Transformation of Light Alkanes into High-Value Aromatics
Abstract
:1. Introduction
2. Results and Discussion
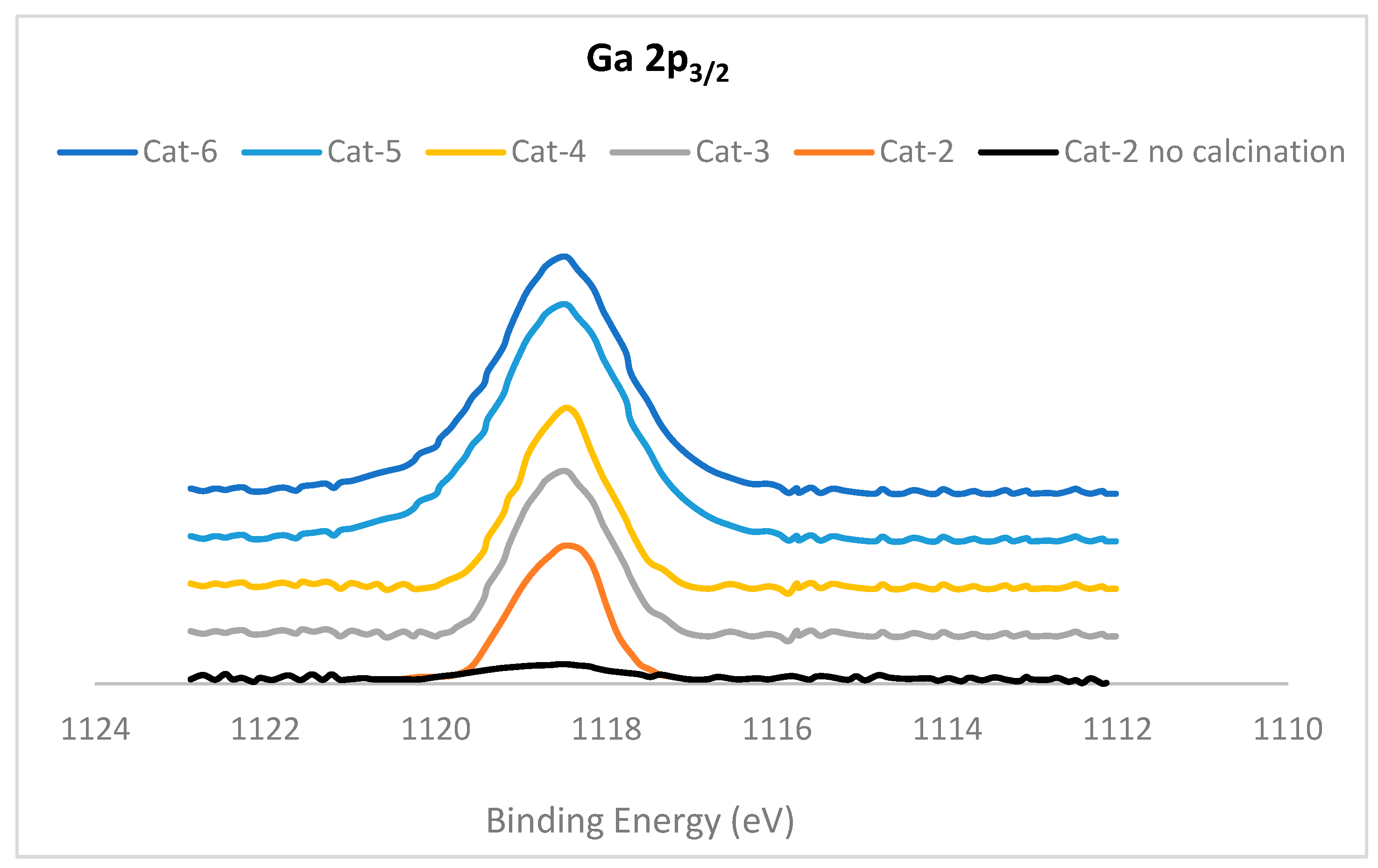
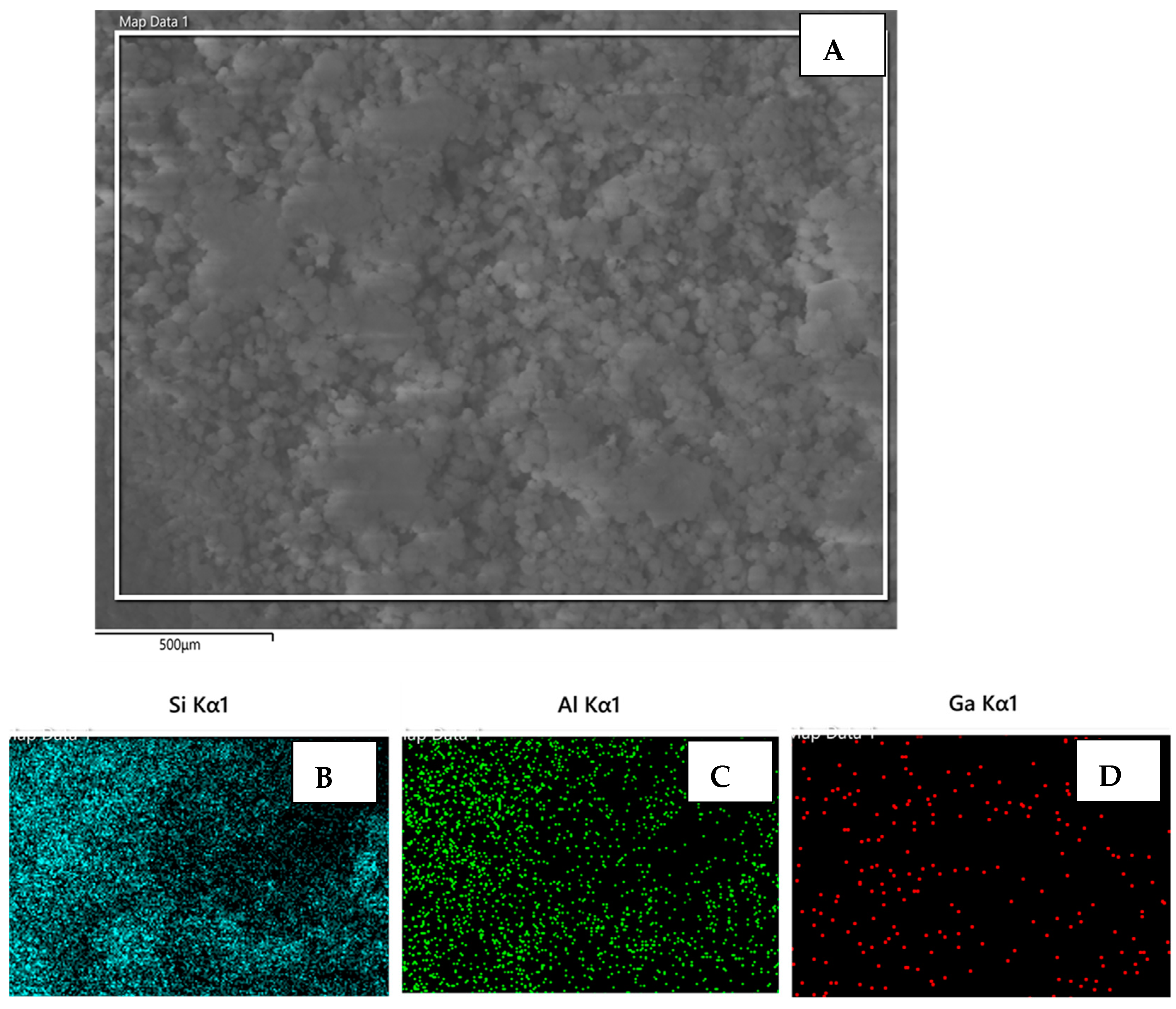
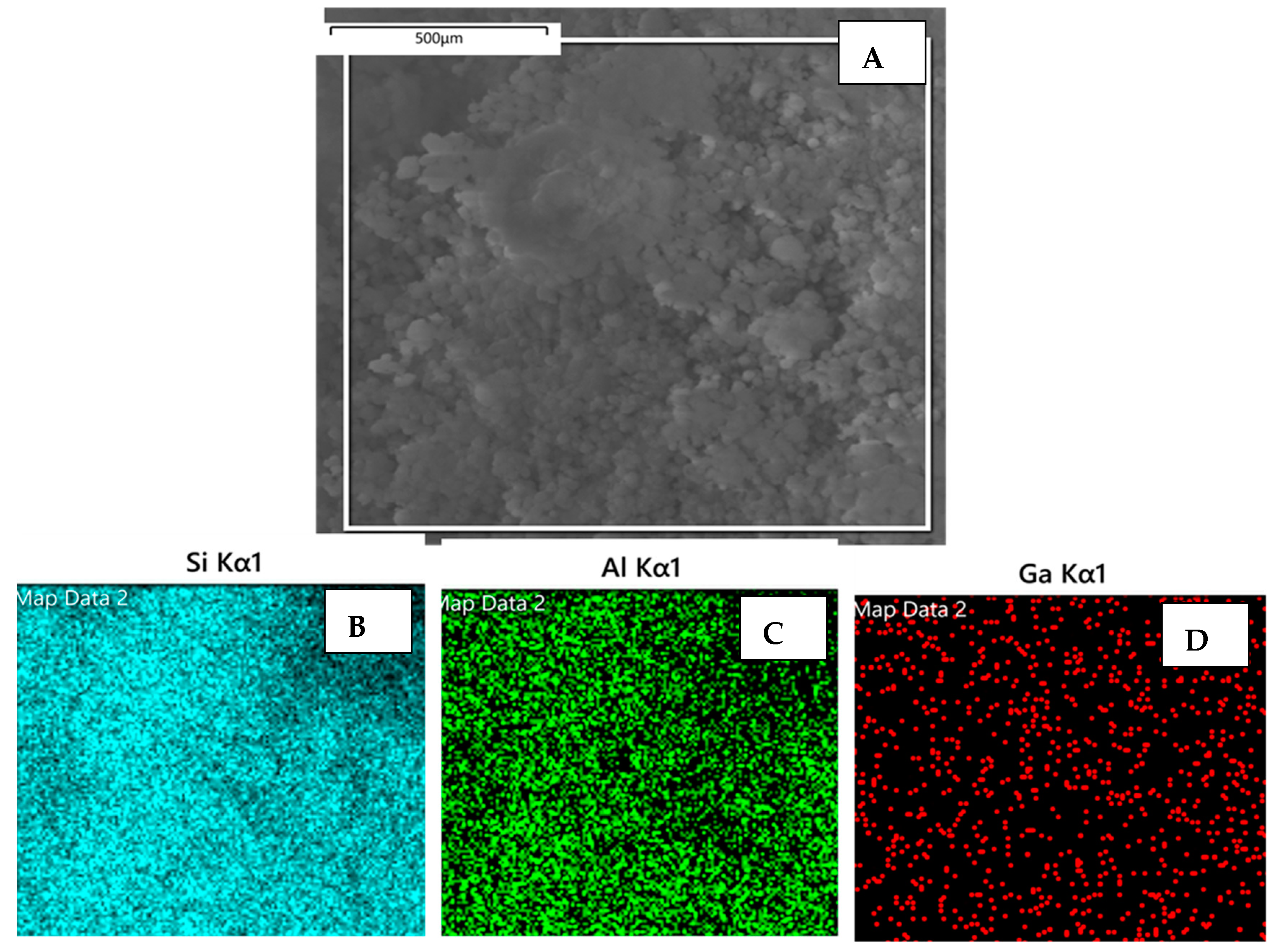
2.1. Characterization of Catalysts
2.2. Evaluation of Catalysts
2.2.1. Impact of Ga/(Al+Ga) Ratio
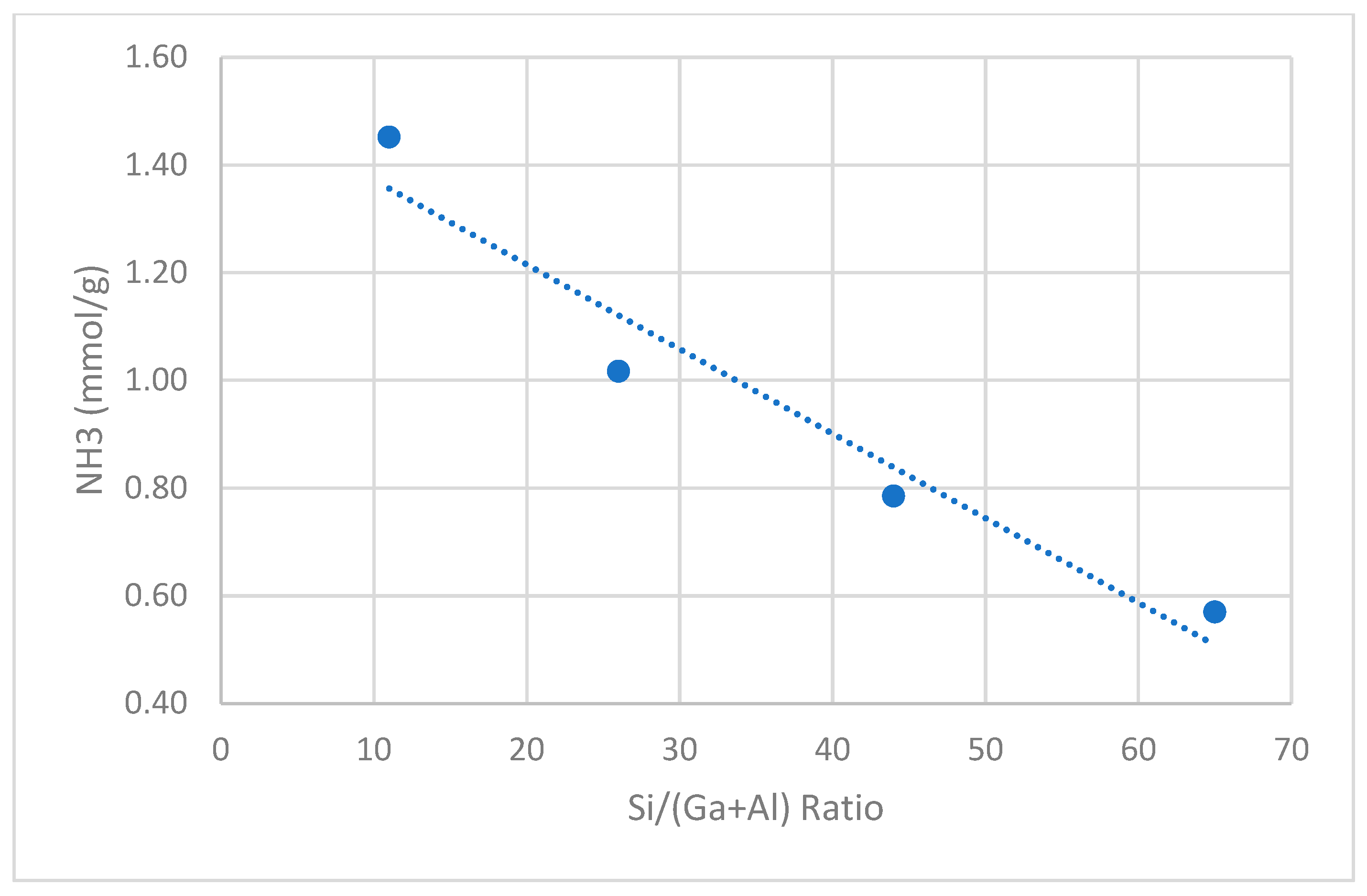
2.2.2. Effect of Si/(Al+Ga) Ratio
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, Y.H.; Kim, H.; Lee, H.; Nam, K.; Ryu, H.W.; Kim, D.H. Application of HCl treatment to tailor the Ga species and propane aromatization performance of various Ga incorporated MFI catalysts. J. Catal. 2023, 422, 24–35. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal. Rev. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Liu, D.; Cao, L.; Zhang, G.; Zhao, L.; Gao, J.; Xu, C. Catalytic conversion of light alkanes to aromatics by metal-containing HZSM-5 zeolite catalysts—A review. Fuel Process. Technol. 2021, 216, 106770–106786. [Google Scholar] [CrossRef]
- Rodrigues, V.D.O.; Vasconcellos, F.J.; Júnior, A.D.C.F. Mechanistic studies through H–D exchange reactions: Propane aromatization in HZSM5 and Ga/ HZSM5 catalysts. J. Catal. 2016, 344, 252–262. [Google Scholar] [CrossRef]
- Fang, Y.J.; Su, X.F.; Bai, X.F.; Wu, W.; Wang, G.L.; Xiao, L.F.; Yu, A.R. Aromatization over nanosized Ga-containing ZSM-5 zeolites prepared by different methods: Effect of acidity of active Ga species on the catalytic performance. J. Energy Chem. 2017, 26, 768–775. [Google Scholar] [CrossRef]
- Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Pinard, L. Propane aromatization on hierarchical Ga/HZSM-5 catalysts. J. Catal. 2018, 366, 223–236. [Google Scholar] [CrossRef]
- Krishnamurthy, G.; Bhan, A.; Delgass, W.N. Identity and chemical function of gallium species inferred from microkinetic modeling studies of propane aromatization over Ga/HZSM-5 catalysts. J. Catal. 2010, 271, 370–385. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Hu, M.; Qi, G.; Wang, Q.; Li, S.; Xu, J.; Deng, F. Unraveling hydrocarbon pool boosted propane aromatization on gallium/ZSM-5 zeolite by solid-state nuclear magnetic resonance spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 23630–23634. [Google Scholar] [CrossRef]
- Dauda, I.B.; Yusuf, M.; Gbadamasi, S.; Bello, M.; Atta, A.Y.; Aderemi, B.O.; Jibril, B.Y. Highly Selective Hierarchical ZnO/ZSM-5 Catalysts for Propane Aromatization. Rev. Chem. Eng. 2023, 39, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Pham, H.N.; Alcala, R.; Datye, A.K.; Miller, J.T. Dehydroaromatization Pathway of Propane on PtZn/SiO2 + ZSM-5 Bifunctional Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 394–409. [Google Scholar] [CrossRef]
- Chen, G.; Griffin, A.; Qiang, A.; Toghiani, H.; Xiang, Y. Ethane and propane dehydroaromatization on Pt/HZSM-5 catalyst: Influence of Pt loading ≤ 500 ppm. Appl. Catal. A Gen. 2023, 665, 119368–119377. [Google Scholar] [CrossRef]
- Lim, Y.H.; Ryu, H.W.; Jung, W.; Nam, K.; Hwang, Y.; Kim, D.H. Direct non-oxidative conversion of shale gas to aromatics over active metal-modified ZSM-5 catalysts. Fuel 2023, 339, 126946–126959. [Google Scholar] [CrossRef]
- Nozik, D.; Tinga, F.M.P.; Bell, A.T. Propane Dehydrogenation and Cracking over Zn/H-MFI Prepared by Solid-State Ion Exchange of ZnCl2. ACS Catal. 2021, 11, 14489–14506. [Google Scholar] [CrossRef]
- Noh, G.; Shi, Z.; Zones, S.I.; Iglesia, E. Isomerization and β-Scission Reactions of Alkanes on Bifunctional Metal- Acid Catalysts: Consequences of Confinement and Diffusional Constraints on Reactivity and Selectivity. J. Catal. 2018, 368, 389–410. [Google Scholar] [CrossRef]
- Liu, R.-l.; Zhu, H.-q.; Wu, Z.-w.; Qin, Z.-f.; Fan, W.-b.; Wang, J.-g. Aromatization of propane over Ga-modified ZSM-5 catalysts. J. Fuel Chem. Technol. 2015, 43, 961–969. [Google Scholar] [CrossRef]
- Ono, Y. Transformation of Lower Alkanes into Aromatic Hydrocarbons over ZSM-5 Zeolites. Catal. Rev.-Sci. Eng. 1992, 34, 179–226. [Google Scholar] [CrossRef]
- Nowak, I.; Quartararo, J.; Derouane, E.G.; Védrine, J.C. ffect of H2–O2 pre-treatments on the state of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. Appl. Catal. A Gen. 2003, 251, 107–120. [Google Scholar] [CrossRef]
- Li, L.; Chalmers, J.A.; Bare, S.R.; Scott, S.L.; Vila, F.D. Rigorous Oxidation State Assignments for Supported Ga-Containing Catalysts Using Theory-Informed X-ray Absorption Spectroscopy Signatures from Well-Defined Ga(I) and Ga(III) Compounds. ACS Catal. 2023, 13, 6549–6561. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K.; Sivadinarayana, C. Influence of zeolite factors affecting zeolitic acidity on the propane aromatization activity and selectivity of Ga/H–ZSM-5. Microporous Mesoporous Mater. 2000, 37, 1–8. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Vahnova, T. Aromatization activity of gallium containing MFI and TON zeolite catalysts in n-butane conversion: Effects of gallium and reaction conditions. Appl. Catal. A. Gen. 2007, 316, 61–67. [Google Scholar] [CrossRef]
- Bayense, C.R.; VanderPol, A.; Galliasso, R. Aromatization of propane over MFI-gallosilicates. Appl. Catal. 1991, 72, 81–98. [Google Scholar] [CrossRef]
- Bayense, C.R.; VanHoof, J.H.C. Aromatization of propane over gallium-containing H-ZSM-5 zeolites: Influence of the preparation method on the product selectivity and the catalytic stability. Appl. Catal. 1991, 79, 127–140. [Google Scholar] [CrossRef]
- Giannetto, G.; Montes, A.; Gnep, N.S.; Florentino, A.; Cartraud, P.; Guisnet, M. Conversion of light alkanes into aromatic hydrocarbons. VII. Aromatization of propane on gallosilicates: Effect of calcination in dry air. J. Catal. 1993, 145, 86–95. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Kinage, A.K.; Banerjee, S.; Choudhary, V.R. Effect of temperature on the product selectivity and aromatics distribution in aromatization of propane over H-GaAlMFI zeolite. Microporous Mesoporous Mater. 2004, 70, 37–42. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Kinage, A.K.; Banerjee, S.; Choudhary, V.R. Influence of space velocity on product selectivity and distribution of aromatics in propane aromatization over H-GaAlMFI zeolite. J. Mol. Catal. A Chem. 2006, 246, 79–84. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Kinage, A.K.; Banerjee, S.; Choudhary, V.R. Influence of hydrothermal pretreatment on acidity and activity of H-GaAlMFI zeolite for the propane aromatization reaction. Microporous Mesoporous Mater. 2005, 87, 23–32. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.; Zbudniewek, K.; Rynkowski, J.; Keller, V.; Grams, J.; Ruppert, A.M.; Keller, N. Wide band gap Ga2O3 as efficient UV-C photocatalyst for gas-phase degradation applications. Environ. Sci. Pollut. Res. 2017, 24, 26792–26805. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Al-Khattaf, S. Physicochemical properties and catalytic performance of galloaluminosilicate in aromatization of lower alkanes: A comparative study with Ga/HZSM-5. J. Porous Mater. 2012, 19, 943–960. [Google Scholar] [CrossRef]
- Man, P.P.; Klinowski, J. Quadrupole nutation 27Al NMR studies of isomorphous substitution of aluminium in the framework of zeolite Y. J. Chem. Phys. Lett. 1988, 147, 581–584. [Google Scholar] [CrossRef]
- Bigey, C.; Su, B.L. Propane as alkylating agent for alkylation of benzene on HZSM-5 and Ga-modified HZSM-5 zeolites. J. Mol. Catal. A 2004, 209, 179–187. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Gobbi, G.C.; Hartman, J.S.; Klinowski, J.; Thomas, J.M. Solid-state magic-angle spinning. Aluminum-27 nuclear magnetic resonance studies of zeolites using a 400-MHz high-resolution spectrometer. J. Phys. Chem. 1982, 86, 1247–1250. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Janssens, T.V.W.; Jakobsen, H.J.; Skibsted, J. Zeolites by confined space synthesis—Characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and 27Al/29Si-MAS NMR spectroscopy. Microporous Mesoporous Mater. 2000, 39, 393–401. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Mezari, B.; Pestman, R.; Wannapakdee, W.; Hensen, J.M. On the Role of Acidity in Bulk and Nanosheet [T]MFI (T=Al3+, Ga3+, Fe3+, B3+)Zeolites in the Methanol-to Hydrocarbons Reaction. Chem. Cat. Chem. 2017, 9, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Leth, K.T.; Rovik, A.K.; Holm, M.S.; Brorson, M.; Jakobsen, H.J.; Skibsted, J.; Christensen, C.H. Synthesis and characterization of conventional and mesoporous Ga-MFI for ethane dehydrogenation. Appl. Catal. A 2008, 348, 257–265. [Google Scholar] [CrossRef]
- Chao, K.J.; Sheu, S.P.; Lin, L.H.; Genet, M.J.; Feng, M.H. Characterization of incorporated gallium in beta zeolite. Zeolites 1997, 18, 18–24. [Google Scholar] [CrossRef]
- Kimura, T.; Hata, N.; Sakashita, K.; Asaoka, s. Production of aromatics from heavier n-paraffins on hybrid cracking-reforming catalyst. Catal. Today 2012, 185, 119–125. [Google Scholar] [CrossRef]
- Viswandham, N.; Muralidhar, G.; Rao, T.S.R.P. Cracking and aromatization properties of some metal modified ZSM-5 catalysts for light alkane conversions. J. Mol. Catal. A Chem. 2004, 223, 269–274. [Google Scholar] [CrossRef]
- Phatansri, S.; Praserthdam, P.; Sripusitto, A. Aromatization of light paraffins over Ga-containing MFI-type catalyst. Korean J. Chem. Eng. 2000, 17, 409–413. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Aitani, A.M.; Ummer, A.C.; Alasiri, H.S. Review on the Catalytic Conversion of Naphtha to Aromatics: Advances and Outlook. Energy Fuels 2023, 37, 2586–2607. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determination of Relative Crystallinity of Zeolite ZSM-5 by X-ray Diffraction. Available online: https://www.astm.org/d5758-01r21.html (accessed on 4 September 2022).
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]










| Catalyst Code | Mass% | Molar Ratio | |||
|---|---|---|---|---|---|
| Al (%) | Si (%) | Ga (%) | Si/(Ga+Al) | Ga/(Ga+Al) | |
| Cat-1 | 1.20 | 22.24 | 0.00 | 17.79 | 0.00 |
| Cat-2 | 1.14 | 22.19 | 0.48 | 16.06 | 0.14 |
| Cat-3 | 1.22 | 23.13 | 1.00 | 13.80 | 0.24 |
| Cat-4 | 1.09 | 23.78 | 1.15 | 14.88 | 0.29 |
| Cat-5 | 0.65 | 22.19 | 1.43 | 17.69 | 0.46 |
| Cat-6 | 0.47 | 22.85 | 1.61 | 20.12 | 0.57 |
| Cat-7 | 1.54 | 23.48 | 1.55 | 10.53 | 0.28 |
| Cat-8 | 0.87 | 34.21 | 0.97 | 26.32 | 0.30 |
| Cat-9 | 0.90 | 58.22 | 0.99 | 43.58 | 0.30 |
| Cat-10 | 0.84 | 81.03 | 0.93 | 65.00 | 0.30 |
| Catalyst Code | Crystallinity (XRD) | Textural Properties | ||||
|---|---|---|---|---|---|---|
| SBET (m2g−1) | Micro Pore Area (m2g−1) | External Area (m2g−1) | VTotal (cm3g−1) | VMicro (cm3g−1) | ||
| Cat-1 | 90 | 285.0 | 138.0 | 147.0 | 0.560 | 0.060 |
| Cat-2 | 86 | 281.6 | 133.0 | 148.7 | 0.580 | 0.070 |
| Cat-3 | 84 | 283.0 | 134.2 | 148.8 | 0.692 | 0.063 |
| Cat-4 | 83 | 281.8 | 132.3 | 148.9 | 0.768 | 0.070 |
| Cat-5 | 80 | 290.0 | 126.6 | 163.5 | 0.792 | 0.068 |
| Cat-6 | 76 | 289.4 | 121.8 | 168.1 | 0.761 | 0.066 |
| Cat-7 | 87 | 289.0 | 135.0 | 154.0 | 0.570 | 0.060 |
| Cat-8 | 89 | 292.0 | 136.0 | 156.0 | 0.56 | 0.059 |
| Cat-9 | 85 | 280.0 | 138.0 | 142.0 | 0.580 | 0.062 |
| Cat-10 | 88 | 284.0 | 133.0 | 151.0 | 0.530 | 0.061 |
| Catalyst Code | Relative Crystallinity (XRD) | NH3 TPD Results (mmol NH3 g−1) | Acidity Based on Pyridine Adsorbed FTIR (mmol Pyr/g) | ||||
|---|---|---|---|---|---|---|---|
| LTP | HTP | Total | Bronsted | Lewis | Total (B+L) | ||
| Cat-1 | 90 | 0.70 | 0.41 | 1.20 | 1.46 | 0.15 | 1.61 |
| Cat-2 | 86 | 0.78 | 0.49 | 1.26 | 1.41 | 0.19 | 1.60 |
| Cat-3 | 84 | 0.85 | 0.48 | 1.33 | 1.37 | 0.26 | 1.63 |
| Cat-4 | 83 | 0.94 | 0.51 | 1.45 | 1.33 | 0.32 | 1.65 |
| Cat-5 | 80 | 1.02 | 0.49 | 1.51 | 1.30 | 0.38 | 1.68 |
| Cat-6 | 76 | 1.05 | 0.52 | 1.57 | 1.26 | 0.45 | 1.71 |
| Cat-7 | 87 | 0.94 | 0.51 | 1.45 | |||
| Cat-8 | 89 | 0.56 | 0.45 | 1.02 | |||
| Cat-9 | 85 | 0.40 | 0.38 | 0.78 | |||
| Cat-10 | 88 | 0.26 | 0.31 | 0.57 | |||
| Catalyst Code | Cat-1 | Cat-2 | Cat-3 | Cat-4 | Cat-5 | Cat-6 |
|---|---|---|---|---|---|---|
| Ratio Ga/(Ga+Al) | 0.00 | 0.14 | 0.20 | 0.30 | 0.46 | 0.57 |
| C3 Conversion | 63.0 | 77.0 | 79.0 | 83.0 | 83.0 | 83.0 |
| C1 | 12.0 | 12.2 | 12.2 | 12.7 | 11.4 | 12.2 |
| C2 | 5.2 | 8.2 | 8.1 | 8.4 | 7.6 | 8.1 |
| C2= | 5.8 | 3.9 | 3.9 | 4.0 | 3.6 | 3.9 |
| C3 | 37.0 | 23.0 | 21.0 | 17.0 | 17.0 | 17.0 |
| C3= | 4.1 | 3.7 | 3.7 | 3.9 | 3.5 | 3.7 |
| C4+C4= | 8.0 | 5.0 | 4.0 | 2.0 | 1.9 | 1.0 |
| Total Aromatics | 28.0 | 44.0 | 47.0 | 52.0 | 55.0 | 54.0 |
| Catalyst Code | Cat-7 | Cat-8 | Cat-9 | Cat-10 |
|---|---|---|---|---|
| Ratio Si/(Ga+Al) | 11.0 | 26.0 | 44.0 | 65.0 |
| C3 Conversion | 80.0 | 60.0 | 36.0 | 18.3 |
| C1 | 7.1 | 5.5 | 5.5 | 2.4 |
| C2 | 7.5 | 6.5 | 5.2 | 2.3 |
| C2= | 6.0 | 4.1 | 2.3 | 1.0 |
| C3 | 20.0 | 40.0 | 64.0 | 81.7 |
| C3= | 8.4 | 7.8 | 3.1 | 1.3 |
| C4+C4= | 1.0 | 1.1 | 2.0 | 2.2 |
| Total Aromatics | 50.0 | 35.0 | 18.0 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.N. Transformation of Light Alkanes into High-Value Aromatics. Catalysts 2024, 14, 196. https://doi.org/10.3390/catal14030196
Akhtar MN. Transformation of Light Alkanes into High-Value Aromatics. Catalysts. 2024; 14(3):196. https://doi.org/10.3390/catal14030196
Chicago/Turabian StyleAkhtar, Muhammad Naseem. 2024. "Transformation of Light Alkanes into High-Value Aromatics" Catalysts 14, no. 3: 196. https://doi.org/10.3390/catal14030196