Origin of the Increase in the Selectivity of Ru Catalysts with the Addition of Amines in the Presence of ZnSO4 for the Selective Hydrogenation of Benzene to Cyclohexene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization




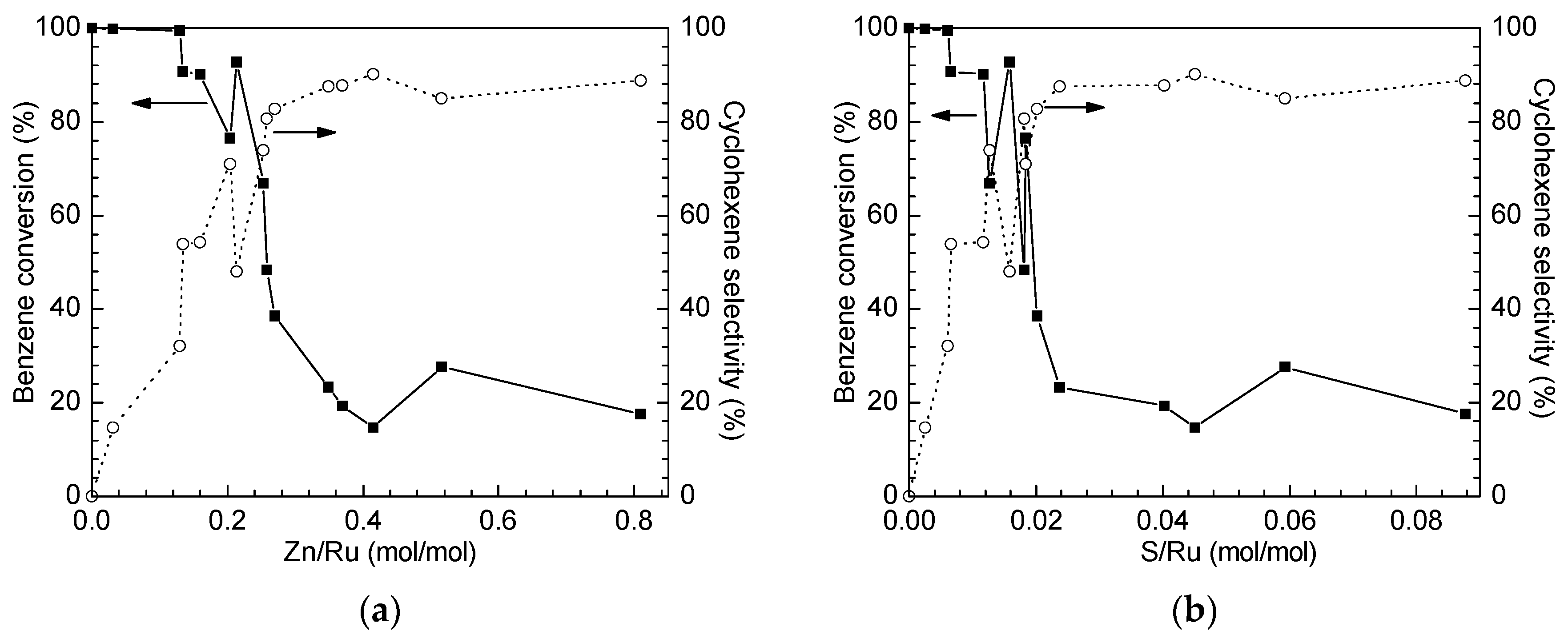
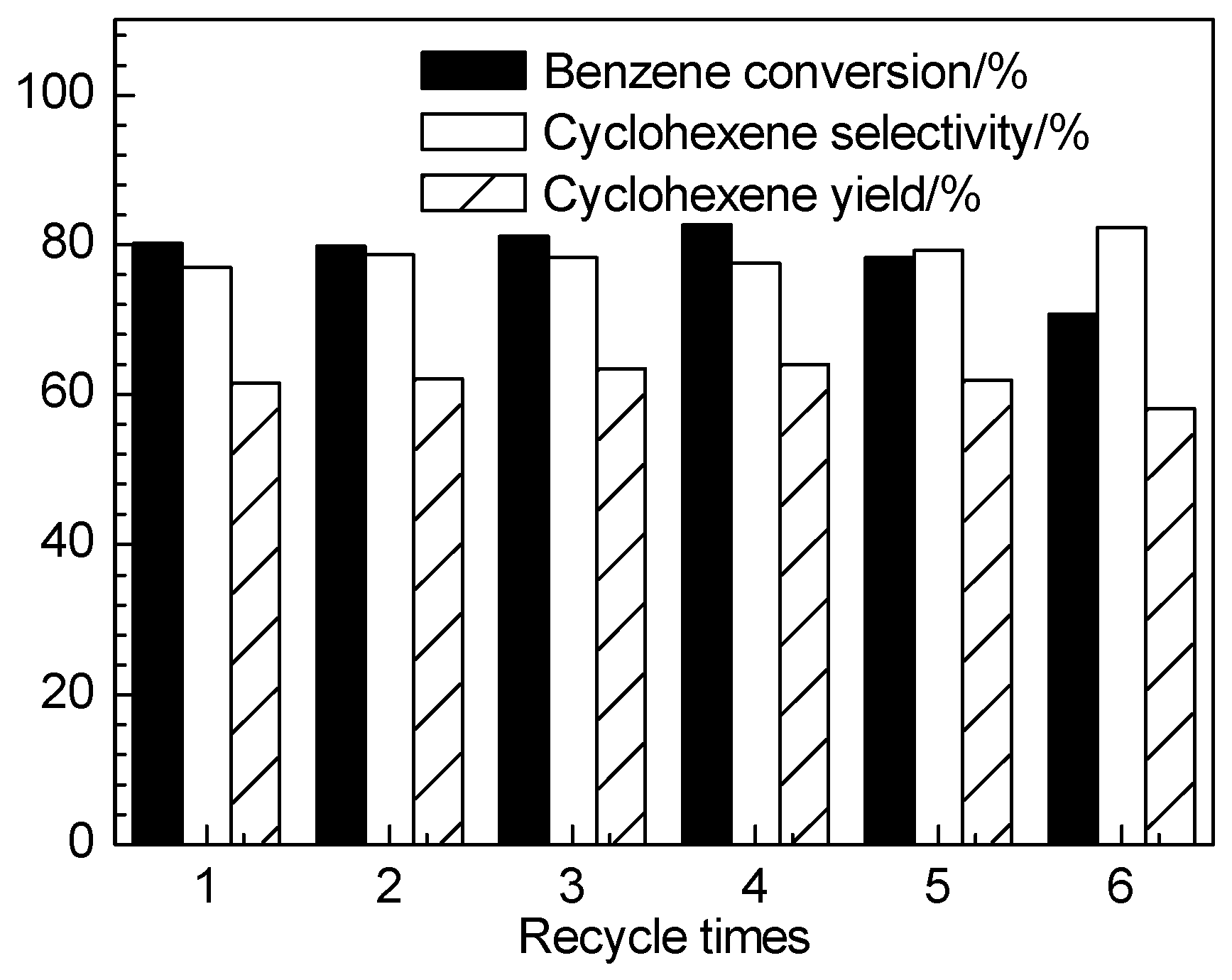
2.2. Catalytic Performance
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalytic Experimental Procedure
3.3. Catalyst Characterization Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, J.C.; Dari, M.D.; Tang, X.Q.; Yuan, P.Q. Diffusion of benzene through water film confined in silica mesopores: Effect of competitive adsorption of solvent. Chem. Eng. Sci. 2020, 224, 115793. [Google Scholar] [CrossRef]
- Zhou, G.B.; Jiang, L.; He, D.P. Nano particulate Ru on TiO2 exposed the {100} facets: Support facet effect on selective hydrogenation of benzene to cyclohexene. J. Catal. 2019, 369, 352–362. [Google Scholar] [CrossRef]
- Zhou, G.B.; Jiang, L.; Dong, Y.L.; He, D.P. Engineering the exposed facets and open-coordinated sites of brookite TiO2 to boost the loaded Ru nanoparticle efficiency in benzene selective hydrogenation. Appl. Surf. Sci. 2019, 486, 187–197. [Google Scholar] [CrossRef]
- Gonçalves, A.H.A.; Soares, J.C.S.; Araújo, L.R.R.; Zotin, F.M.Z.; Mendes, F.M.T.; Gaspar, A.B. Surface investigation by X-ray photoelectron spectroscopy of Ru-Zn catalysts for the partial hydrogenation of benzene. Mol. Catal. 2020, 483, 110710. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Dabbawala, A.A.; Chakraborty, M.; Bajaj, H.C.; Mukhopadhyay, S.; Shenoy, K.T. Partial hydrogenation of benzene to cyclohexene over Ru/gamma-Al2O3 nanocatalyst via w/o microemulsion using boric acid and ethanolamine additives. Res. Chem. Intermed. 2016, 42, 1557–1569. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Wang, H.X.; Dong, Y.Y.; Liu, Z.Y.; Liu, S.C. Selective Hydrogenation of Benzene to Cyclohexene over a Ru-Zn catalyst with Diethanolamine as an Additive. Chin. J. Catal. 2012, 33, 610–620. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, Z.H.; Guo, W.; Zhou, X.L.; Liu, Z.Y.; Liu, S.C. Effect of Organic Additives on the Performance of Nano-sized Ru-Zn Catalyst. Chin. J. Chem. 2011, 29, 369–373. [Google Scholar] [CrossRef]
- Struijk, J.; Scholten, J.J.F. Selectivity to cyclohexenes in the liquid phase hydrogenation of benzene and toluene over ruthenium catalysts, as influenced by reaction modifiers. Appl. Catal. A 1992, 82, 277–287. [Google Scholar] [CrossRef]
- Spinacé, E.V.; Vaz, J.M. Liquid-phase hydrogenation of benzene tocyclohexene catalyzed by Ru/SiO2 in the presence ofwater–organic mixtures. Catal. Commun. 2003, 4, 91–96. [Google Scholar] [CrossRef]
- Struijk, J.; Moene, R.; Kamp, T.V.D.; Scholten, J.J.F. Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution II. Influence of various salts on the performance of the catalyst. Appl. Catal. A Gen. 1992, 89, 77–102. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121/122, 448–451. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, Y.; Liu, J.; Pei, Y.; Li, Z.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Discrimination of the roles of CdSO4 and ZnSO4 in liquid phase hydrogenation of benzene to cyclohexene. J. Catal. 2009, 268, 100–105. [Google Scholar] [CrossRef]
- Wu, J.M.; Yang, Y.F.; Chen, J.L. Study on the causes of catalyst inactivation of benzene semi-hydrogenation. Chem. Ind. Eng. Prog. 2003, 22, 295–297. [Google Scholar]
- Zhang, P.; Wu, T.B.; Jiang, T.; Wang, W.T.; Liu, H.Z.; Fan, H.L.; Zhang, Z.F.; Han, B.X. Ru–Zn supported on hydroxyapatite as an effective catalyst for partial hydrogenation of benzene. Green Chem. 2013, 15, 152–159. [Google Scholar] [CrossRef]
- Yuan, P.Q.; Wang, B.Q.; Ma, Y.M.; He, H.M.; Cheng, Z.M.; Yuan, W.K. Hydrogenation of cyclohexene over Ru-Zn/Ru(0001) surface alloy: A first principles density functional study. J. Mol. Catal. A 2009, 301, 140–145. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, Y.Z.; Xie, S.H.; Qiao, M.H.; Li, H.X.; Fan, K.N. Partial hydrogenation of benzene to cyclohexene on a Ru-Zn/m-ZrO2nanocomposite catalyst. Appl. Catal. A Gen. 2004, 272, 29–36. [Google Scholar] [CrossRef]
- Hu, S.C.; Chen, Y.W. Partial hydrogenation of benzene on Ru-Zn/SiO2 catalysts. Ind. Eng. Chem. Res. 2001, 40, 6099–6104. [Google Scholar] [CrossRef]
- Suppino, R.S.; Landers, R.; Cobo, A.J.G. Partial hydrogenation of benzene on Ru catalysts: Effects of additives in the reaction medium. Appl. Catal. A Gen 2013, 452, 9–16. [Google Scholar] [CrossRef]
- Fan, G.Y.; Li, R.X.; Li, X.J.; Chen, H. Effect of organic additives on partial hydrogenation of benzene. Catal. Commun. 2008, 9, 1394–1397. [Google Scholar] [CrossRef]
- Bu, J.; Liu, J.L.; Chen, X.Y.; Zhuang, J.H.; Yan, S.R.; Qiao, M.H.; He, H.Y.; Fan, K.N. Ru/SBA-15 catalysts for partial hydrogenation of benzene to cyclohexene: Tuning the Ru crystallite size by Ba. Catal. Commun. 2008, 9, 2612–2615. [Google Scholar] [CrossRef]
- Ramos-Fernándze, E.V.; Ferreira, A.F.P.; Sepúlveda-Escribano, A.; Kapteijn, F.; Rodríguez-Reinoso, F. Enhancing the catalytic performance of Pt/ZnO in the selective hydrogenation of cinnamaldehyde by Cr addition to the support. J. Catal. 2008, 258, 52–60. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Jiang, H.B.; Li, S.H.; Zhang, Y.X.; Liu, S.C.; Liu, Z.Y. Effect of transition metals (Cr, Mn, Fe, Co, Ni, Cu and Zn) on the hydrogenation properties of benzene over Ru-based catalyst. Appl. Catal. A Gen. 2013, 464–465, 1–9. [Google Scholar] [CrossRef]
- Su, F.B.; Lee, F.Y.; Lv, L.; Liu, J.; Tian, X.N.; Zhao, X.S. Sandwiched Ruthenium/Carbon Nanostructures for Highly Active Heterogeneous Hydrogenation. Adv. Funct. Mater. 2007, 17, 1926–1931. [Google Scholar] [CrossRef]
- Sun, H.J.; Wang, H.X.; Jiang, H.B.; Li, S.H.; Liu, S.C.; Liu, Z.Y.; Yuan, X.M.; Yang, K.J. Eeffect of (Zn(OH)2)3(ZnSO4)(H2O)5 on the performance of Ru-Zn catalyst for benzene selective hydrogenation to cyclohexene. Appl. Catal. A 2013, 450, 160–168. [Google Scholar] [CrossRef]
- Milone, C.; Neri, G.; Donato, A.; Musolino, M.G.; Mercadante, L. Selective Hydrogenation of Benzene to Cyclohexene on Ru/γ -Al2O3. J. Catal. 1996, 159, 253–258. [Google Scholar] [CrossRef]
- Ronchin, L.; Luigi, T. Selective hydrogenation of benzene to cyclohexene using a suspendedRu catalyst in a mechanically agitated tetraphase reactor. Catal. Today 1999, 48, 255–264. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Z.; Chen, L.; Li, H.; Peng, Z.; Liu, Z.; Liu, S. Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant. Catalysts 2018, 8, 513. [Google Scholar] [CrossRef]
- Prasad, K.H.V.K.; Prasad, B.S.; Mallikarjunan, M.M.; Vaidyeswaran, R. Self-poisoning and rate multiplicity in hydrogenation of benzene. J. Catal. 1983, 84, 65–73. [Google Scholar] [CrossRef]
- Rochin, L.; Tonilolo, L. Kinetics of liquid-phase hydrogenation reactions oversupported metal catalysts-a review. Appl. Catal. A 2001, 208, 77–89. [Google Scholar]
- Shimizu, H.; Christmann, K.; Ertl, G. Model studies on bimetallic Cu/Ru catalysts: II. Adsorption of hydrogen. J. Catal. 1980, 61, 412–419. [Google Scholar] [CrossRef]
- Lu, K.; Tatarchuk, B.J. Activated chemisorption of hydrogen on supported ruthenium: II. Effects of crystallite size and adsorbed chlorine on accurate surface area measurements. J. Catal. 1987, 106, 176–187. [Google Scholar] [CrossRef]
- Lu, K.; Tatarchuk, B.J. Activated chemisorption of hydrogen on supported ruthenium: I. Influence of adsorbed chlorine on accurate surface area measurements. J. Catal. 1987, 106, 166–175. [Google Scholar] [CrossRef]
- Liu, H.Z.; Jiang, T.; Han, B.X.; Liang, S.G.; Zhou, Y.X. Selective Phenol Hydrogenation to Cyclohexanone Over a Dual Supported Pd–Lewis Acid Catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Struijk, J.; d’Angremond, M.; Lucas-de Regt, W.J.M.; Scholten, J.J.F. Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution I. Preparation, characterization of the catalyst and study of a number of process variables. Appl. Catal. A 1992, 83, 263–295. [Google Scholar] [CrossRef]
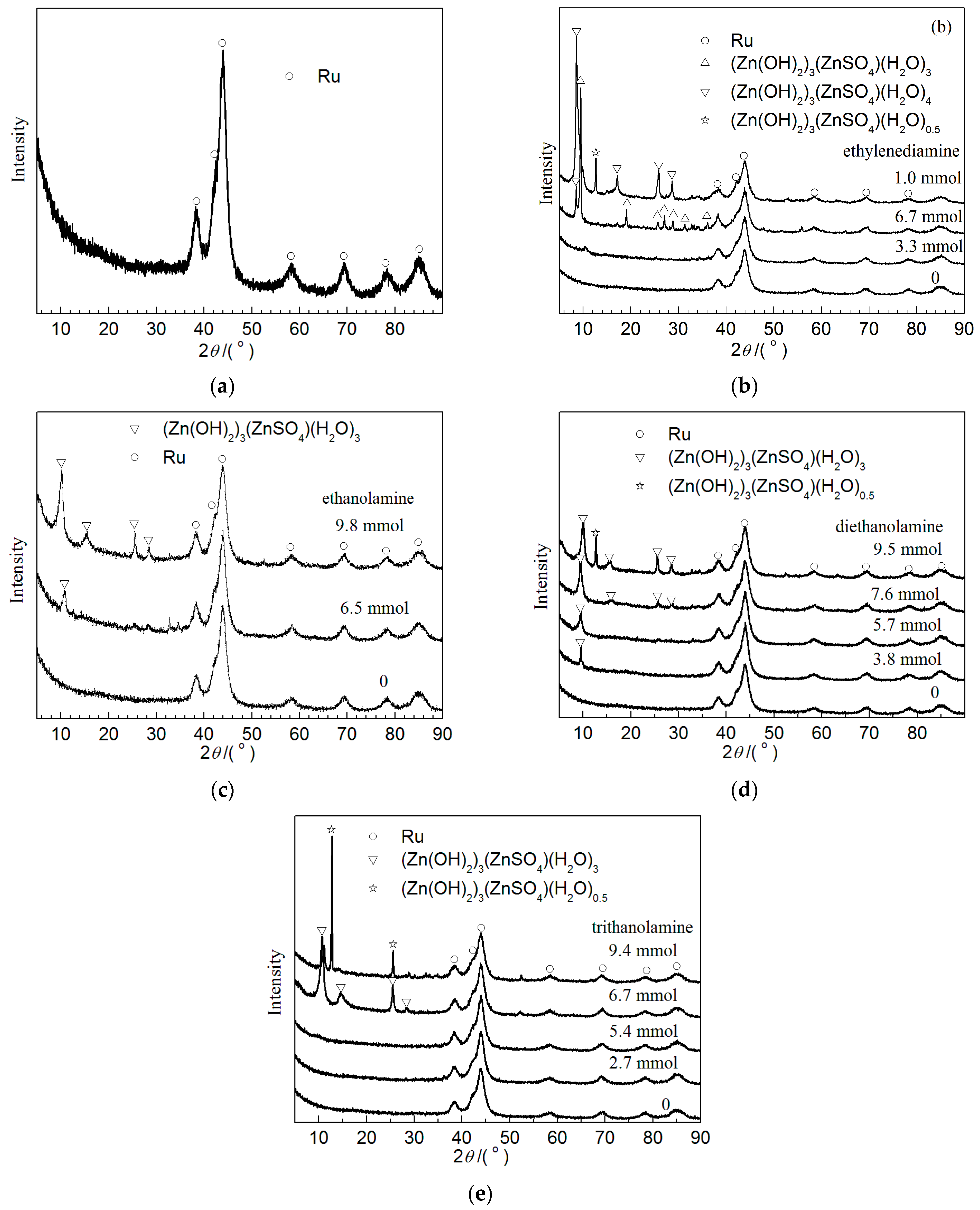
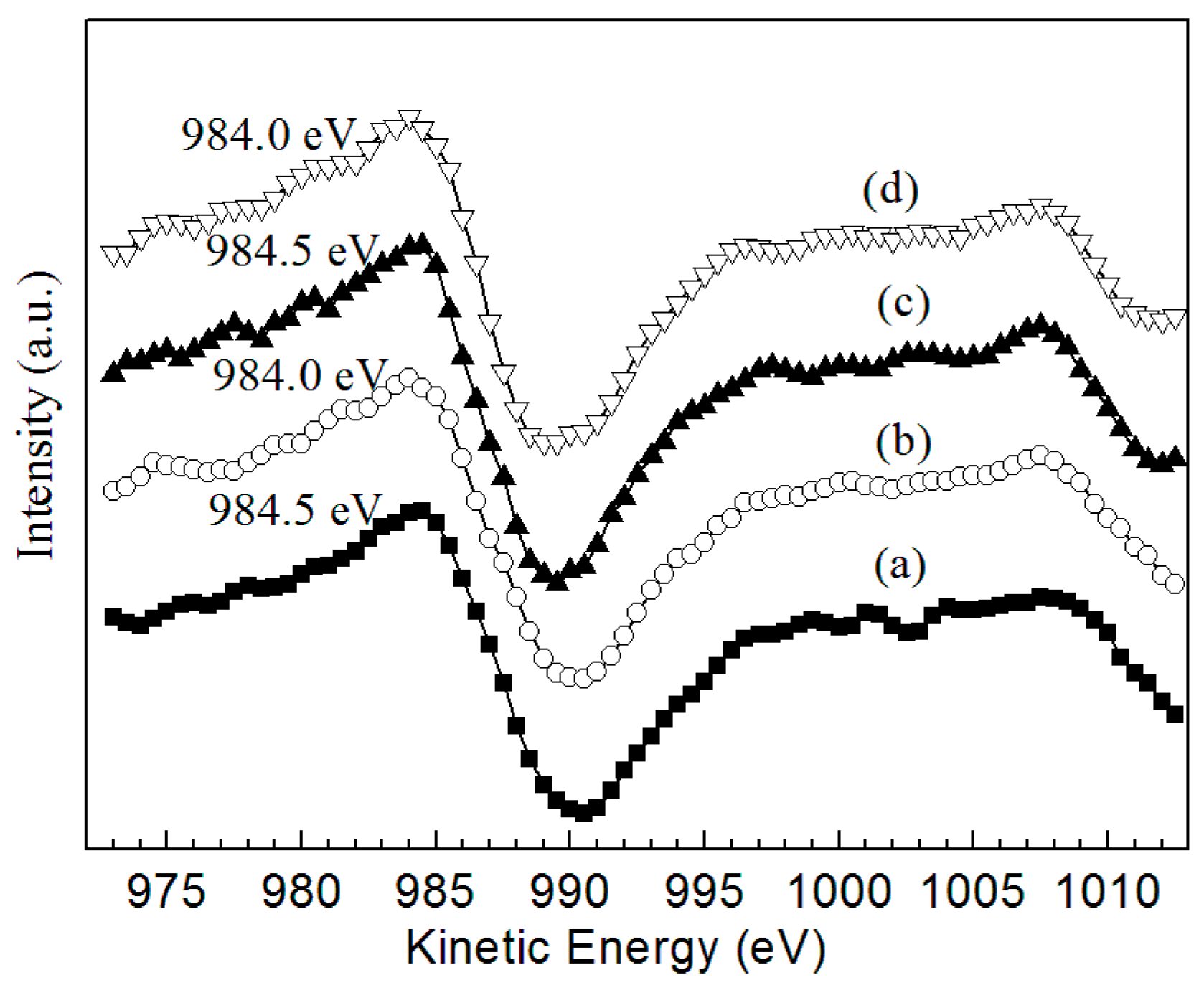

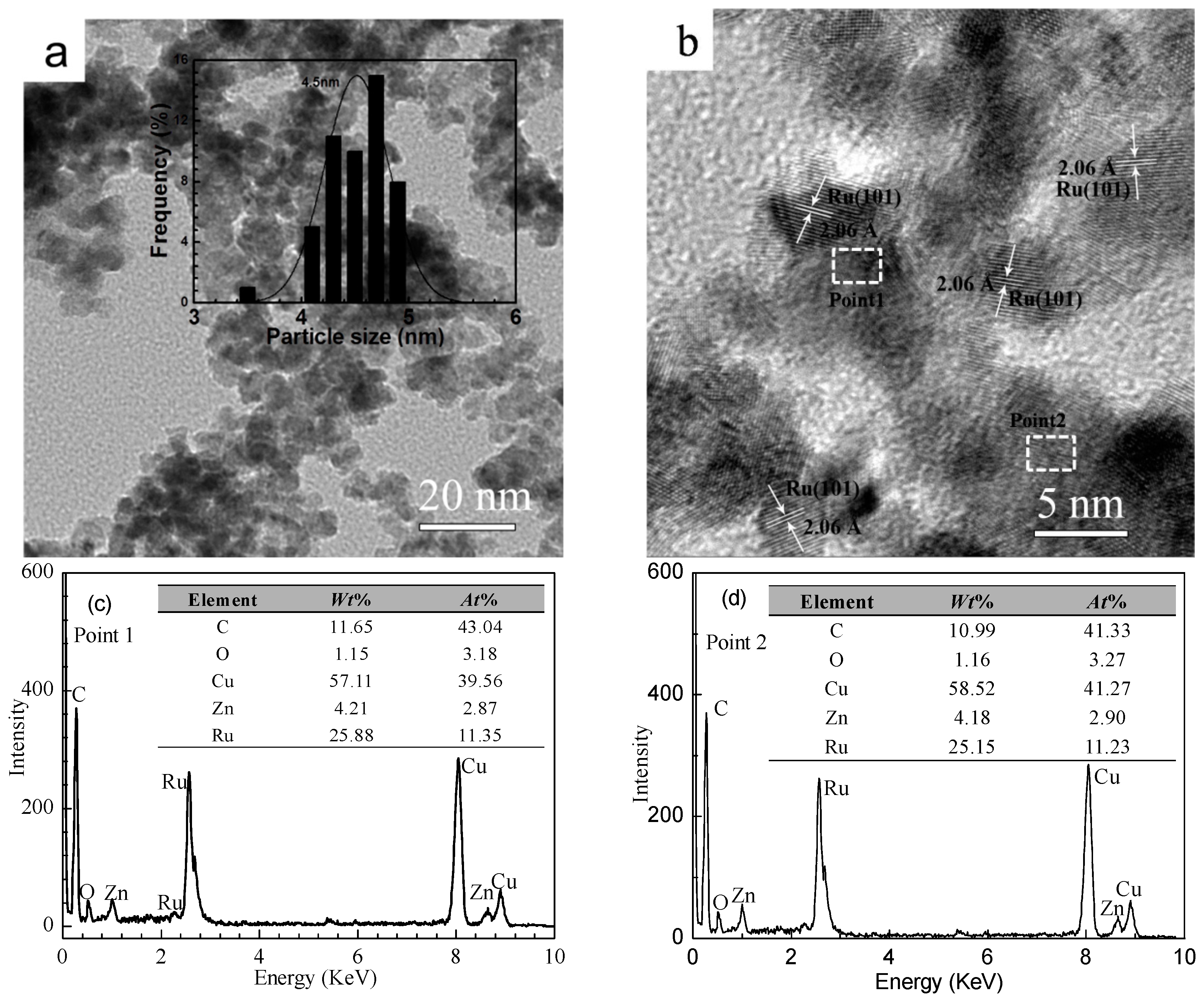
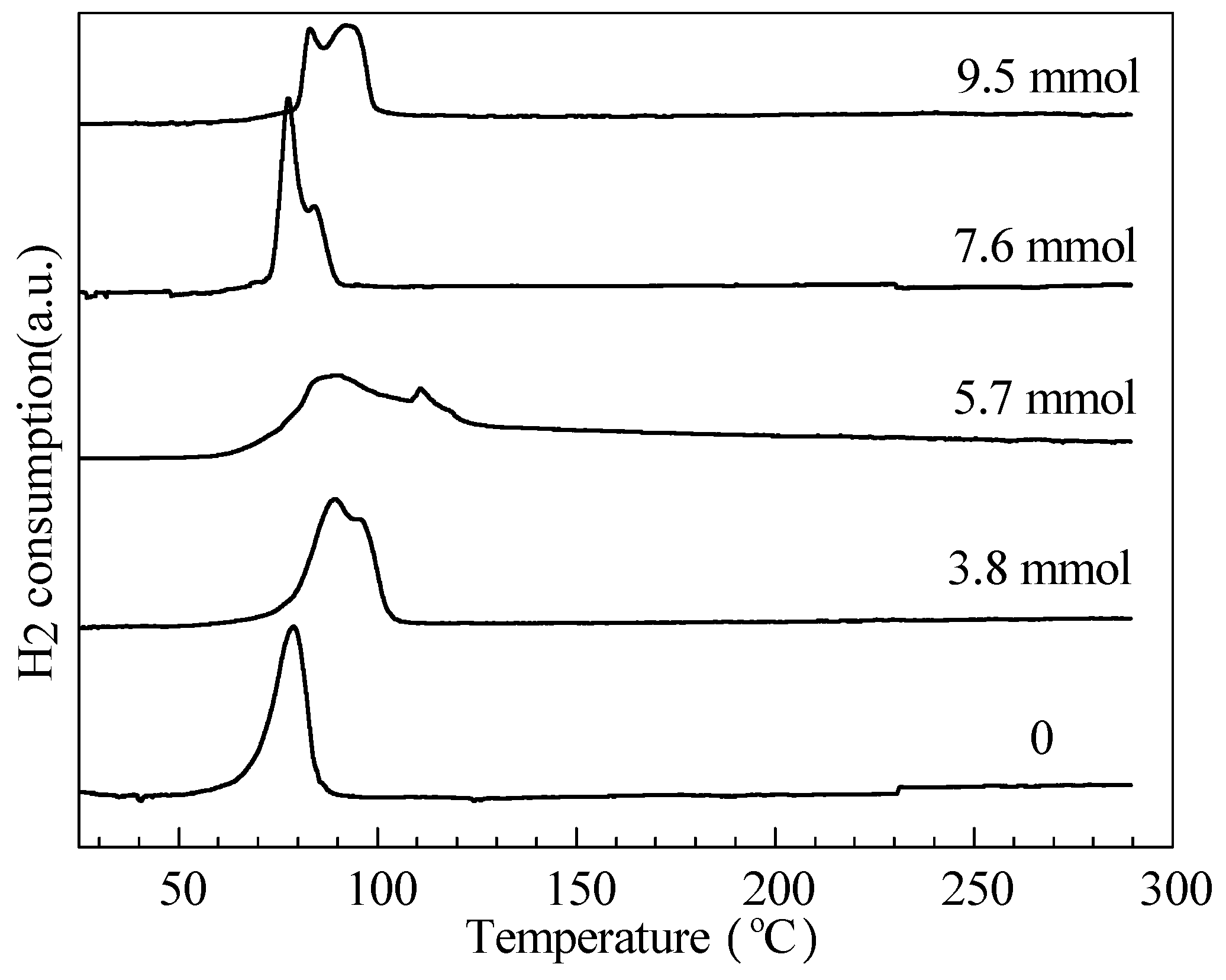



| Amines | n(Zn)/n(Ru) (mol/mol) | n(S)/n(Ru) (mol/mol) | pH Value | Ru Crystallite Size (nm) |
|---|---|---|---|---|
| Ru catalyst | 0 | 0 | 4.7 | |
| Blank a | 0.0313 | 0.0026 | 5.53 | 4.5 |
| 3.3 mmol ethylenediamine a | 0.2133 | 0.0159 | 6.03 | 4.3 |
| 6.7 mmol ethylenediamine a | 0.5155 | 0.0593 | 6.04 | 4.3 |
| 1.0 mmol ethylenediamine a | 0.8090 | 0.0877 | 6.28 | 5.0 |
| 6.5 mmol ethanolamine a | 0.2535 | 0.0127 | 6.00 | 4.1 |
| 9.8 mmol ethanolamine a | 0.3694 | 0.0403 | 6.20 | 4.9 |
| 3.8 mmol diethanolamine a | 0.1597 | 0.0117 | 6.04 | 4.8 |
| 5.7 mmol diethanolamine a | 0.2042 | 0.0184 | 6.02 | 4.8 |
| 7.6 mmol diethanolamine a | 0.2695 | 0.0202 | 6.17 | 4.5 |
| 7.6 mmol diethanolamine b | 0 | 0 | 10.57 | 4.6 |
| 9.5 mmol diethanolamine a | 0.4150 | 0.0450 | 5.98 | 4.6 |
| 2.7 mmol triethanolamine a | 0.1300 | 0.0061 | 6.25 | 4.5 |
| 5.4 mmol triethanolamine a | 0.1337 | 0.0066 | 6.21 | 4.8 |
| 6.7 mmol triethanolamine a | 0.2576 | 0.0181 | 6.16 | 4.7 |
| 9.4 mmol triethanolamine a | 0.3488 | 0.0238 | 6.19 | 4.6 |
| Addtive | A/(m2/g) | d/nm | V/(cm3/g) |
|---|---|---|---|
| Blank | 70 | 10.44 | 0.191 |
| 0.4 g diethanol amine | 60 | 8.94 | 0.134 |
| 0.6 g diethanol amine | 59 | 10.21 | 0.152 |
| 0.8 g diethanol amine | 50 | 10.41 | 0.130 |
| 1.0 g diethanol amine | 49 | 8.11 | 0.099 |
| Amines | Conversion (%) 2 | Selectivity (%) 2 | Yield (%) 2 | Time (min) 2 |
|---|---|---|---|---|
| Blank test 1 | 70.7 | 46.7 | 33.0 | 5 |
| 3.3 mmol ethanediamine 1 | 92.7 | 48.0 | 44.5 | 15 |
| 6.7 mmol ethanediamine 1 | 35.5 | 86.4 | 30.7 | 25 |
| 10.0 mmol ethanediamine 1 | 28.7 | 86.9 | 24.9 | 25 |
| 6.5 mmol ethanolamine 1 | 85.0 | 66.9 | 56.9 | 25 |
| 9.8 mmol ethanolamine 1 | 31.1 | 86.0 | 26.8 | 25 |
| 3.8 mmol diethanolamine 1 | 90.2 | 54.2 | 48.8 | 15 |
| 5.7 mmol diethanolamine 1 | 84.6 | 67.1 | 56.7 | 20 |
| 7.6 mmol diethanolamine 1 | 57.0 | 84.5 | 48.6 | 25 |
| 7.6 mmol diethanolamine 3 | 100 | 0 | 0 | 5 |
| 9.5 mmol diethanolamine 1 | 29.1 | 88.5 | 25.8 | 25 |
| 2.7 mmol triethanolamine 1 | 94.3 | 44.0 | 41.5 | 10 |
| 5.4 mmol triethanolamine 1 | 90.6 | 53.9 | 48.9 | 15 |
| 6.7 mmol triethanolamine 1 | 69.9 | 75.1 | 52.4 | 25 |
| 9.4 mmol triethanolamine 1 | 40.2 | 84.5 | 34.0 | 25 |
| Experiment a | Reaction Substrate |
|---|---|
| Blank | 1.96 g catalyst, 280 mL of 0.6 mol/L ZnSO4·7H2O aqueous solution |
| Test1 | 1.96 g catalyst, 280 mL of 0.6 mol/L ZnSO4·7H2O aqueous solution, 7.6 mmol diethanolamine |
| Test2 | Slurry in Test 1 (including amine and 280 mL of 0.6 mol/L ZnSO4·7H2O aqueous solution), 1.96 g catalyst |
| Test3 | Spent catalyst in Test 1 (covered with chemisorbed Zn salts), 280 mL H2O |
| Test4 | Spent catalyst in Test 1 (covered with chemisorbed Zn salts), 280 mL of 0.6 mol/L ZnSO4·7H2O aqueous solution |
| ZrO2 Dosage (g) | Benzene Conversion b (%) | Cyclohexenes Electivity b (%) | Cyclohexene Yield b (%) | Time b (min) |
|---|---|---|---|---|
| 0 | 57.7 | 84.5 | 48.6 | 25 |
| 5 | 77.5 | 74.6 | 57.7 | 25 |
| 10 | 80.1 | 77.0 | 61.6 | 25 |
| 15 | 87.9 | 64.5 | 56.7 | 25 |
| 20 | 94.8 | 54.0 | 56.2 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, W.; Wang, X.; Chen, Z.; Peng, Z. Origin of the Increase in the Selectivity of Ru Catalysts with the Addition of Amines in the Presence of ZnSO4 for the Selective Hydrogenation of Benzene to Cyclohexene. Catalysts 2024, 14, 194. https://doi.org/10.3390/catal14030194
Sun H, Zhang W, Wang X, Chen Z, Peng Z. Origin of the Increase in the Selectivity of Ru Catalysts with the Addition of Amines in the Presence of ZnSO4 for the Selective Hydrogenation of Benzene to Cyclohexene. Catalysts. 2024; 14(3):194. https://doi.org/10.3390/catal14030194
Chicago/Turabian StyleSun, Haijie, Wen Zhang, Xiaohui Wang, Zhihao Chen, and Zhikun Peng. 2024. "Origin of the Increase in the Selectivity of Ru Catalysts with the Addition of Amines in the Presence of ZnSO4 for the Selective Hydrogenation of Benzene to Cyclohexene" Catalysts 14, no. 3: 194. https://doi.org/10.3390/catal14030194





