Kinetic and Mechanistic Study of Aldose Conversion to Functionalized Furans in Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
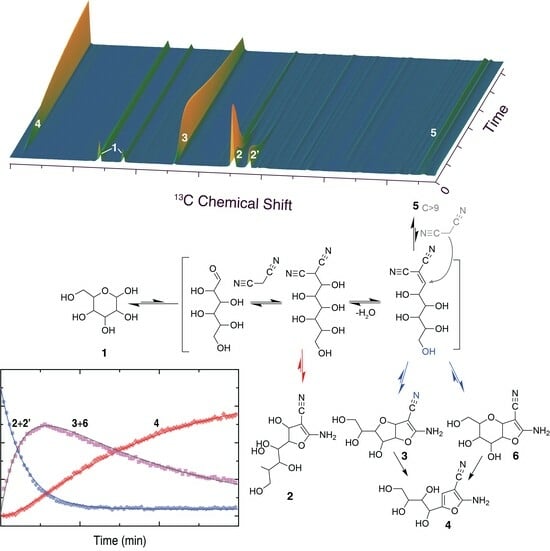
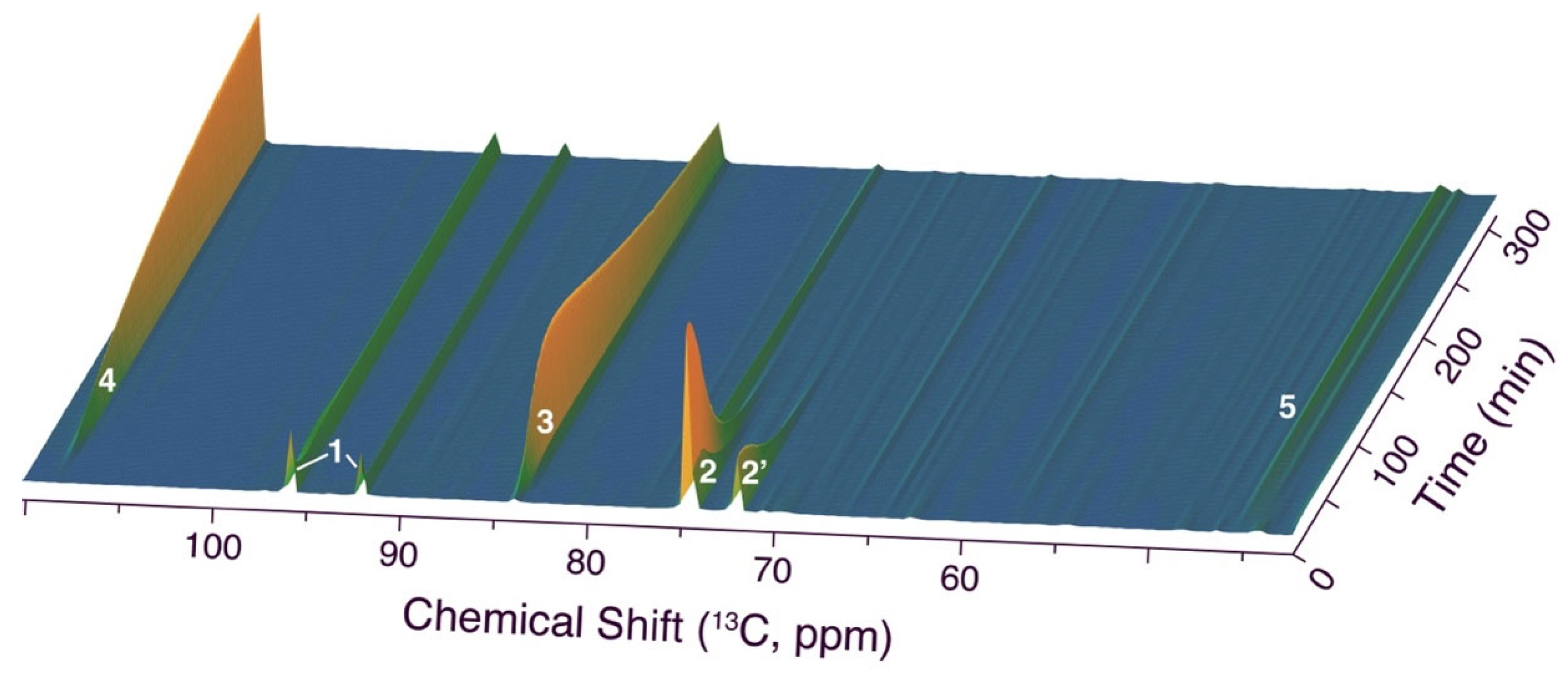
2.1. Stable Aldoses Can Be Converted in a Surprisingly Fast Reaction via Two Intermediates, One of Them Previously Not Anticipated
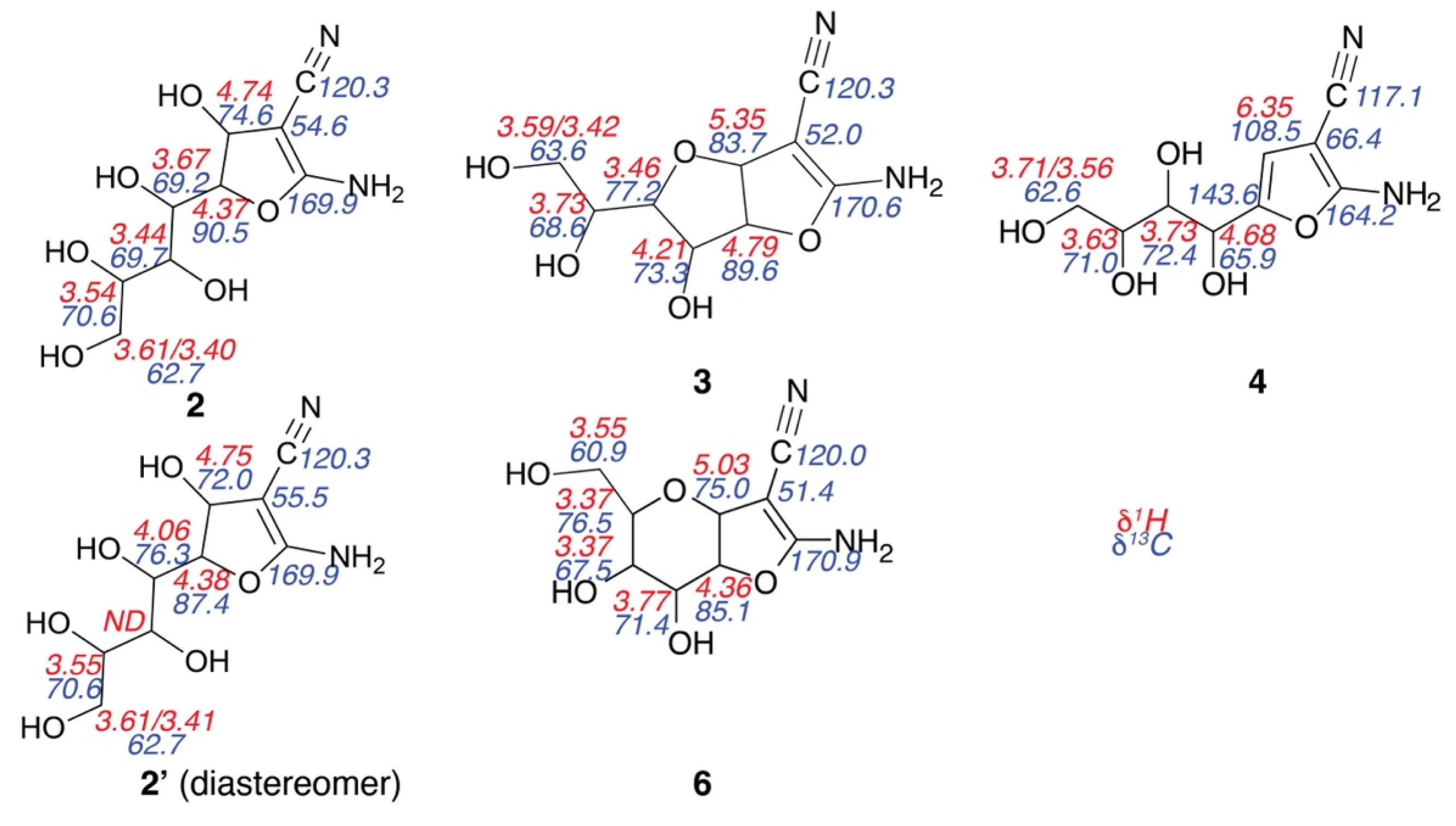
2.2. Assignment of the Detected Species in a Complex Reaction Mixture
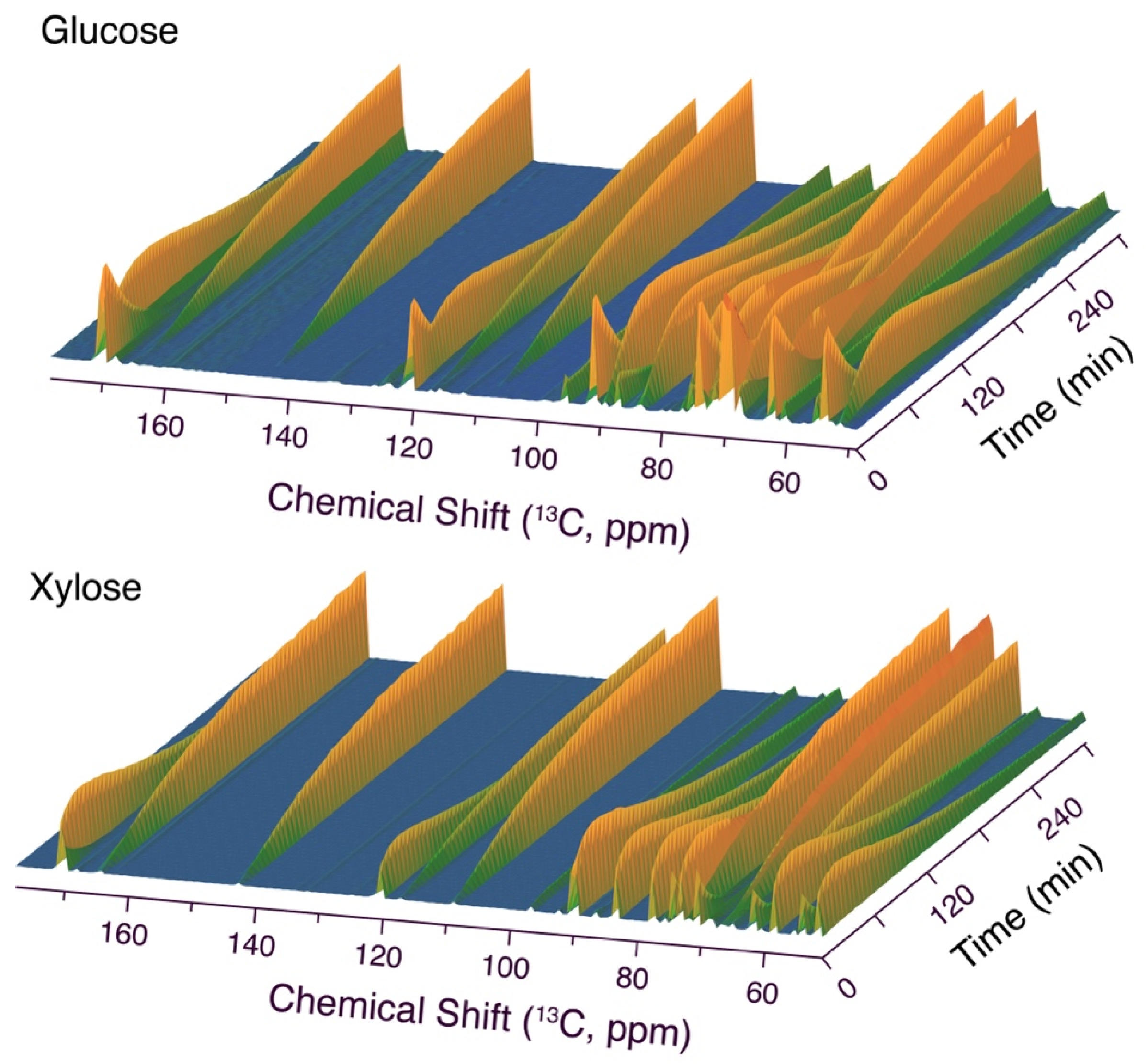
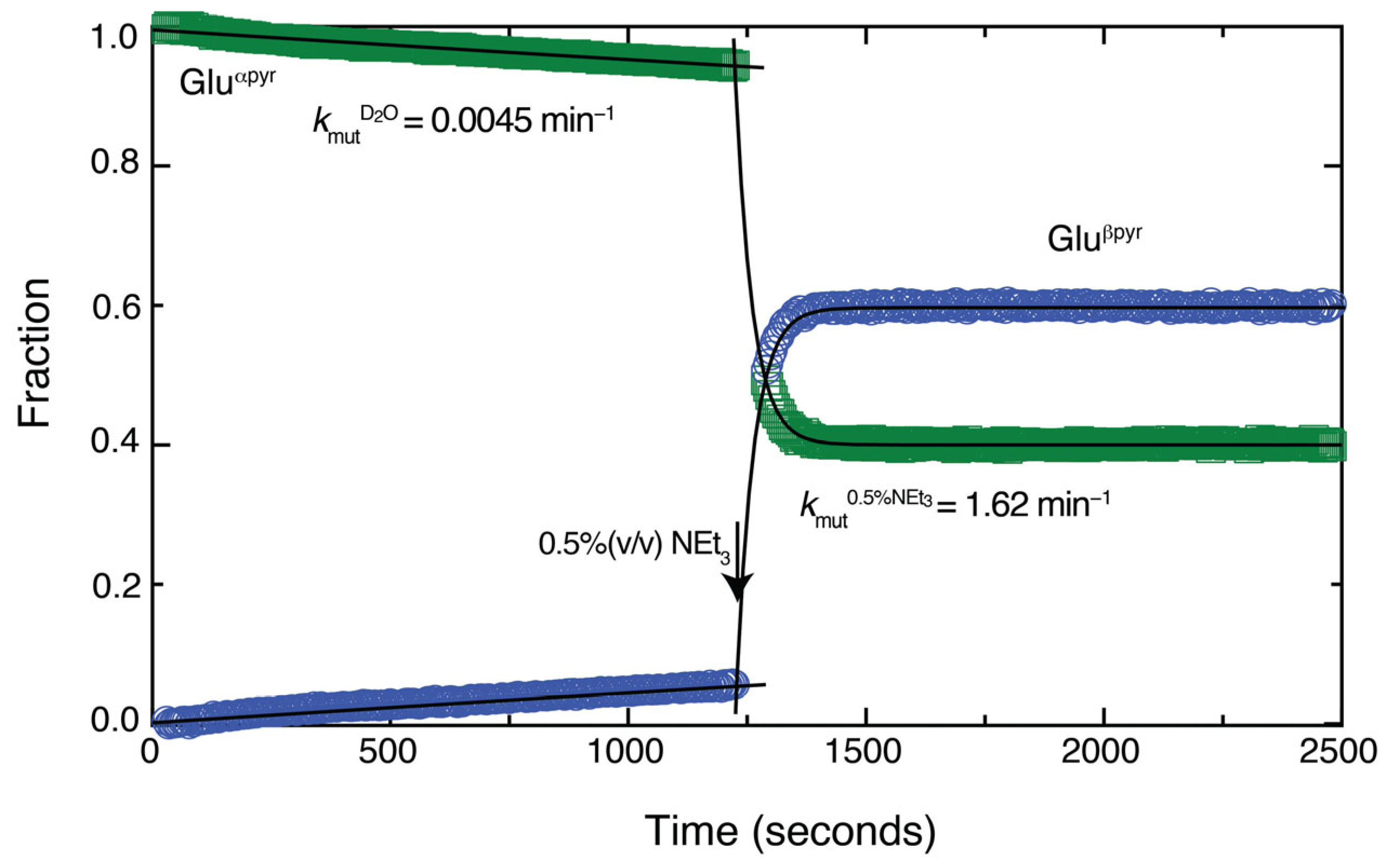
2.3. Isotope Tracking to Validate Assignments and Clarify Mechanisms
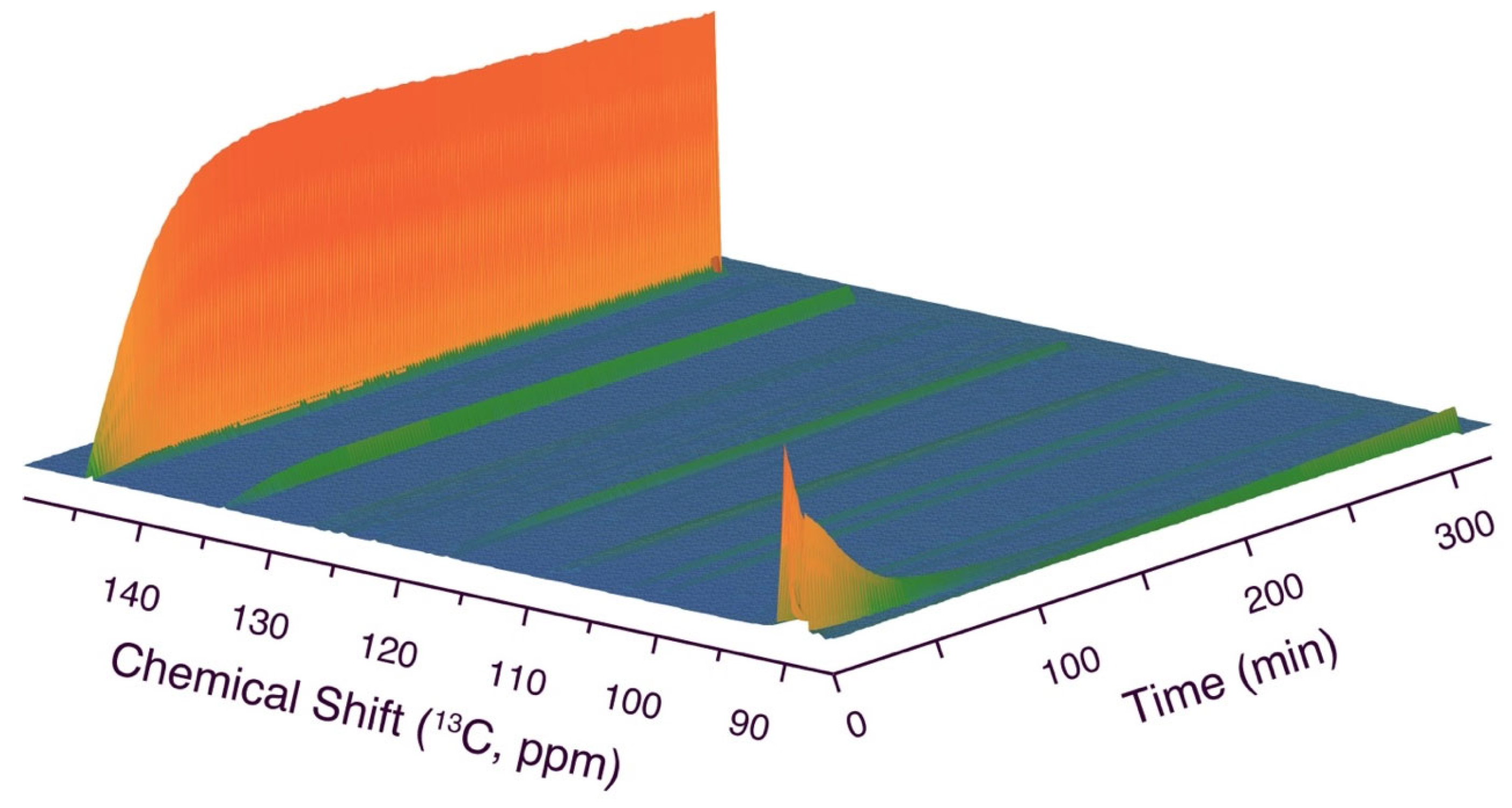
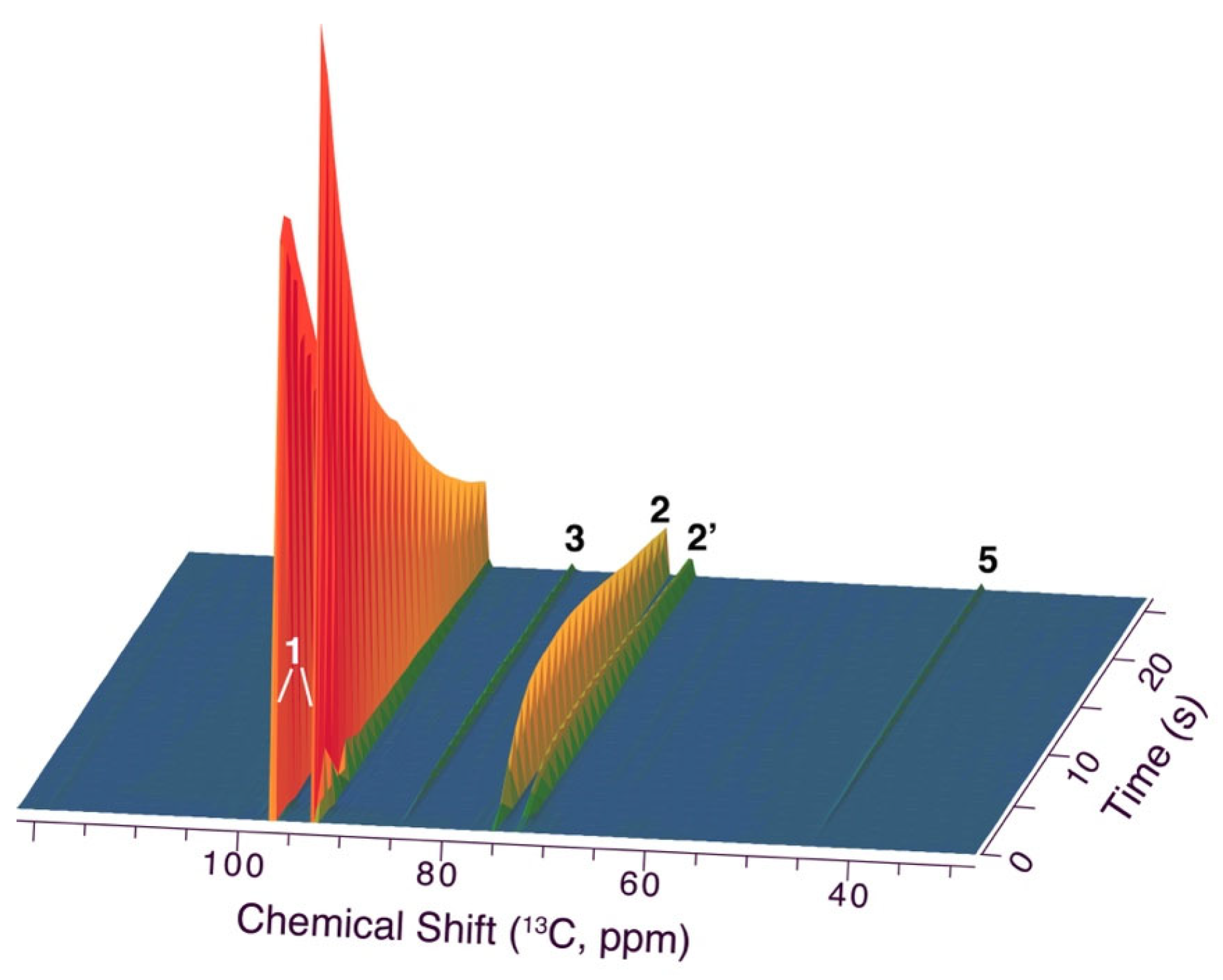
2.4. DNP NMR Investigates Rapid Steps and Shows the Transient Nature of Acyclic Intermediates
2.5. Role of Aldose Ring Opening and Correlation of Reactivity with Open Populations
2.6. Plausible Mechanism and Kinetic Analysis Are Mutually Consistent
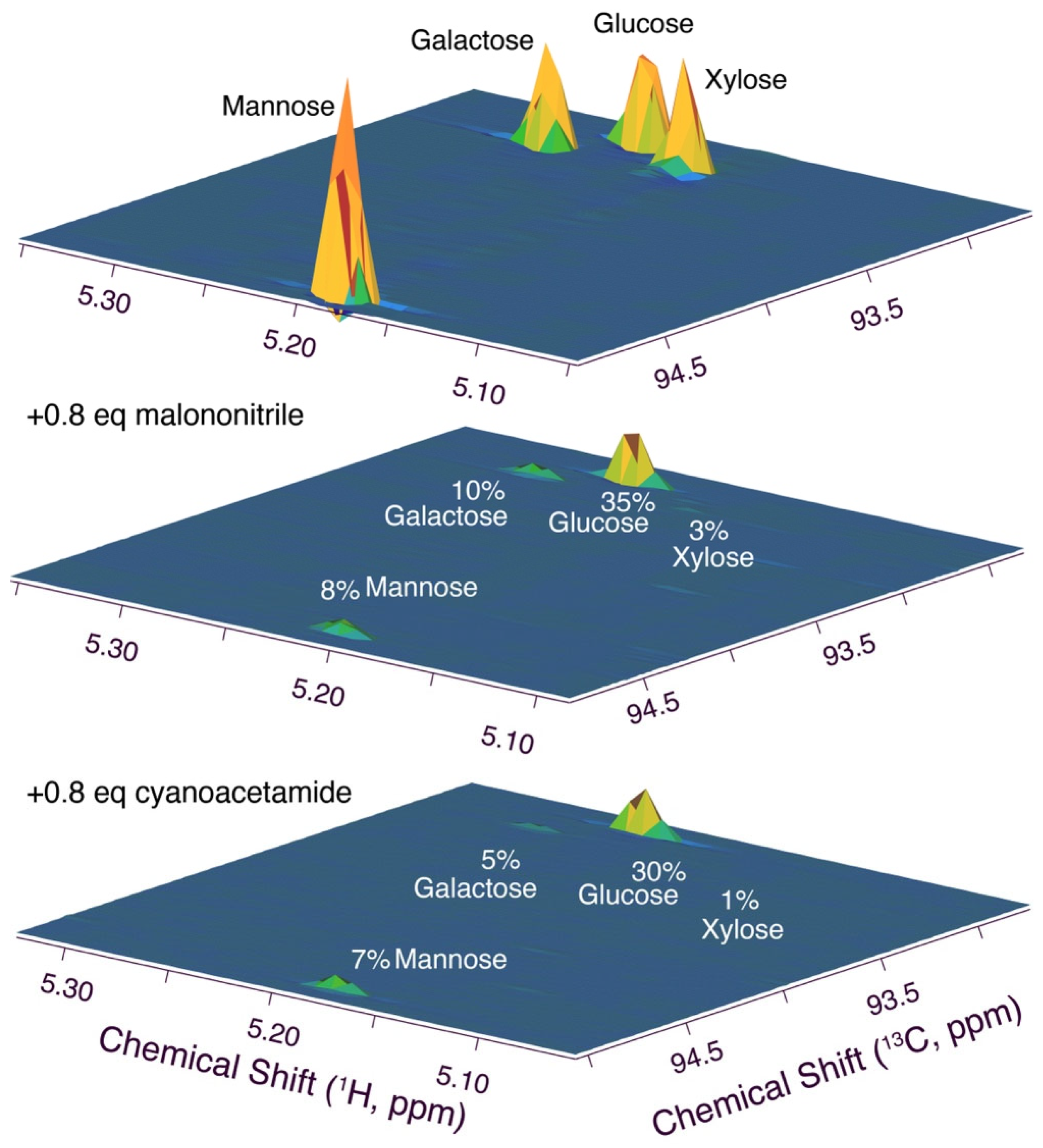
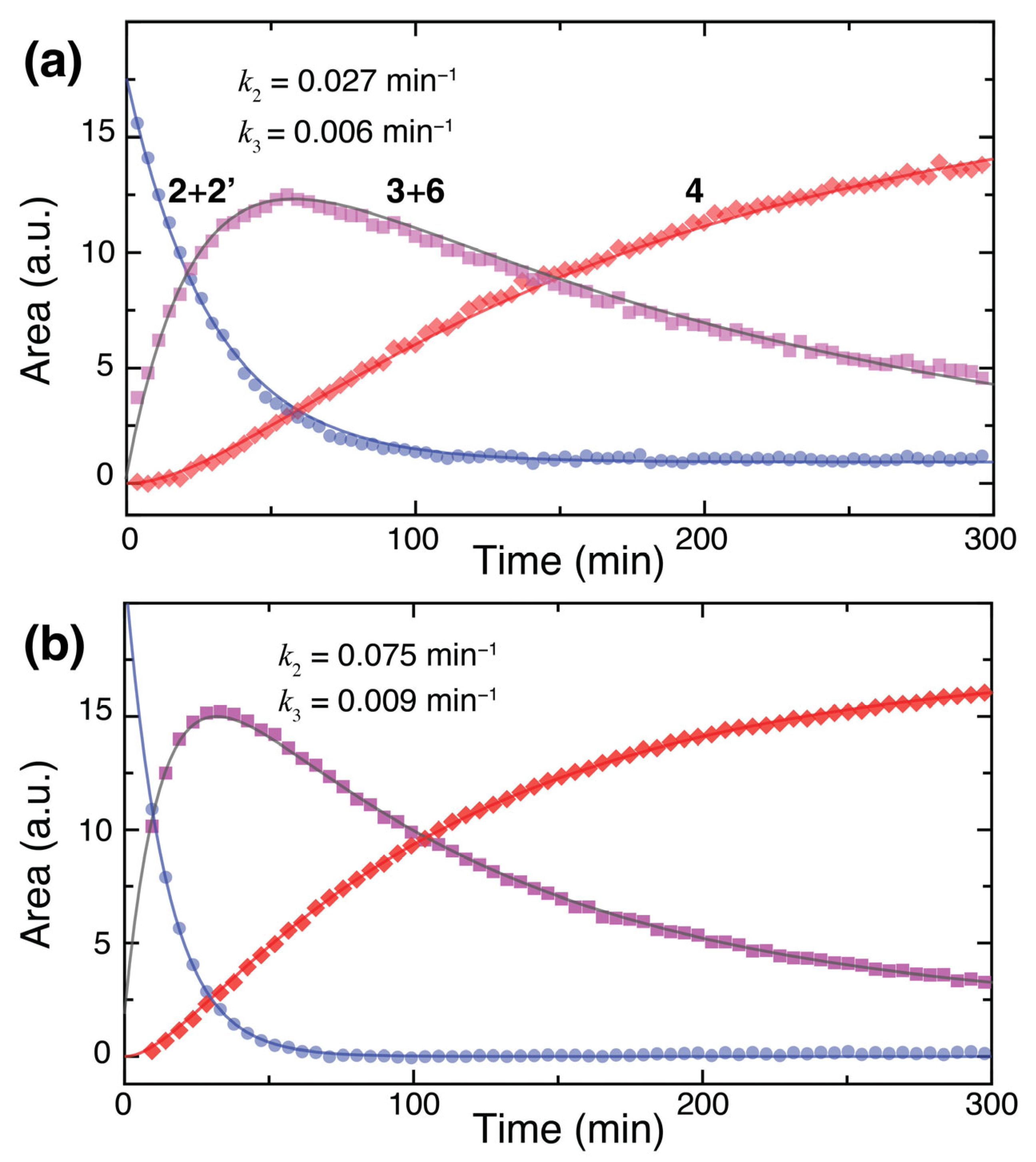
2.7. Kinetics of Desired Product Formation and of Some Competing Reactions
3. Materials and Methods
3.1. Chemicals
3.2. Reaction Procedure
3.3. NMR Spectroscopy
3.4. dDNP-NMR Experiments
3.5. Fitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christensen, C.H.; Rass-Hansen, J.; Marsden, C.C.; Taarning, E.; Egeblad, K. The Renewable Chemicals Industry. ChemSusChem 2008, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tolborg, S.; Meier, S.; Sádaba, I.; Elliot, S.G.; Kristensen, S.K.; Saravanamurugan, S.; Riisager, A.; Fristrup, P.; Skrydstrup, T.; Taarning, E. Tin-Containing Silicates: Identification of a Glycolytic Pathway via 3-Deoxyglucosone. Green Chem. 2016, 18, 3360–3369. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Taarning, E.; Sádaba, I.; Jensen, P.R.; Meier, S. Discovery and Exploration of the Efficient Acyclic Dehydration of Hexoses in Dimethyl Sulfoxide/Water. ChemSusChem 2019, 12, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.G.; Tolborg, S.; Sádaba, I.; Taarning, E.; Meier, S. Quantitative NMR Approach to Optimize the Formation of Chemical Building Blocks from Abundant Carbohydrates. ChemSusChem 2017, 10, 2990–2996. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic Oxidation of Carbohydrates into Organic Acids and Furan Chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, S.; Fesseler, J.; Gorte, O.; Hahn, T.; Zibek, S.; Syldatk, C.; Ochsenreither, K. Sustainable Carbon Sources for Microbial Organic Acid Production with Filamentous Fungi. Biotechnol. Biofuels 2017, 10, 242. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Liu, H.; Luo, N.; Zhao, Z.; Wang, F. Sustainable Productions of Organic Acids and Their Derivatives from Biomass via Selective Oxidative Cleavage of C–C Bond. ACS Catal. 2018, 8, 2129–2165. [Google Scholar] [CrossRef]
- De Clippel, F.; Dusselier, M.; Van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and Selective Sugar Conversion to Alkyl Lactate and Lactic Acid with Bifunctional Carbon–Silica Catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable Bio-Ethanol Production from Agro-Residues: A Review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Ghosh, D.; Dasgupta, D.; Agrawal, D.; Kaul, S.; Adhikari, D.K.; Kurmi, A.K.; Arya, P.K.; Bangwal, D.; Negi, M.S. Fuels and Chemicals from Lignocellulosic Biomass: An Integrated Biorefinery Approach. Energy Fuels 2015, 29, 3149–3157. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Lebarbier Dagle, V.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- Iroegbu, A.O.; Hlangothi, S.P. Furfuryl Alcohol a Versatile, Eco-Sustainable Compound in Perspective. Chem. Afr. 2019, 2, 223–239. [Google Scholar] [CrossRef]
- Sannelli, F.; Jensen, P.R.; Meier, S. In-Cell NMR Approach for Real-Time Exploration of Pathway Versatility: Substrate Mixtures in Nonengineered Yeast. Anal. Chem. 2023, 95, 7262–7270. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Han, J.; Lee, J. Renewable Butanol Production via Catalytic Routes. Int. J. Environ. Res. Public Health 2021, 18, 11749. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Meylemans, H.A. The Role of Butanol in the Development of Sustainable Fuel Technologies. J. Chem. Technol. Biotechnol. 2011, 86, 2–9. [Google Scholar] [CrossRef]
- Park, S.-H.; Hahn, J.-S. Development of an Efficient Cytosolic Isobutanol Production Pathway in Saccharomyces Cerevisiae by Optimizing Copy Numbers and Expression of the Pathway Genes Based on the Toxic Effect of α-Acetolactate. Sci. Rep. 2019, 9, 3996. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical Review on the Current Scenario and Significance of Crude Glycerol Resulting from Biodiesel Industry towards More Sustainable Renewable Energy Industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; Da Silva César, A. Glycerol from Biodiesel Production: Technological Paths for Sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Binder, J.B.; Blank, J.J.; Cefali, A.V.; Raines, R.T. Synthesis of Furfural from Xylose and Xylan. ChemSusChem 2010, 3, 1268–1272. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Meier, S.; Hansen, A.R.; Jensen, P.R. Probing the Role of Individual OH Sites in Carbohydrate Conversion Suggests Strategies for Increasing Product Selectivity and Avoiding Humins. ACS Sustain. Chem. Eng. 2023, 11, 1027–1036. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A Versatile, Biomass-Derived Platform Chemical for the Production of Renewable Chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical Conversion Pathways for Carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Abou-Yousef, H.; Hassan, E.B. Efficient Utilization of Aqueous Phase Bio-Oil to Furan Derivatives through Extraction and Sugars Conversion in Acid-Catalyzed Biphasic System. Fuel 2014, 137, 115–121. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A Critical Review of Recent Advances in the Production of Furfural and 5-Hydroxymethylfurfural from Lignocellulosic Biomass through Homogeneous Catalytic Hydrothermal Conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Brownlee, H.J.; Miner, C.S. Industrial Development of Furfural. Ind. Eng. Chem. 1948, 40, 201–204. [Google Scholar] [CrossRef]
- Garrett, E.R.; Dvorchik, B.H. Kinetics and Mechanisms of the Acid Degradation of the Aldopentoses to Furfural. J. Pharm. Sci. 1969, 58, 813–820. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Zhu, B.; Zhou, Z.; El-Hout, S.I.; Yang, J.; Zhang, J. 2,5-Furandicarboxylic Acid Production via Catalytic Oxidation of 5-Hydroxymethylfurfural: Catalysts, Processes and Reaction Mechanism. J. Energy Chem. 2021, 54, 528–554. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Monrad, R.N.; Madsen, R. Modern Methods for Shortening and Extending the Carbon Chain in Carbohydrates at the Anomeric Center. Tetrahedron 2011, 67, 8825–8850. [Google Scholar] [CrossRef]
- Rodrigues, F.; Canac, Y.; Lubineau, A. A Convenient, One-Step, Synthesis of β-C-Glycosidic Ketones in Aqueous Media. Chem. Commun. 2000, 20, 2049–2050. [Google Scholar] [CrossRef]
- Sato, S.; Naito, Y.; Aoki, K. Scandium Cation-Exchanged Montmorillonite Catalyzed Direct C-Glycosylation of a 1,3-Diketone, Dimedone, with Unprotected Sugars in Aqueous Solution. Carbohydr. Res. 2007, 342, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Ronaghi, N.; Jones, E.V.; Moon, H.J.; Park, S.J.; Jones, C.W.; France, S. Selective Conversion of Malononitrile and Unprotected Carbohydrates to Bicyclic Polyhydroxyalkyl Dihydrofurans Using Magnesium Oxide as a Recyclable Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 5966–5975. [Google Scholar] [CrossRef]
- Zhang, R.; Eronen, A.; Du, X.; Ma, E.; Guo, M.; Moslova, K.; Repo, T. A Catalytic Approach via Retro-Aldol Condensation of Glucose to Furanic Compounds. Green Chem. 2021, 23, 5481–5486. [Google Scholar] [CrossRef]
- Lambu, M.R.; Judeh, Z.M.A. Efficient, One-Step, Cascade Synthesis of Densely Functionalized Furans from Unprotected Carbohydrates in Basic Aqueous Media. Green Chem. 2019, 21, 821–829. [Google Scholar] [CrossRef]
- Ronaghi, N.; Fialho, D.M.; Jones, C.W.; France, S. Conversion of Unprotected Aldose Sugars to Polyhydroxyalkyl and C -Glycosyl Furans via Zirconium Catalysis. J. Org. Chem. 2020, 85, 15337–15346. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, B. Recent Advances in the Chemical Synthesis of C -Glycosides. Chem. Rev. 2017, 117, 12281–12356. [Google Scholar] [CrossRef]
- Scherrmann, M.-C. Knoevenagel Reaction of Unprotected Sugars. In Carbohydrates in Sustainable Development II; Rauter, A.P., Vogel, P., Queneau, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–18. ISBN 978-3-64215-161-3. [Google Scholar]
- Gröger, H.; Gallou, F.; Lipshutz, B.H. Where Chemocatalysis Meets Biocatalysis: In Water. Chem. Rev. 2022, 123, 5262–5296. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef]
- Simon, M.-O.; Li, C.-J. Green Chemistry Oriented Organic Synthesis in Water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.-P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Brønsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef]
- Meier, S.; Karlsson, M.; Jensen, P.R. Detecting Elusive Intermediates in Carbohydrate Conversion: A Dynamic Ensemble of Acyclic Glucose–Catalyst Complexes. ACS Sustain. Chem. Eng. 2017, 5, 5571–5577. [Google Scholar] [CrossRef]
- Meier, S. Mechanism and Malleability of Glucose Dehydration to HMF: Entry Points and Water-Induced Diversions. Catal. Sci. Technol. 2020, 10, 1724–1730. [Google Scholar] [CrossRef]
- Zhao, E.W.; Liu, T.; Jónsson, E.; Lee, J.; Temprano, I.; Jethwa, R.B.; Wang, A.; Smith, H.; Carretero-González, J.; Song, Q.; et al. In Situ NMR Metrology Reveals Reaction Mechanisms in Redox Flow Batteries. Nature 2020, 579, 224–228. [Google Scholar] [CrossRef]
- Blasco, T. Insights into Reaction Mechanisms in Heterogeneous Catalysis Revealed by in Situ NMR Spectroscopy. Chem. Soc. Rev. 2010, 39, 4685. [Google Scholar] [CrossRef]
- Bertz, S.H.; Cope, S.; Murphy, M.; Ogle, C.A.; Taylor, B.J. Rapid Injection NMR in Mechanistic Organocopper Chemistry. Preparation of the Elusive Copper(III) Intermediate. J. Am. Chem. Soc. 2007, 129, 7208–7209. [Google Scholar] [CrossRef]
- Voigt, B.; Matviitsuk, A.; Mahrwald, R. Organocatalyzed Knoevenagel-Addition—Simple Access to Carbon Chain-Elongated Branched Carbohydrates. Tetrahedron 2013, 69, 4302–4310. [Google Scholar] [CrossRef]
- Eger, K.; Storz, T.; Spätling, S. The First Condensation Product of Malononitrile with Ribose. Liebigs Ann. Chem. 1989, 1989, 1049. [Google Scholar] [CrossRef]
- Van Beurden, K.; De Koning, S.; Molendijk, D.; Van Schijndel, J. The Knoevenagel Reaction: A Review of the Unfinished Treasure Map to Forming Carbon–Carbon Bonds. Green Chem. Lett. Rev. 2020, 13, 349–364. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Karlsson, M.; Jensen, P.R.; Duus, J.Ø.; Meier, S.; Lerche, M.H. Development of Dissolution DNP-MR Substrates for Metabolic Research. Appl. Magn. Reson. 2012, 43, 223–236. [Google Scholar] [CrossRef]
- Drew, K.N.; Zajicek, J.; Bondo, G.; Bose, B.; Serianni, A.S. 13C-Labeled Aldopentoses: Detection and Quantitation of Cyclic and Acyclic Forms by Heteronuclear 1D and 2D NMR Spectroscopy. Carbohydr. Res. 1998, 307, 199–209. [Google Scholar] [CrossRef]
- Zhu, Y.; Zajicek, J.; Serianni, A.S. Acyclic Forms of [1-13C]Aldohexoses in Aqueous Solution: Quantitation by 13C NMR and Deuterium Isotope Effects on Tautomeric Equilibria. J. Org. Chem. 2001, 66, 6244–6251. [Google Scholar] [CrossRef] [PubMed]
- Isbell, H.S.; Pigman, W. Mutarotation of Sugars in Solution: Part II. In Advances in Carbohydrate Chemistry and Biochemistry; Wolfrom, M.L., Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 24, pp. 13–65. ISBN 0065-2318. [Google Scholar]
- Petersen, B.O.; Hindsgaul, O.; Meier, S. Profiling of Carbohydrate Mixtures at Unprecedented Resolution Using High-Precision 1H-13C Chemical Shift Measurements and a Reference Library. Analyst 2013, 139, 401–406. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warthegau, S.S.; Karlsson, M.; Madsen, R.; Jensen, P.R.; Meier, S. Kinetic and Mechanistic Study of Aldose Conversion to Functionalized Furans in Aqueous Solutions. Catalysts 2024, 14, 199. https://doi.org/10.3390/catal14030199
Warthegau SS, Karlsson M, Madsen R, Jensen PR, Meier S. Kinetic and Mechanistic Study of Aldose Conversion to Functionalized Furans in Aqueous Solutions. Catalysts. 2024; 14(3):199. https://doi.org/10.3390/catal14030199
Chicago/Turabian StyleWarthegau, Stefan S., Magnus Karlsson, Robert Madsen, Pernille Rose Jensen, and Sebastian Meier. 2024. "Kinetic and Mechanistic Study of Aldose Conversion to Functionalized Furans in Aqueous Solutions" Catalysts 14, no. 3: 199. https://doi.org/10.3390/catal14030199