Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review
Abstract
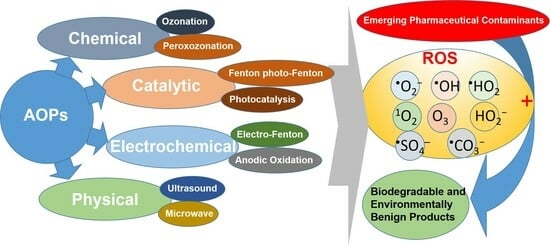
:1. Introduction
2. Pharmaceuticals in the Environment
3. Degradation of Pharmaceuticals by Advanced Oxidation Processes in Aqueous Solution
3.1. UV–Hydrogen Peroxide
3.2. Fenton, Photo-Fenton, and Electro-Fenton
3.3. Ozone-Based Processes
3.4. Ultrasonic Irradiation
3.5. Photocatalysis
4. Parameters Governing Advanced Oxidation Processes in the Degradation of Pharmaceuticals
5. A Combination of Advanced Oxidation Processes
6. Reported Studies of the Advanced Oxidation Processes of Pharmaceuticals
7. Pilot Test of Advanced Oxidation Processes on Industrial Wastewater
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peña, O.I.G.; Zavala, M.Á.L.; Ruelas, H.C. Pharmaceuticals market, consumption trends and disease incidence are not driving the pharmaceutical research on water and wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Kuster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical wastewater as emerging contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Rogowska, J.; Zimmermann, A. Household pharmaceutical waste disposal as a global problem—A review. Int. J. Environ. Res. Public Health 2022, 19, 15798. [Google Scholar] [CrossRef] [PubMed]
- Aliste, M.; Garrido, I.; Hernandez, V.; Flores, P.; Hellín, P.; Navarro, S.; Fenoll, J. Assessment of reclaimed agro-wastewater polluted with insecticide residues for irrigation of growing lettuce (Lactuca sativa L.) using solar photocatalytic technology. Environ. Pollut. 2022, 292, 118367. [Google Scholar] [CrossRef] [PubMed]
- Frascaroli, G.; Reid, D.; Hunter, C.; Roberts, J.; Helwig, K.; Spencer, J.; Escudero, A. Pharmaceuticals in wastewater treatment plants: A systematic review on the substances of greatest concern responsible for the development of antimicrobial resistance. Appl. Sci. 2021, 11, 6670. [Google Scholar] [CrossRef]
- Shigei, M.; Assayed, A.; Hazaymeh, A.; Dalahmeh, S.S. Pharmaceutical and antibiotic pollutant levels in wastewater and the waters of the Zarqa river, Jordan. Appl. Sci. 2021, 11, 8638. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake. Environ. Pollut. 2017, 222, 356–366. [Google Scholar] [CrossRef]
- Yang, H.; Lu, G.; Yan, Z.; Liu, J.; Dong, H.; Bao, X.; Zhang, X.; Sun, Y. Residues, bioaccumulation, and trophic transfer of pharmaceuticals and personal care products in highly urbanized rivers affected by water diversion. J. Hazard. Mater. 2020, 391, 122245. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Sithole, T.; Mufamadi, S.; Mazhandu, Z.; Sillanpaa, M.; Kaushik, A.; Mashifana, T. Efficient detection and treatment of pharmaceutical contaminants to produce clean water for better health and environmental. J. Clean. Prod. 2023, 387, 135798. [Google Scholar] [CrossRef]
- Gil, A.; Taoufik, N.; Gacía, A.M.; Korili, A.S. Comparative removal of emerging contaminants from aqueous solution by adsorption on an activated carbon. Environ. Technol. 2019, 40, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Shahrin, E.W.E.S.; Narudin, N.A.H.; Shahri, N.N.M.; Nur, M.; Lim, J.-W.; Bilad, M.R.; Mahadi, A.H.; Hobley, J.; Usman, A. A comparative study of adsorption behavior of rifampicin, streptomycin, and ibuprofen contaminants from aqueous solutions onto chitosan: Dynamic interactions, kinetics, diffusions, and mechanisms. Emerg. Contam. 2023, 9, 100199. [Google Scholar] [CrossRef]
- Majid, A.F.A.; Dewi, R.; Shahri, N.N.M.; Shahri, E.W.E.S.; Kusrini, E.; Shamsuddin, N.; Lim, J.-W.; Thongratkaew, S.; Kajornsak Faungnawakij, K.; Usman, A. Enhancing adsorption performance of alkali activated kaolinite in the removal of antibiotic rifampicin from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132209. [Google Scholar] [CrossRef]
- Foureaux, A.F.S.; Reis, E.O.; Lebron, Y.; Moreira, V.; Santos, L.V.; Amaral, M.S.; Lange, L.C. Rejection of pharmaceutical compounds from surface water by nanofiltration and reverse osmosis. Sep. Purif. Technol. 2019, 212, 171–179. [Google Scholar] [CrossRef]
- Moreira, J.B.; Santos, T.D.; Zaparoli, M.; de Almeida, A.C.A.; Costa, J.A.V.; de Morais, M.G. An overview of nanofiltration and nanoadsorption technologies to emerging pollutants treatment. Appl. Sci. 2022, 12, 8352. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Surendra, K.C.; Wu, D.; Lu, H.; Wong, J.W.C.; Khana, S.K. Anaerobic membrane bioreactors for pharmaceutical-laden wastewater treatment: A critical review. Bioresour. Technol. 2022, 361, 127667. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Azimian-Kivi, M.; Jafarzadeh, Y.; Yegani, R. Pharmaceutical wastewater treatment using polypropylene membranes incorporated with carboxylated and PEG-grafted nanodiamond in membrane bioreactor (MBR). Water Environ. J. 2021, 35, 1249–1259. [Google Scholar] [CrossRef]
- Dhiman, N.; Chaudhary, S.; Singh, A.; Chauhan, A.; Kumar, R. Sustainable degradation of pharmaceutical waste using different fungal strains: Enzyme induction, kinetics and isotherm studies. Environ. Technol. Innov. 2022, 25, 102156. [Google Scholar] [CrossRef]
- Moghaddam, A.; Khayatan, D.; Barzegar, P.E.F.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Ghaleh, H.E.G.; Tebyaniyan, H. Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, R.; Kumar, A.; Shankar, R.; Khan, N.A.; Gupta, K.N.; Ram, M.; Arya, R.K. Pharmaceutical waste-water treatment via advanced oxidation based integrated processes: An engineering and economic perspective. J. Water Process Eng. 2023, 54, 103977. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. NPJ Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of pharmaceuticals from water using sewage sludge-derived biochar: A review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Shafi, T.; Dubey, B.K.; Chowdhury, S. Biochar-mediated removal of pharmaceutical compounds from aqueous matrices via adsorption. Waste Dispos. Sustain. Energy 2023, 5, 37–62. [Google Scholar] [CrossRef]
- Ilavský, J.; Barloková, D. The removal of selected pharmaceuticals from water by adsorption with granular activated carbons. Eng. Proc. 2023, 57, 33. [Google Scholar]
- Kryuchkova, M.; Batasheva, S.; Akhatova, F.; Babaev, V.; Buzyurova, D.; Vikulina, A.; Volodkin, D.; Fakhrullin, R.; Rozhina, E. Pharmaceuticals removal by adsorption with montmorillonite nanoclay. Int. J. Mol. Sci. 2021, 22, 9670. [Google Scholar] [CrossRef]
- Ganthavee, V.; Trzcinski, A.P. Removal of pharmaceutically active compounds from wastewater using adsorption coupled with electrochemical oxidation technology: A critical review. J. Ind. Eng. Chem. 2023, 126, 20–35. [Google Scholar] [CrossRef]
- Kummerer, K. Antibiotics in the aquatic environment—A review. Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: A review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments—Occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Lakhani, S.; Acharya, D.; Sakariya, R.; Sharma, D.; Patel, P.; Shah, M.; Prajapati, M. A comprehensive study of bioremediation for pharmaceutical wastewater treatment. Clean. Chem. Eng. 2022, 4, 100073. [Google Scholar] [CrossRef]
- Yu, S.Y.; Xie, Z.H.; Wu, X.; Zheng, Y.Z.; Shi, Y.; Xiong, Z.K.; Zhou, P.; Liu, Y.; He, C.S.; Pan, Z.C.; et al. Review of advanced oxidation processes for treating hospital sewage to achieve decontamination and disinfection. Chin. Chem. Lett. 2024, 35, 108714. [Google Scholar] [CrossRef]
- Lupu, G.-I.; Orbeci, C.; Bobirica, L.; Bobirica, C.; Pascu, L.F. Key principles of advanced oxidation processes: A systematic analysis of current and future perspectives of the removal of antibiotics from wastewater. Catalysts 2023, 13, 1280. [Google Scholar] [CrossRef]
- Verinda, S.B.; Muniroh, M.; Yulianto, E.; Maharani, N.; Gunawan, G.; Amalia, N.F.; Hobley, J.; Usman, A.; Nur, M. Degradation of ciprofloxacin in aqueous solution using ozone microbubbles: Spectroscopic, kinetics, and antibacterial analysis. Heliyon 2022, 8, e10137. [Google Scholar] [CrossRef]
- Chung, J.; Chung, S.; Lee, G.; Lee, Y.-W. Application of wastewater reuse with photocatalyst prepared by sol-gel method and its kinetics on the decomposition of low molecular weight pollutants. Int. J. Environ. Res. Public Health 2020, 17, 4203. [Google Scholar] [CrossRef]
- Friedmann, D. A general overview of heterogeneous photocatalysis as a remediation technology for wastewaters containing pharmaceutical compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced oxidation process: A remediation technique for organic and non-biodegradable pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Tawfik, A.F.; Alajmi, A.M.; Badr, M.Y.; Al-Jedai, A.; Almozain, N.H.; Bukhary, H.A.; Halwani, A.A.; Al Awadh, S.A.; Alshamsan, A.; et al. Localizing pharmaceuticals manufacturing and its impact on drug security in Saudi Arabia. Saudi Pharm. J. 2022, 30, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Pouzola, T.; Levi, Y.; Bertrand-Krajewski, J.-L. Modelling daily and hourly loads of pharmaceuticals in urban wastewater. Int. J. Hyg. Environ. Health 2020, 229, 113552. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of pharmaceuticals and personal care products in the water environment of Poland: A review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Hernández-Tenorio, R.; González-Juárez, E.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Review of occurrence of pharmaceuticals worldwide for estimating concentration ranges in aquatic environments at the end of the last decade. J. Hazard. Mater. Adv. 2002, 8, 100172. [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Ge, J.; Yin, H. Fate and transport of pharmaceuticals in water systems: A processes review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Pindling, S.; Azulai, D.; Zheng, B.; Dahan, D.; Perron, G.G. Dysbiosis and early mortality in zebrafish larvae exposed to subclinical concentrations of streptomycin. FEMS Microbiol. Lett. 2018, 365, fny188. [Google Scholar] [CrossRef]
- Matijević, G.; Babić, S.; Maršavelski, A.; Stipaničev, D.; Repec, S.; Čož-Rakovac, R.; Klobučar, G. Estimating risk of cardiovascular pharmaceuticals in freshwaters using zebrafish embryotoxicity test—Statins threat revealed. Chemosphere 2023, 313, 137574. [Google Scholar] [CrossRef]
- Walter, R.B.; Milano, F.; Brasky, T.M.; White, E. Long-term use of acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of hematologic malignancies: Results from the prospective Vitamins and Lifestyle (VITAL) study. J. Clin. Oncol. 2011, 29, 2424–2431. [Google Scholar] [CrossRef]
- Porretti, M.; Arrigo, F.; Di Bella, G.; Faggio, C. Impact of pharmaceutical products on zebrafish: An effective tool to assess aquatic pollution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109439. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, C.E.; Ramírez-Morales, D.; Masis-Mora, M.; Montiel-Mora, J.R.; Soto-Garita, C.; Araya-Valverde, E.; Cambronero-Heinrichs, J.C.; Sànchez-Melsió, A.; Briceño-Guevara, S.; Mendez-Rivera, M.; et al. Occurrence and risk assessment of pharmaceuticals in hospital wastewater in Costa Rica. Chemosphere 2023, 339, 139746. [Google Scholar] [CrossRef] [PubMed]
- Manyahi, J.; Moyo, S.; Aboud, S.; Langeland, N.; Blomberg, B. High rate of antimicrobial resistance and multiple mutations in the dihydrofolate reductase gene among Streptococcus pneumoniae isolated from HIV-infected adults in a community setting in Tanzania. J. Glob. Antimicrob. Resist. 2020, 22, 749–753. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Chaurasia, D.; Pandey, A.; Gupta, P. Co-occurrence of multidrug resistance, b-lactamase and plasmid mediated AmpC genes in bacteria isolated from river Ganga, northern India. Environ. Pollut. 2020, 267, 115502. [Google Scholar] [CrossRef] [PubMed]
- Stachurová, T.; Sýkorová, N.; Semerád, J.; Malachová, K. Resistant genes and multidrug-resistant bacteria in wastewater: A study of their transfer to the water reservoir in the Czech Republic. Life 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, K.M. Functional materials based on molecules with hydrogen-bonding ability: Applications to drug co-crystals and polymer complexes. R. Soc. Open Sci. 2018, 5, 180564. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Aziz, K.H.H. Heterogeneous catalytic activation of peroxydisulfate toward degradation of pharmaceuticals diclofenac and ibuprofen using scrap printed circuit board. RSC Adv. 2023, 13, 115. [Google Scholar] [CrossRef]
- Giri, A.S.; Golder, A.K.; Chakma, S. Kinetics, degradation mechanisms and antibiotic activity reduction of chloramphenicol in aqueous solution by UV/H2O2 process. Water Sci. Technol. 2021, 84, 524. [Google Scholar] [CrossRef]
- Cataldo, F. Hydrogen peroxide photolysis with different UV light sources including a new UV-LED light source. New Front. Chem. 2014, 23, 99–110. [Google Scholar]
- Scaria, J.; Nidheesh, P.V. Comparison of Hydroxyl-Radical-Based Advanced Oxidation Processes with Sulfate Radical-Based Advanced Oxidation Processes. Curr. Opin. Chem. Eng. 2022, 36, 1–2. [Google Scholar] [CrossRef]
- Sievers, M. Advanced oxidation processes. Treatise Water Sci. 2011, 4, 377–408. [Google Scholar]
- Barroso-Martinez, J.S.; Romo, A.I.B.; Pudar, S.; Putnam, S.T.; Bustos, E.; Rodriguez-Lopez, J. Real-time detection of hydroxyl radical generated at operating electrodes via redox-active adduct formation using scanning electrochemical microscopy. J. Am. Chem. Soc. 2022, 144, 18896–18907. [Google Scholar] [CrossRef] [PubMed]
- Kulišťáková, A. Removal of pharmaceutical micropollutants from real wastewater matrices by means of photochemical advanced oxidation processes—A review. J. Water Process Eng. 2023, 53, 103727. [Google Scholar] [CrossRef]
- Litter, M.I.; Mariel Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 20160164. [Google Scholar] [CrossRef]
- Lin, P.J.; Yeh, C.H.; Jiang, J.C. Theoretical insight into hydroxyl production via H2O2 decomposition over the Fe3O4(311) surface. RSC Adv. 2021, 11, 36257. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Moreira, M.M.; Figueiredo, S.A.; Delerue-Matos, C.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Electro-Fenton degradation of a ternary pharmaceutical mixture and its application in the regeneration of spent biochar. J. Electroanal. Chem. 2021, 886, 2–9. [Google Scholar] [CrossRef]
- Patel, S.; Mondal, S.; Majumder, S.K.; Das, P.; Ghosh, P. Treatment of a pharmaceutical industrial effluent by a hybrid process of advanced oxidation and adsorption. ACS Omega 2020, 5, 32305–32317. [Google Scholar] [CrossRef]
- Du, J.; Wang, C.; Zhao, Z.; Cui, F.; Ou, Q.; Liu, J. Role of oxygen and superoxide radicals in promoting H2O2 production during VUV/UV radiation of water. Chem. Eng. Sci. 2021, 241, 116683. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Nakada, N.; Shinohara, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef]
- Orge, C.A.; Graça, C.A.L.; Restivo, J.; Pereira, M.F.R.; Soares, O.S.G.P. Catalytic ozonation of pharmaceutical compounds using carbon-based catalysts. Catal. Commun. 2024, in press. [Google Scholar] [CrossRef]
- Francoeur, M.; Yacou, C.; Petit, E.; Granier, D.; Flaud, V.; Gaspard, S.; Brosillon, S.; Ayral, A. Removal of antibiotics by adsorption and catalytic ozonation using magnetic activated carbons prepared from Sargassum sp. J. Water Process Eng. 2023, 53, 103602. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhuo, Q.; Dai, L.; Guan, B. Mn anchored zeolite molecular nest for enhanced catalytic ozonation of cephalexin. Chemosphere 2023, 335, 139058. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, J.; Wang, L.; Zhang, Y.; Zhuang, T.; Wang, H.; Cui, X.; Wang, Z. A power-triggered preparation strategy of nanostructured inorganics: Sonosynthesis. Nanoscale Adv. 2021, 3, 2423. [Google Scholar] [CrossRef] [PubMed]
- Fetyan, N.A.H.; Attia, T.M.S. Water purification using ultrasound waves: Application and challenges. Arab. J. Basic Appl. Sci. 2020, 27, 194–207. [Google Scholar] [CrossRef]
- Sakkas, P.M.; Schneider, O.; Sourkouni, G.; Argirusis, C. Sonochemistry in the service of SOFC research. Ultrason. Sonochem. 2014, 21, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V.; Landi, M.; Scannapieco, D.; Belgiorno, V. Sonochemical degradation of twenty-three emerging contaminants in urban wastewater. Desalin. Water Treat. 2013, 51, 6601–6608. [Google Scholar] [CrossRef]
- Alishiri, M.; Abdollahi, S.M.; Neysari, A.N.; Ranjbar, S.F.; Abdoli, N.; Afsharjahanshahi, M. Removal of ciprofloxacin and cephalexin antibiotics in water environment by magnetic graphene oxide nanocomposites; optimization using response surface methodology. Results Eng. 2023, 20, 101507. [Google Scholar] [CrossRef]
- Hjazi, A.; Almajidi, Y.Q.; Kadhum, W.R.; Aly, M.; Malviya, J.; Fenjan, M.N.; Alawadi, A.; Alsaalamy, A.; Chandramauli, A.; Baharinikoo, L. Optimization of removal of sulfonamide antibiotics by magnetic nanocomposite from water samples using central composite design. Water Resour. Ind. 2023, 30, 100229. [Google Scholar] [CrossRef]
- Kong, C.P.Y.; Suhaimi, N.A.A.; Shahri, N.N.M.; Lim, J.-W.; Nur, M.; Hobley, J.; Usman, A. Auramine O UV photocatalytic degradation on TiO2 nanoparticles in a heterogeneous aqueous solution. Catalysts 2022, 12, 975. [Google Scholar] [CrossRef]
- Suhaimi, N.A.A.; Kong, C.P.Y.; Shahri, N.N.M.; Nur, M.; Hobley, J.; Usman, A. Dynamics of diffusion- and immobilization-limited photocatalytic degradation of dyes by metal oxide nanoparticles in binary or ternary solutions. Catalysts 2022, 12, 1254. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Owolabi, T.O.; Olubi, O.E.; Hezam, A. State-of-the-art, challenges and prospects of heterogeneous tandem photocatalysis. Coord. Chem. Rev. 2023, 492, 2–20. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, J.; Qiu, Y. Efficient degradation of organic pollutants by peroxymonosulfate activated with MgCuFe-layered double hydroxide. RSC Adv. 2019, 9, 2284. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, J.; Yang, D.-Q. A durable superhydrophilic self-cleaning coating based on TiO2–SiO2-PAA nanocomposite for photovoltaic applications: Long-term outdoor study. Sol. Energy Mater. Sol. Cells 2024, 268, 112731. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A systematic review of metal oxide applications for energy and environmental sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Zulmajdi, S.L.N.; Zamri, N.I.I.; Yasin, H.M.; Kusrini, E.; Hobley, J.; Usman, A. Comparative study on the adsorption, kinetics, and thermodynamics of the photocatalytic degradation of six different synthetic dyes on TiO2 nanoparticles. React. Kinet. Mech. Catal. 2020, 129, 519–534. [Google Scholar] [CrossRef]
- Suhaimi, N.A.A.; Shahri, N.N.M.; Samat, J.H.; Kusrini, E.; Lim, J.-W.; Hobley, J.; Usman, A. Domination of methylene blue over rhodamine B during simultaneous photocatalytic degradation by TiO2 nanoparticles in an aqueous binary solution under UV irradiation. React. Kinet. Mech. Catal. 2022, 135, 511–527. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic degradation of methylene blue by titanium dioxide: Experimental and modeling study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, S.; Choi, W. Dye decolorization test for the activity assessment of visible light photocatalysts: Realities and limitations. Catal. Today 2014, 224, 21–28. [Google Scholar] [CrossRef]
- Gatti, T.; Lamberti, F.; Mazzaro, R.; Kriegel, I.; Schlettwein, D.; Enrichi, F.; Lago, N.; Di Maria, E.; Meneghesso, G.; Vomiero, A.; et al. Opportunities from doping of non-critical metal oxides in last generation light-conversion devices. Adv. Energy Mater. 2021, 11, 2101041. [Google Scholar] [CrossRef]
- Sakuna, P.; Ketwong, P.; Ohtani, B.; Trakulmututa, J.; Kobkeatthawin, T.; Luengnaruemitchai, A.; Smit, S.M. The influence of metal-doped graphitic carbon nitride on photocatalytic conversion of acetic acid to carbon dioxide. Front. Chem. 2022, 10, 825786. [Google Scholar] [CrossRef]
- Ali, H.; Masar, M.; Yasir, M.; Machovsky, M.; Monteiro, O.C.; Kuritka, I. Current trends in environmental and energy photocatalysis and ISO standardization. J. Environ. Chem. Eng. 2023, 11, 111541. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Zhang, Y.; Qiu, L.; Xing, Y. A 0D/2D Bi4V2O11/g-C3N4 S-scheme heterojunction with rapid interfacial charges migration for photocatalytic antibiotic degradation. Acta Phys. Chim. Sin. 2022, 38, 2112027. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, Modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]
- Qu, S.; Wang, W.; Pan, X.; Li, C. Improving the Fenton catalytic performance of FeOCl using an electron mediator. J. Hazard. Mater. 2020, 384, 121494. [Google Scholar] [CrossRef]
- Pastor, E.; Sachs, M.; Selim, S.; Durrant, J.R.; Bakulin, A.A.; Walsh, A. Electronic defects in metal oxide photocatalysts. Nat. Rev. Mater. 2022, 7, 503–521. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef]
- Suma, D.; Deka, T.; Ningthoukhongjam, P.; Chowdhury, A.; Nair, R.G. A critical review on prospects and challenges of metal-oxide embedded g-C3N4-based direct Z-scheme photocatalysts for water splitting and environmental remediation. Appl. Surf. Sci. Adv. 2022, 11, 100273. [Google Scholar]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Gan, W.; Fu, X.; Guo, J.; Zhang, M.; Yu, H.; Ding, C.; Qi, S.; Cao, X.; Sun, Z. Facile synthesis of mesoporous hierarchical TiO2 micro-flowers serving as the scaffolding of 0D Ag3PO4 nanoparticles for the ultra-fast degradation of organic pollutants. J. Alloys Compd. 2022, 909, 164737. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Armenise, S.; García-Bordejé, E.; Valverde, J.L.; Romeo, E.; Monzón, A. A Langmuir-Hinshelwood approach to the kinetic modelling of catalytic ammonia decomposition in an integral reactor. Phys. Chem. Chem. Phys. 2013, 15, 12104–12117. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Xian, T.; Liu, G.; Yang, H. Photocatalytic purification of simulated dye wastewater in different pH environments by using BaTiO3/Bi2WO6 heterojunction photocatalysts. Opt. Mater. 2021, 113, 110853. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2•− and OH• radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A General 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Hassan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El-Nemr, A. Principles of photocatalysts and their different applications: A review. Top. Curr. Chem. 2023, 31, 381. [Google Scholar] [CrossRef]
- Wang, J.; van Ree, T.; Wu, Y.; Zhang, P.; Gao, L. Metal oxide semiconductors for solar water splitting. In Metal Oxides Energy Technologies; Wu, Y., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 205–249. [Google Scholar]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, J.; Vidwans, N.A.; Gentry, T.; Vaddiraju, S. Catalyst recovery, regeneration and reuse during large-scale disinfection of water using photocatalysis. Water 2021, 13, 2623. [Google Scholar] [CrossRef]
- Suhaimi, N.A.A.; Umar, M.K.H.; Lau, H.L.H.; Roslan, N.N.; Lim, J.-W.; Hobley, J.; Nur, M.; Usman, A. An insight into the photocatalytic degradation of the antibiotic rifampicin by titanium dioxide nanoparticles in aqueous solution under UV light irradiation. Reac. Kinet. Mech. Cat. 2024, in press. [Google Scholar] [CrossRef]
- Yousefi, A.; Nezamzadeh-Ejhieh, A. Characterization of BiOCl/BiOI binary catalyst and its photocatalytic activity towards rifampin. J. Photochem. Photobiol. A Chem. 2022, 433, 114135. [Google Scholar] [CrossRef]
- Kadıoğlu, E.N.; Öztürk, H.; Eroğlu, H.A.; Akbal, F.; Kuleyin, A.; Özkaraova, E.B. Artificial neural network modeling of Fenton-based advanced oxidation processes for recycling of textile wastewater. J. Ind. Eng. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Naddeo, V.; Uyguner-Demirel, C.S.; Prado, M.; Cesaro, A.; Belgiorno, V.; Ballesteros, F. Enhanced ozonation of selected pharmaceutical compounds by sonolysis. Environ. Technol. 2015, 36, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Haza, U.J.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Singh, A.K.; Jain, B.; Yadav, S.; Carabineiro, S.A.C.; Susan, M.A.B.H. Lochrome dark blue azo dye removal by sonophotocatalysis using Mn2+ doped ZnS quantum dots. Catalysts 2021, 11, 1025. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent advancements in the sonophotocatalysis (SPC) and doped-sonophotocatalysis (DSPC) for the treatment of recalcitrant hazardous organic water pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Santos, O.; Cruz-Alcalde, A.; López-Vinent, N.; Zanganas, D.; Sans, C. Photocatalytic degradation of sulfamethoxazole using TiO2 in simulated seawater: Evidence for direct formation of reactive halogen species and halogenated by-products. Sci. Total Environ. 2020, 736, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Wong, K.T.; Choong, C.E.; Kim, J.R.; Kim, H.; Kim, S.H.; Jeon, B.H.; Yoon, Y.; Snyder, S.A.; Jang, M. Degradation synergism between sonolysis and photocatalysis for organic pollutants with different hydrophobicity: A perspective of mechanism and application for high mineralization efficiency. J. Hazard. Mater. 2021, 416, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Issaka, E.; AMU-Darko, J.N.O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants―A review. Chemosphere 2022, 289, 2–15. [Google Scholar] [CrossRef]
- Huang, B.; Yang, C.; Zeng, H.; Zhou, L. Multivalent iron-based magnetic porous biochar from peach gum polysaccharide as a heterogeneous Fenton catalyst for degradation of dye pollutants. Int. J. Biol. Macromol. 2023, 253, 2–8. [Google Scholar] [CrossRef]
- Dhawle, R.; Frontistis, Z.; Mantzavinos, D.; Lianos, P. Production of hydrogen peroxide with a photocatalytic fuel cell and its application to UV/H2O2 degradation of dyes. Chem. Eng. J. Adv. 2021, 6, 100109. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Nickheslat, A.; Amin, M.M.; Izanloo, H.; Fatehizadeh, A.; Mousavi, S.M. Phenol photocatalytic degradation by advanced oxidation process under ultraviolet radiation using titanium dioxide. J. Environ. Public Health 2013, 2013, 815310. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Zulmajdi, S.L.N.; Zamri, N.I.I.; Mahadi, A.H.; Rosli, M.Y.H.; Ja’afar, F.; Yasin, H.M.; Kusrini, E.; Hobley, J.; Usman, A. Sol-gel preparation of different crystalline phases of TiO2 nanoparticles for photocatalytic degradation of methylene blue in aqueous solution. Am. J. Nanomater. 2019, 7, 39–45. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 2–22. [Google Scholar] [CrossRef]
- Yilmaz, E.; Salem, S.; Sarp, G.; Aydin, S.; Sahin, K.; Korkmaz, I.; Yuvali, D. TiO2 nanoparticles and C-nanofibers modified magnetic Fe3O4 nanospheres (TiO2@Fe3O4@C–NF): A multifunctional hybrid material for magnetic solid-phase extraction of ibuprofen and photocatalytic degradation of drug molecules and azo dye. Talanta 2020, 213, 1–9. [Google Scholar] [CrossRef]
- Khani, Z.; Schieppati, D.; Bianchi, C.L.; Boffito, D.C. The sonophotocatalytic degradation of pharmaceuticals in water by MnOx-TiO2 systems with tuned band-gaps. Catalysts 2019, 9, 949. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.N.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.M.; Poyatos, J.M. Removal of carbamazepine, ciprofloxacin and ibuprofen in real urban wastewater by using light-driven advanced oxidation processes. Int. J. Environ. Sci. Technol. 2019, 16, 6005–6018. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K. Photocatalytic degradation of the antibiotic levofloxacin using highly crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- An, J.; Li, G.; An, T.; Song, W.; Feng, H.; Lu, Y. Photocatalytic degradation of three amantadine antiviral drugs as well as their eco-toxicity evolution. Catal. Today 2015, 258, 602–609. [Google Scholar] [CrossRef]
- Abbood, N.S.; Ali, N.S.; Khader, E.H.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Photocatalytic degradation of cefotaxime pharmaceutical compounds onto a modified nanocatalyst. Res. Chem. Intermed. 2023, 49, 43–56. [Google Scholar] [CrossRef]
- Wang, Z.; Srivastava, V.; Ambat, I.; Safaei, Z.; Sillanpää, M. Degradation of Ibuprofen by UV-LED/catalytic advanced oxidation process. J. Water Process Eng. 2019, 31, 100808. [Google Scholar] [CrossRef]
- Akter, S.; Islam, M.S.; Kabir, M.H.; Shaikh, M.A.A.; Gafur, M.A. UV/TiO2 photodegradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: An optimization and kinetic study. Arab. J. Chem. 2022, 15, 103900. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A. Synthesis and characterization of B-doped TiO2 and their performance for the degradation of metoprolol. Catal. Today 2015, 252, 27–34. [Google Scholar] [CrossRef]
- Stets, S.; do Amaral, B.; Schneider, J.T.; de Barros, I.R.; de Liz, M.V.; Ribeiro, R.R.; Nagata, N.; Peralta-Zamora, P. Antituberculosis drugs degradation by UV-based advanced oxidation processes. J. Photochem. Photobiol. A Chem. 2018, 353, 26–33. [Google Scholar] [CrossRef]
- Shankaraiah, G.; Poodari, S.; Bhagawan, D.; Himabindu, V.; Vidyavathi, S. Degradation of antibiotic norfloxacin in aqueous solution using advanced oxidation processes (AOPs)—A comparative study. Desalin. Water Treat. 2016, 57, 27804–27815. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.; Hu, S.; Zhang, G.; Lan, H.; Peng, J.; Liu, H. Evaluation of degradation performance toward antiviral drug ribavirin using advanced oxidation process and its relations to ecotoxicity evolution. Sci. Total Environ. 2022, 850, 157851. [Google Scholar] [CrossRef] [PubMed]
- Papac, J.; Ballesteros, S.C.; Tonkovic, S.; Kovacic, M.; Tomic, A.; Cvetnić, M.; Kusic, H.; Senta, I.; Terzić, S.; Ahel, M.; et al. Degradation of pharmaceutical memantine by photo-based advanced oxidation processes: Kinetics, pathways and environmental aspects. J. Environ. Chem. Eng. 2023, 11, 109334. [Google Scholar] [CrossRef]
- Meribout, R.; Zuo, Y.; Khodja, A.A.; Piram, A.; Lebarillier, S.; Cheng, J.; Wang, C.; Wong-Wah-Chung, P. Photocatalytic degradation of antiepileptic drug carbamazepine with bismuth oxychlorides (BiOCl and BiOCl/AgCl compsite) in water: Efficiency evaluation and elucidation degradation pathways. J. Photochem. Photobiol. A Chem. 2016, 328, 105–113. [Google Scholar] [CrossRef]
- Chiron, S.; Minero, C.; Vione, D. Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters. Environ. Sci. Technol. 2006, 40, 5977–5983. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wu, Z.; Chen, C.; Fang, J. UV/Chlorine Process: An efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc. Chem. Res. 2022, 55, 286–297. [Google Scholar] [CrossRef]
- Sanches-Simões, E.; Testolin, R.C.; Müller, F.M.; Gerlach, O.M.S.; Pimentel-Almeida, W.; Conti-Lampert, A.D.; Corrêa, A.X.R.; Almerindo, G.I.; González, S.Y.G.; Radetski, C.M. Metronidazole degradation by physico-chemical and advanced oxidative processes: Influence of pH and nTiO2-functionalized macroporous support. Water Air Soil Pollut. 2022, 233, 466. [Google Scholar] [CrossRef]
- Kundu, P.; Kaur, A.; Mehta, S.K.; Kansal, S.K. Removal of ofloxacin from aqueous phase using Ni-doped TiO2 nanoparticles under solar irradiation. J. Nanosci. Nanotechnol. 2014, 14, 6991–6995. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Pham, T.H.; Viet, N.M.; Thang, P.Q.; Rajagopal, R.; Sathya, R.; Jung, S.H.; Kim, T. Improved photodegradation of antibiotics pollutants in wastewaters by advanced oxidation process based on Ni-doped TiO2. Chemosphere 2022, 302, 134837. [Google Scholar] [CrossRef]
- Shokri, M.; Jodat, A.; Modirshahla, N.; Behnajady, M.A. Photocatalytic degradation of chloramphenicol in an aqueous suspension of silver-doped TiO2 nanoparticles. Environ. Technol. 2013, 34, 1161–1166. [Google Scholar] [CrossRef]
- Malakootian, M.; Olama, N.; Malakootian, M.; Nasiri, A. Photocatalytic degradation of metronidazole from aquatic solution by TiO2-doped Fe3+ nano-photocatalyst. Int. J. Environ. Sci. Technol. 2019, 16, 4275–4284. [Google Scholar] [CrossRef]
- Surenjan, A.; Sambandam, B.; Pradeep, T.; Philip, L. Synthesis, characterization and performance of visible light active C-TiO2 for pharmaceutical photodegradation. J. Environ. Chem. Eng. 2017, 5, 757–767. [Google Scholar] [CrossRef]
- Ntelane, T.S.; Feleni, U.; Mthombeni, N.H.; Kuvarega, A.T. Sulfate radical-based advanced oxidation process (SR-AOP) on titania supported mesoporous dendritic silica (TiO2/MDS) for the degradation of carbamazepine and other water pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130276. [Google Scholar] [CrossRef]
- Abdullah, M.; Iqbal, J.; Rehman, M.S.U.; Khalid, U.; Mateen, F.; Arshad, S.N.; Al-Sehemi, A.G.; Algarni, H.; Al-Hartomy, O.A.; Fazal, T. Removal of ceftriaxone sodium antibiotic from pharmaceutical wastewater using an activated carbon based TiO2 composite: Adsorption and photocatalytic degradation evaluation. Chemosphere 2023, 317, 137834. [Google Scholar] [CrossRef]
- Czech, B.; Tyszczuk-Rotko, K. Visible-light-driven photocatalytic removal of acetaminophen from water using a novel MWCNT-TiO2-SiO2 photocatalysts. Sep. Purif. Technol. 2018, 206, 343–355. [Google Scholar] [CrossRef]
- Ali, H.; Yasir, M.; Ngwabebhoh, F.A.; Sopik, T.; Zandraa, O.; Sevcik, J.; Masar, M.; Machovsky, M.; Kuritka, I. Boosting photocatalytic degradation of estrone hormone by silica-supported g-C3N4/WO3 using response surface methodology coupled with Box-Behnken design. J. Photochem. Photobiol. A Chem. 2023, 441, 114733. [Google Scholar] [CrossRef]
- Janssens, R.; Cristovao, M.B.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J.; Luis, P. Coupling of nanofiltration and UV, UV/TiO2 and UV/H2O2 processes for the removal of anti-cancer drugs from real secondary wastewater effluent. J. Environ. Chem. Eng. 2019, 7, 103351. [Google Scholar] [CrossRef]
- Aram, M.; Farhadian, M.; Nazar, A.R.S.; Tangestaninejad, S.; Eskandari, P.; Byong-Hun Jeon, B.-H. Metronidazole and Cephalexin degradation by using of Urea/TiO2/ZnFe2O4/Clinoptiloite catalyst under visible-light irradiation and ozone injection. J. Mol. Liq. 2020, 304, 112764. [Google Scholar] [CrossRef]
- Zainurin, S.N.; Ismail, W.Z.W.; Mahamud, S.N.I.; Ismail, I.; Jamaludin, J.; Ariffin, K.N.Z.; Wan Ahmad Kamil, W.M.W.A. Advancements in monitoring water quality based on various sensing methods: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 14080. [Google Scholar] [CrossRef] [PubMed]
- Cibati, A.; Gonzalez-Olmos, R.; Rodriguez-Mozaz, S.; Buttiglieri, G. Unravelling the performance of UV/H2O2 on the removal of pharmaceuticals in real industrial, hospital, grey and urban wastewaters. Chemosphere 2022, 290, 133315. [Google Scholar] [CrossRef] [PubMed]
- Lumbaque, E.C.; Cardoso, R.M.; de Araújo Gomes, A.; Malato, S.; Sánchez Pérez, J.A.; Sirtori, C. Removal of pharmaceuticals in hospital wastewater by solar photo-Fenton with Fe3+-EDDS using a pilot raceway pond reactor: Transformation products and in silico toxicity assessment. Microchem. J. 2021, 164, 106014. [Google Scholar] [CrossRef]
- Pan, J.; Niu, X.-Z.; Yang, H.; Zheng, X.; Guan, B.; Wang, H. Pilot test of Mn-Fe/Al2O3 catalytic ozonation in tertiary wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 111937. [Google Scholar] [CrossRef]
- Majumder, A.; Otter, P.; Röher, D.; Bhatnagar, A.; Khalil, N.; Gupta, A.K.; Bresciani, R.; Arias, C.A. Combination of advanced biological systems and photocatalysis for the treatment of real hospital wastewater spiked with carbamazepine: A pilot-scale study. J. Environ. Manag. 2024, 351, 119672. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, P.; Li, X.; Ge, L.; Niu, J. Insight into typical photo-assisted AOPs for the degradation of antibiotic micropollutants: Mechanisms and research gaps. Chemosphere 2023, 343, 140211. [Google Scholar] [CrossRef] [PubMed]


| Pharmaceutical | Chemical Formula | pKa | Therapeutic Applications |
|---|---|---|---|
| Acetaminophen (Paracetamol) | C8H9NO2 | 9.4 | Analgesic and antipyretic pharmaceuticals widely used to treat pain and fever. |
| Aspirin (Acetylsalicylic acid) | C9H8O4 | 3.5 | An NSAID to reduce pain, fever, and/or inflammation. |
| Amantadine | C10H17N | 10.5 | An antidyskinetic medicine to treat dyskinesia and Parkinson’s disease. |
| Ampicillin | C16H19N3O4S | 2.5 | An antibiotic to treat acute otitis media caused by susceptible organisms. |
| Amoxicillin | C16H19N3O5S | 3.2, 11.7 | An antibiotic widely used to treat bacterial infections, including chest infections, such as pneumonia and odontogenic abscesses |
| Azithromycin | C38H72N2O12 | 8.7 | An antibiotic prescribed to children for the treatment of acute otitis media caused by Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae. |
| Bupropion | C13H18ClNO | 8.2 | An antidepressant medication used for treating conditions like depression and mental disorders and aiding in smoking cessation. |
| Carbamazepine | C15H12N2O | 13.9 | An anticonvulsant or anti-epileptic drug for the treatment of nerve pain and seizures. |
| Cefotaxime | C16H17N5O7S2 | 3.75 | A β-lactam antibiotic used to treat gram-positive, gram-negative, and anaerobic bacteria. |
| Cephalexin | C16H17N3O4S | 4.5 | A β-lactam antibiotic used to treat bacterial infections caused by bacteria such as pneumonia. |
| Chloramphenicol | C11H12Cl2N | 1.14 | An antibiotic useful for the treatment of severe bacterial infections. |
| Ceftriaxone | C18H18N8O7S3 | 3.0 | A cephalosporin antibiotic used to treat various bacterial infections. |
| Cetirizine | C21H27Cl3N2O3 | 3.6, 7.6 | A non-drowsy antihistamine used to relieve allergy symptoms. |
| Ciprofloxacin | C17H18FN3O3 | 6.1, 8.7 | A fluoroquinolone antibiotic used to treat various bacterial infections. |
| Clarithromycine | C38H69NO13 | 8.5 | An antibiotic used to treat skin problems and chest infections. |
| Cloxacillin | C19H18ClN3O5S | 2.8 | A penicillin-type antibiotic to treat a wide range of bacterial infections. |
| Diclofenac | C14H11Cl2NO2 | 4.15 | An NSAID used to treat pain and inflammation, such as gout and arthritis. |
| Erythromycin | C37H67NO13 | 8.88 | An antibiotic used to treat a variety of bacterial infections, such as skin infections and respiratory tract infections. |
| Fenofibrate | C20H21ClO4 | 4.0 | A fibrate class of medication used to treat abnormal blood lipid levels. |
| Hydrochlorothiazide | C7H8ClN3O4S2 | 7.9, 9.2 | A diuretic drug used to treat high blood pressure, edema, and swelling due to fluid build-up. |
| Ibuprofen | C13H18O2 | 4.85 | An NSAID used to relieve fever, pain, and inflammation. |
| Isoniazid | C6H7N3O | 1.82 | An antibiotic used in the treatment of latent mycobacterium tuberculosis infection. |
| Ketoprofen | C16H14O3 | 3.88 | An NSAID used to treat inflammation, pain, swelling, stiffness, rheumatoid arthritis, and osteoarthritis. |
| Levofloxacin | C18H20FN3O4 | 5.7, 7.9 | A fluoroquinolone antibiotic used to treat accute sinusitis and pneumonia bacterial infections. |
| Memantine | C12H21N | 10.7 | An antiparkinson agent used to suppress memory loss, dementia, and Alzheimer’s disease. |
| Metoprolol | C12H21N | 13.9 | A β-blocker and antihypertensive medication used to treat high blood pressure, fast heart rate, and chest pain. |
| Metamizole | C13H17N3O4S | 1.4 | An antipyretic, analgesic, painkiller drug used to relieve severe and persistent fever and pain. |
| Metronidazole | C6H9N3O3 | 2.38 | An antibiotic and antiprotozoal medication used to treat bacterial infections and inflammatory diseases. |
| Nabumetone | C15H16O2 | 4.8 | An NSAID used to treat mild to moderate pain and help to relieve symptoms of arthritis and reduce pain. |
| Naproxen | C14H14O3 | 4.15 | An NSAID used to treat pain, fever, rheumatoid arthritis, and inflammatory diseases. |
| Norfloxacin | C16H18FN3O3 | 6.34, 8.75 | A fluoroquinolone antibiotic used in the treatment of a variety of bacterial infections. |
| Oflaxacin | C18H20FN3O4 | 5.45 | A quinolone antibiotic used for the treatment of various bacterial infections. |
| Oxytetracycline | C22H24N2O9 | 3.22, 7.46, 8.94 | A tetracycline class of antibiotic used to treat various infectious diseases. |
| Promethazine | C17H20N2S | 9.05 | Antihistamine used to relieve allergy symptoms. |
| Ranitidine | C13H22N4O3S | 8.4 | Antihistamine used to decrease acid produced in the stomach. |
| Ribavirin | C8H12N4O5 | 12.25 | An antiviral drug for the treatment of hepatitis C and respiratory viral infections. |
| Rifampicin | C43H58N4O12 | 1.7, 7.9 | An antibiotic used to treat a variety of mycobacterial and gram-positive bacterial infections. |
| Rimantadine | C12H21N | 10.4 | An antiviral drug used to prevent and treat respiratory tract infections caused by influenza A virus. |
| Simvastatin | C25H38O5 | 4.7 | Hydroxymethylglutaryl-CoA reductase inhibitors used to treat high cholesterol and reduce the risk of heart disease. |
| Streptomycin | C21H39N7O12 | 7.4, 13.5 | An antibiotic isolated from Streptomyces griseus used to inhibit gram-positive and gram-negative bacteria and that is useful to treat cavitary lung disease. |
| Sulfamethoxazole | C10H11N3O3S | 3.9 | An antibiotic used to treat bacterial infections and is useful against gram-positive and gram-negative bacteria. |
| Tetracycline | C22H24N2O8 | 3.3, 7.8, 9.6 | An antibiotic used to treat a variety of infections, acne, brucellosis, cholera, malaria, and plague. |
| Tramadol | C16H25NO2 | 9.2 | An opioid painkiller to treat moderately severe pain. |
| Trimethoprim | C14H18N4O3 | 7.6, 8.3 | An antibiotic used to treat and prevent different types of infections. |
| Valsartan | C24H29N5O3 | 3.9, 4.7 | An angiotensin receptor blocker used to treat high blood pressure, heart failure, and diabetic kidney disease. |
| AOP Method | Wastewater | Light Source | Catalyst | Results | Ref. |
|---|---|---|---|---|---|
| UV-H2O2 | Northeast of Spain: two from urban wastewater plants. One from graywater, a psychiatric hospital, and the pharmaceutical industry. The wastewater was composed of 30 pharmaceuticals and 13 transformation products. | UV-C lamp (30 min) | None | The pharmaceuticals were approximately 6–86% removed from urban wastewater plants, and 59% from graywater, with electrical energy per order (EEO) values of 0.9–1.5 kWh/(m3·order). The removals were lower for hospitals and industries (36% and 17%), with electrical energy per order (EEO) values of 7.3–9.1 kWh/(m3·order). | [168] |
| Photo-Fenton | Porto Alegre City, local hospital: paracetamol (297 μg/L) and dipyrone (55 μg/L). | Solar irradiation (30 min) | Fe3+-EDDS (1:2 ratio) | 77% pharmaceutical removal. Most of the transformation products show low toxicity, mutagenicity, and bioaccumulation. | [169] |
| Catalytic ozonation | Zhejiang Province, China, industrial treatment plant: 8% pharmaceutical waste. | None | Mn-Fe/Al2O3 | 99% removal of DOC and 14.5 mg/L average removal of COD. The effluent has a low total transcriptional effect level index value of ≤1.50. | [170] |
| Biological-photocatalysis | Kharagpur Subdivisional Hospital, India: containing carbamazepine. | UV-A irradiation | Al–ZnO/Fe | 85% carbamazepine removal from aerated horizontal flow-constructed wetland. The biological method removed 30% carbamazepine. | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslan, N.N.; Lau, H.L.H.; Suhaimi, N.A.A.; Shahri, N.N.M.; Verinda, S.B.; Nur, M.; Lim, J.-W.; Usman, A. Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review. Catalysts 2024, 14, 189. https://doi.org/10.3390/catal14030189
Roslan NN, Lau HLH, Suhaimi NAA, Shahri NNM, Verinda SB, Nur M, Lim J-W, Usman A. Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review. Catalysts. 2024; 14(3):189. https://doi.org/10.3390/catal14030189
Chicago/Turabian StyleRoslan, Nur Nabaahah, Harry Lik Hock Lau, Nurul Amanina A. Suhaimi, Nurulizzatul Ningsheh M. Shahri, Sera Budi Verinda, Muhammad Nur, Jun-Wei Lim, and Anwar Usman. 2024. "Recent Advances in Advanced Oxidation Processes for Degrading Pharmaceuticals in Wastewater—A Review" Catalysts 14, no. 3: 189. https://doi.org/10.3390/catal14030189