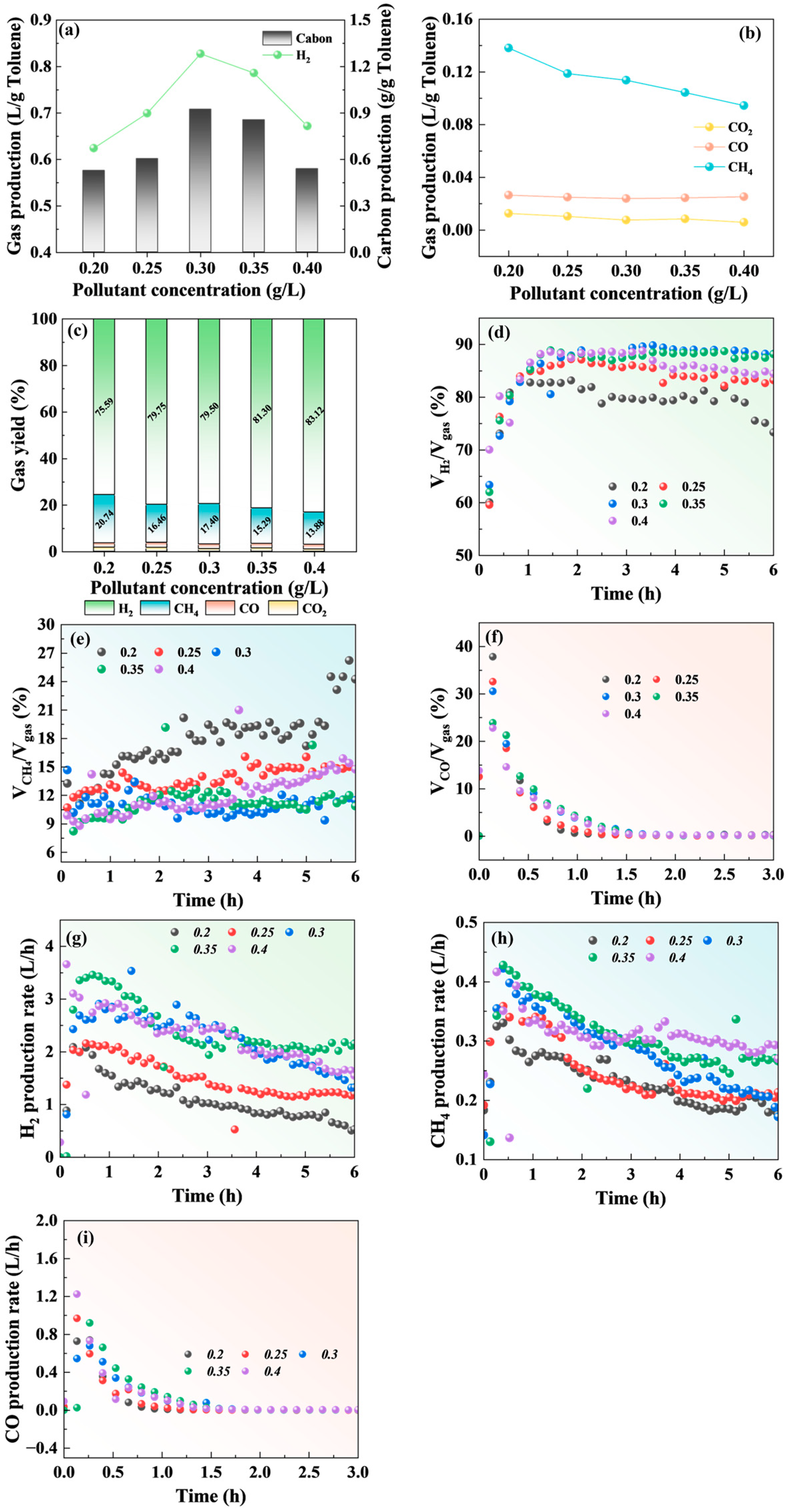
2.1. Effect of Pollutant Concentrations
2.2. Effect of Residence Time
2.3. Effect of Weight Hourly Space Velocity
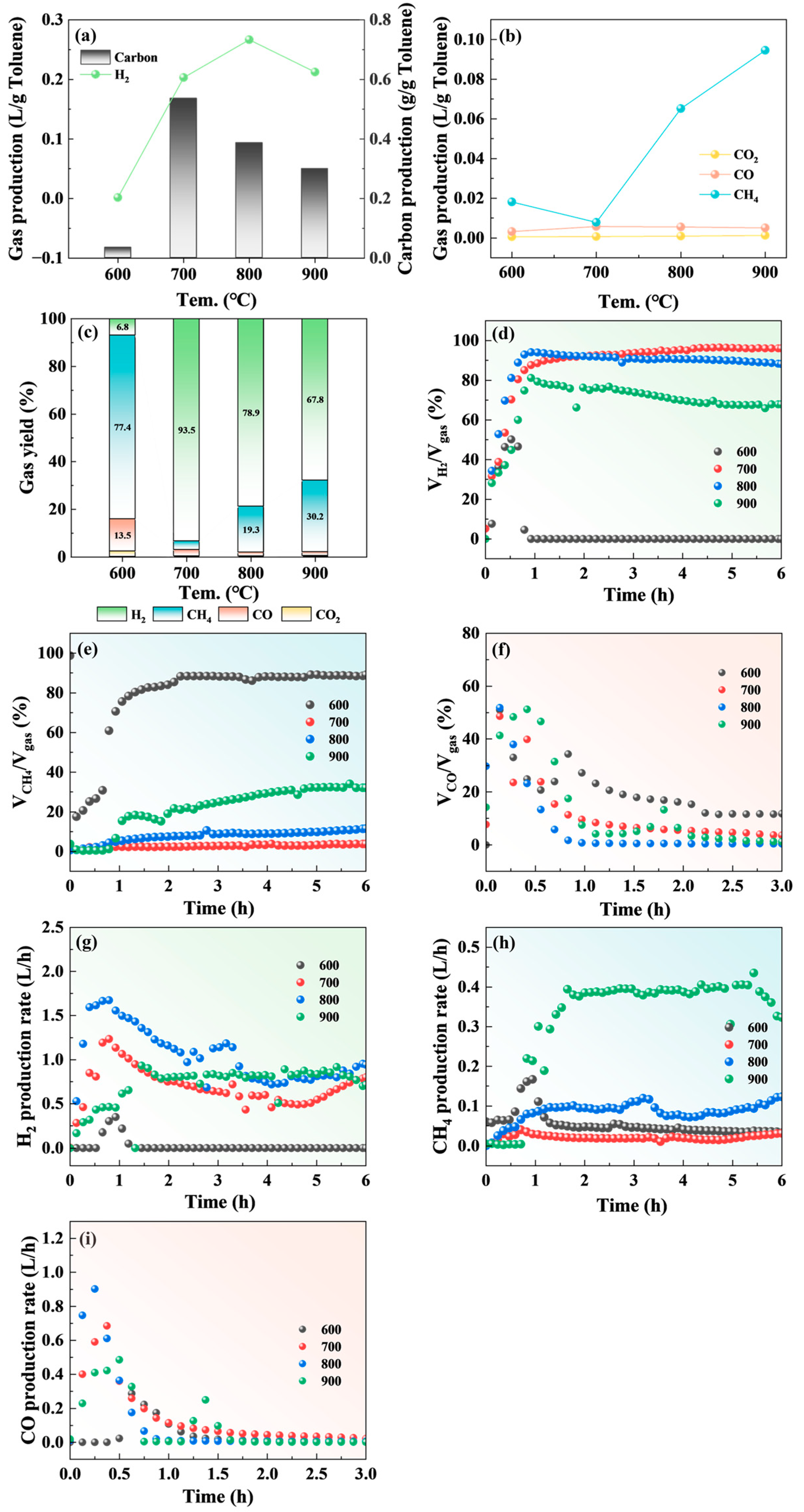
2.4. The Influence of Reaction Temperature
2.5. Comparison of Catalytic Performance
2.6. Catalyst Stability Evaluation
3. Material and Methods
3.1. Preparation of Perovskite
3.2. Catalyst Activity Testing
3.3. Characterizations
3.4. Data Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, M.; Li, G.Y.; Guan, Y.L.; Li, C.M.; Zhou, R.Q.; Zarnegar, A.M. Hydrogen and syngas production from steam gasification of biomass using cement as catalyst. Biomass. Convers. Bior. 2019, 10, 119–124. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Osazuwa, O.U.; Othman, N.H.; Setiabudi, H.D.; Sulaiman, S. Recent progress on catalyst development in biomass tar steam reforming: Toluene as a biomass tar model compound. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Ngo, T.N.L.T.; Chiang, K.Y.; Liu, C.F.; Chang, Y.H.; Wan, H.P. Hydrogen production enhancement using hot gas cleaning system combined with prepared Ni-based catalyst in biomass gasification. Int. J. Hydrogen Energy 2021, 46, 11269–11283. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Kumar, A.; Bellmer, D.D.; Huhnke, R.L. Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas. Renew. Energy 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Abou Rached, J.; El Hayek, C.; Dahdah, E.; Gennequin, C.; Aouad, S.; Tidahy, H.L.; Estephane, J.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Ni based catalysts promoted with cerium used in the steam reforming of toluene for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12829–12840. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Feng, Y.; Chen, T.; Chen, D.; Zheng, A.; Wei, G.; He, F.; Zhao, Z.; Wu, J.; et al. Exploring the Conversion Mechanisms of Toluene as a Biomass Tar Model Compound on NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 16539–16548. [Google Scholar] [CrossRef]
- Zhao, M.; Memon, M.Z.; Ji, G.; Yang, X.; Vuppaladadiyam, A.K.; Song, Y.; Raheem, A.; Li, J.; Wang, W.; Zhou, H. Alkali metal bifunctional catalyst-sorbents enabled biomass pyrolysis for enhanced hydrogen production. Renew. Energy 2020, 148, 168–175. [Google Scholar] [CrossRef]
- Wang, T.J.; Chang, J.; Wu, C.Z.; Fu, Y.; Chen, Y. The steam reforming of naphthalene over a nickel-dolomite cracking catalyst. Biomass Bioenergy 2005, 28, 508–514. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified nickel-based catalysts for improved steam reforming of biomass tar: A critical review. Renew. Sust. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Huang, S.; Xu, H.; Li, H.; Guo, Y.; Sun, Z.; Du, Y.; Li, H.; Zhang, J.; Pang, R.; Dong, Q.; et al. Preparation and characterization of char supported Ni Cu nanoalloy catalyst for biomass tar cracking together with syngas-rich gas production. Fuel. Process. Technol. 2021, 218, 106858. [Google Scholar] [CrossRef]
- Li, J.; Tao, J.; Yan, B.; Cheng, K.; Chen, G.; Hu, J. Microwave reforming with char-supported Nickel-Cerium catalysts: A potential approach for thorough conversion of biomass tar model compound. Appl. Energy 2020, 261, 114375. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Z.; Li, Y.; Yang, B.; Shang, J.; Wu, Z. Chemical looping reforming characteristics of methane and toluene from biomass pyrolysis volatiles based on decoupling strategy: Embedding NiFe2O4 in SBA-15 as an oxygen carrier. Chem. Eng. J. 2023, 466, 143228. [Google Scholar] [CrossRef]
- Jurado, L.; Papaefthimiou, V.; Thomas, S.; Roger, A.C. Upgrading syngas from wood gasification through steam reforming of tars over highly active Ni-perovskite catalysts at relatively low temperature. Appl. Catal. B-Environ. 2021, 299, 120687. [Google Scholar] [CrossRef]
- Dong, X.; Zhu, X.; Lin, F.; Yan, B.; Li, J.; Chen, G. UV–vis-enhanced photothermal catalytic reforming of biomass model tar via LaMnxNi1-xO3 perovskite catalyst. Chem. Eng. J. 2023, 457, 14112. [Google Scholar] [CrossRef]
- Gai, D.; Shi, J.; Cui, X.; Zhao, P.; Zuo, W.; Zhang, J.; Jia, G.; Huang, Z. Catalytic performance and mechanism of A-site vacancy deficient perovskite catalyst over tar cracking during biomass pyrolysis. J. Clean. Prod. 2023, 405, 126987. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, B.; Yang, H.; Cui, X.; Zhao, M. Chemical looping partial oxidation (CLPO) of toluene on LaFeO3 perovskites for tunable syngas production. Chem. Eng. J. 2023, 451, 138968. [Google Scholar] [CrossRef]
- Cui, X.; Wu, T.; Cao, J.P.; Tang, W.; Yang, F.L.; Zhu, B.A.; Wang, Z. Mechanism for catalytic cracking of coal tar over fresh and reduced LaNi1-xFexO3 perovskite. Fuel 2021, 288, 119683. [Google Scholar] [CrossRef]
- Gai, D.; Cui, X.; Wu, T.; Shi, J.; Zhao, P.; Zhang, J.; Xia, X. A-site disubstituted of La1−xSrxNi0.8Fe0.2O3 perovskite on coal pyrolysis volatiles catalytic cracking: Activity and reaction mechanism. Process Safe. Environ. 2022, 168, 748–759. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.; Li, H.; Zhao, Z. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane. Int. J. Hydrogen Energy 2014, 39, 3243–3252. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-type oxides LaFe1-xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production. Appl. Energy 2016, 168, 193–203. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Tian, D.; Wang, Y.; Zhu, X.; Wei, Y.; Zheng, M.; Luo, Y. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl. Catal. B-Environ 2017, 202, 51–63. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Xu, J.; Wu, Y. Hydrogen-Rich Gas Production from Two-Stage Catalytic Pyrolysis of Pine Sawdust with Nano-NiO/Al2O3 Catalyst. Catalysts 2022, 12, 256. [Google Scholar] [CrossRef]
- Lu, Q.; Shenfu, Y.; Chen, X.; Li, K.; Qian, T.; Zhao, Y.; Meng, L.; Xie, X.; Zhao, Y.; Zhou, Y. The effect of reaction condition on catalytic cracking of wheat straw pyrolysis volatiles over char-based Fe-Ni-Ca catalyst. Energy 2023, 263, 125722. [Google Scholar] [CrossRef]
- Gu, H.; Yang, J.; Song, G.; Cui, X.; Niu, M.; Zhao, S. LaFe1-xNix as a Robust Catalytic Oxygen Carrier for Chemical Looping Conversion of Toluene. Appl. Sci. 2021, 12, 391. [Google Scholar] [CrossRef]
- Chen, L.; Song, Z.; Zhang, S.; Chang, C.K.; Chuang, Y.C.; Peng, X.; Dun, C.; Urban, J.J.; Guo, J.; Chen, J.L.; et al. Ternary NiMo-Bi liquid alloy catalyst for efficient hydrogen production from methane pyrolysis. Science 2023, 381, 857–861. [Google Scholar] [CrossRef]
- Hojo, H.; Inohara, Y.; Ichitsubo, R.; Einaga, H. Catalytic properties of LaNiO3 and Mn-modified LaNiO3 catalysts for oxidation of CO and benzene. Catal. Today 2023, 410, 127–134. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Yan, B.; Li, J.; Wang, J.; Jiao, L.; Chen, G.; Ahmed, S.; Cao, Y. Chemical looping gasification of digestate: Investigation on the surface and lattice oxygen of perovskite oxygen carrier. Fuel 2022, 318, 123663. [Google Scholar] [CrossRef]
- He, J.; Yang, Q.; Song, Z.; Chang, W.; Huang, C.; Zhu, Y.; Ma, X.; Wang, X. Improving the carbon resistance of iron-based oxygen carrier for hydrogen production via chemical looping steam methane reforming: A review. Fuel 2023, 351, 128864. [Google Scholar] [CrossRef]
- Wang, H.W.; Wu, J.X.; Wang, X.Y.; Wang, H.; Liu, J.R. Formation of perovskite-type LaNiO3 on La-Ni/Al2O3-ZrO2 catalysts and their performance for CO methanation. J. Fuel. Chem. Technol. 2021, 49, 186–197. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Wang, L.; Liu, G. High-performance Rh/CeO2 catalysts prepared by l-lysine-assisted deposition precipitation method for steam reforming of toluene. Fuel 2023, 341, 127736. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Guo, Y.; Lu, G.; Boreave, A.; Retailleau, L.; Baylet, A.; Giroir-Fendler, A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl. Catal. B-Environ. 2014, 148–149, 490–498. [Google Scholar] [CrossRef]
- Vella, L.D.; Villoria, J.A.; Specchia, S.; Mota, N.; Fierro, J.L.G.; Specchia, V. Catalytic partial oxidation of CH4 with nickel-lanthanum-based catalysts. Catal. Today 2011, 171, 84–96. [Google Scholar] [CrossRef]
- Ruocco, C.; de Caprariis, B.; Palma, V.; Petrullo, A.; Ricca, A.; Scarsella, M.; De Filippis, P. Methane dry reforming on Ru perovskites, AZrRuO3: Influence of preparation method and substitution of A cation with alkaline earth metals. J. CO2 Util. 2019, 30, 222–231. [Google Scholar] [CrossRef]
- Shen, Y. Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification. Renew. Sust. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Huang, S.; Wu, S.; Li, Y.; Wu, Y.; Lei, T. Study on the mechanism of syngas production from catalytic pyrolysis of biomass tar by Ni-Fe catalyst in CO2 atmosphere. Fuel 2023, 335, 126705. [Google Scholar] [CrossRef]
- Lei, Z.; Hao, S.; Yang, J.; Lei, Z.; Dan, X. Study on solid waste pyrolysis coke catalyst for catalytic cracking of coal tar. Int. J. Hydrogen Energy 2020, 45, 19280–19290. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, Y.; Zhao, Y.; Meng, L.; Qin, T.; Chen, X.; Li, K.; Yuan, S. Catalytic pyrolysis of pine needle biomass over Fe-Co-K catalyst for H2-rich syngas production: Influence of catalyst preparation. Energy 2022, 244, 122602. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, Y.; Zhang, L.; Xie, X.; Yuan, S. The forms of active sites of Fe/Ca/Ni@C catalyst for the reforming of volatiles of biomass pyrolysis under N2 and CO2 atmosphere. Fuel 2022, 318, 123625. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Li, Y.; Liu, P.; Sun, T.; Yang, Y.; Wang, Z.; Li, H.; Lei, T. Synergistic modification of Ca/Co on Ni/HZSM-5 catalyst for pyrolysis of organic components in biomass tar. J. Anal. Appl. Pyrolysis 2022, 166, 136876. [Google Scholar] [CrossRef]
- Liu, H.; Ye, C.; Ye, Z.; Zhu, Z.; Wang, Q.; Tang, Y.; Luo, G.; Guo, W.; Dong, C.; Li, G.; et al. Catalytic cracking and catalyst deactivation/regeneration characteristics of Fe-loaded biochar catalysts for tar model compound. Fuel 2023, 334, 126810. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Chen, W.; Wu, Y.; Lei, T.; Huang, S.; Li, Y.; Wu, S.; Wang, Z. Catalytic pyrolysis of toluene as biomass tar model component using Ni/HZSM-5 modified by CeO2 and MgO promoters. J. Anal. Appl. Pyrolysis 2022, 162, 105436. [Google Scholar] [CrossRef]
- Guo, F.; Liang, S.; Jia, X.; Peng, K.; Jiang, X.; Qian, L. One-step synthesis of biochar-supported potassium-iron catalyst for catalytic cracking of biomass pyrolysis tar. Int. J. Hydrogen Energy 2020, 45, 16398–16408. [Google Scholar] [CrossRef]
- Shanmuganandam, K.; Thanikaikarasan, S.; Venkata Ramanan, M.; Anichai, J.; Sebastian, P.J. Enhancement of hydrogen energy from Casuarina equisetifolia using NiO-Pr2O3/TiO2 and NiO/TiO2 nano composites. Int. J. Hydrogen Energy 2020, 45, 4152–4160. [Google Scholar] [CrossRef]
- Du, S.; Dong, Y.; Guo, F.; Tian, B.; Mao, S.; Qian, L.; Xin, C. Preparation of high-activity coal char-based catalysts from high metals containing coal gangue and lignite for catalytic decomposition of biomass tar. Int. J. Hydrogen Energy 2021, 46, 14138–14147. [Google Scholar] [CrossRef]
- Yan, J.; Liu, W.; Sun, R.; Jiang, S.; Wang, S.; Shen, L. Chemical looping catalytic gasification of biomass over active LaNixFe1-xO3 perovskites as functional oxygen carriers. Chin. J. Chem. Eng. 2021, 36, 146–156. [Google Scholar] [CrossRef]
- Chava, R.; Roy, B.; Appari, S. Recent advances and perspectives of perovskite-derived Ni-based catalysts for CO2 reforming of biogas. J. CO2 Util. 2022, 65, 102206. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L. Promoting syngas production from steam reforming of toluene using a highly stable Ni/(Mg, Al)Ox catalyst. Appl. Catal. B-Environ. 2022, 300, 120743. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Xi, S.; Kawi, S. LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Convers. Manag. 2020, 206, 112475. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Lu, C.; Li, D.; Li, Z.; Gao, J.; Wei, J.; Li, K. Enhanced performance of LaFeO3 oxygen carriers by NiO for chemical looping partial oxidation of methane. Fuel. Process. Technol. 2022, 236, 107396. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Guan, Y.; Zhang, G.; Xing, D. Hydrogen production and coke resistance characteristic during volatile reforming over Fe2O3-Ce2O3/sludge char catalyst. J. Clean. Prod. 2024, 434, 139836. [Google Scholar] [CrossRef]
- Sun, Z.; Russell, C.K.; Whitty, K.J.; Eddings, E.G.; Dai, J.; Zhang, Y.; Fan, M.; Sun, Z. Chemical looping-based energy transformation via lattice oxygen modulated selective oxidation. Prog. Energy Combust. 2023, 96, 101045. [Google Scholar] [CrossRef]
- Zhang, Z.; Ou, Z.; Qin, C.; Ran, J.; Wu, C. Roles of alkali/alkaline earth metals in steam reforming of biomass tar for hydrogen production over perovskite supported Ni catalysts. Fuel 2019, 257, 116032. [Google Scholar] [CrossRef]
- Sun, R.; Shen, L.; Wang, S.; Bai, H. CO conversion over LaFeO3 perovskite during chemical looping processes: Influences of Ca-doping and oxygen species. Appl. Catal. B-Environ. 2022, 316, 121598. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Soomro, A.; Hu, J.; Sun, Z.; Ma, S.; Xiang, W. Effects of supports on reduction activity and carbon deposition of iron oxide for methane chemical looping hydrogen generation. Appl. Energy 2018, 225, 912–921. [Google Scholar] [CrossRef]
- Shen, X.; Sun, Y.; Wu, Y.; Wang, J.; Jiang, E.; Xu, X.; Su, J.; Jia, Z. The coupling of CH4 partial oxidation and CO2 splitting for syngas production via double perovskite-type oxides LaFexCo1−xO3. Fuel 2020, 268, 117381. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L. Direct Conversion of Syngas Produced from Steam Reforming of Toluene into Methane over a Ni/Biochar Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 11212–11222. [Google Scholar] [CrossRef]





| Catalytic Agent | T/°C | Tar Conversion Rate/% | Product | Time | |
|---|---|---|---|---|---|
| NiFe2O4 | 850 | 96.83 | H2: 0.91 L/g | 1 h | [6] |
| Pyrolysis coke | H2: 0.145 L/g H2: 56.78% | [36] | |||
| Nickel-dolomite | 700 | 94.8 | H2: 50.1% | 1 h | [8] |
| 700 | 84.3 | H2: 51.5% | 6 h | ||
| La1-xSrxNi0.8Fe0.2O3 | 700 | H2: 0.217 L/g CO: 0.258 L/g | 10 min | [15] | |
| Fe-Co-K | 700 | H2: 0.154 L/g CO: 0.108 L/g | 1 h | [37] | |
| 5Fe1.5Ca@C | 600 | H2: 0.299 L/g | [38] | ||
| Ni-4 wt%Ca-0.1 wt%Co | 900 | 98.6 | H2: 0.12 L/g H2: 51.6% | 40 min | [39] |
| Ni-4%Fe/HZSM-5 | 900 | 99.1 | H2: 0.224 L/g H2: 54.47% | 40 min | [35] |
| Fe-loaded biochar | 800 | 94.0 | H2: 81% | 90 min | [40] |
| Ni-4%Ce/HZSM-5 | 800 | 94.5 | H2: 0.04 L/g H2: 37.66% | [41] | |
| Ni-1%Mg/HZSM-5 | 800 | 89.5 | H2: 0.039 L/g H2: 37.64% | [41] | |
| PSC-K2FeO4 | 800 | 94.9 | H2: 0.18 L/g H2: ~30% CO: 0.3 L/g | [42] | |
| Nano-NiO/γ-A12O3 | 800 | 73.06 | H2: 0.47 L/g H2: 48.44% | [22] | |
| NiO-Pr2O3/TiO2 | 850 | 97 | H2: 22% | [43] | |
| Ni-Cu/ASC | 800 | 93.2 | H2: 0.304 L/g H2: 37.82% | [10] | |
| Fe-Ni-Ca | 800 | H2: 0.288 L/g H2: 71.48% | [23] | ||
| Ni-Cu/ASC | 800 | 93.2 | H2: 0.105 L/g H2: 33.87% | [10] | |
| High-activity coal char | 800 | 93.5 | H2: 0.175 L/g H2: 36.84% | [44] | |
| LaNi0.5Fe0.5O3 | 700 | 93.6 | H2 MAX: 1.002 L/g 93.5% | 6 h | This study |
| 800 | 99.4 | ||||
| 900 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-M.; Xi, B.-D.; Li, M.-X.; Ye, M.-Y.; Hou, J.-Q.; Wei, Y.-F.; Yu, C.-Z.; Meng, F.-H. Deciphering the Pivotal Reaction Conditions for Hydrogen Production from Tar Catalytic Cracking by Perovskite. Catalysts 2024, 14, 188. https://doi.org/10.3390/catal14030188
Chen W-M, Xi B-D, Li M-X, Ye M-Y, Hou J-Q, Wei Y-F, Yu C-Z, Meng F-H. Deciphering the Pivotal Reaction Conditions for Hydrogen Production from Tar Catalytic Cracking by Perovskite. Catalysts. 2024; 14(3):188. https://doi.org/10.3390/catal14030188
Chicago/Turabian StyleChen, Wang-Mi, Bei-Dou Xi, Ming-Xiao Li, Mei-Ying Ye, Jia-Qi Hou, Yu-Fang Wei, Cheng-Ze Yu, and Fan-Hua Meng. 2024. "Deciphering the Pivotal Reaction Conditions for Hydrogen Production from Tar Catalytic Cracking by Perovskite" Catalysts 14, no. 3: 188. https://doi.org/10.3390/catal14030188