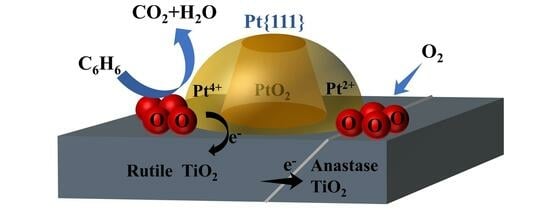
Effect of Interaction between Pt and Different Crystalline Phases of TiO2 on Benzene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
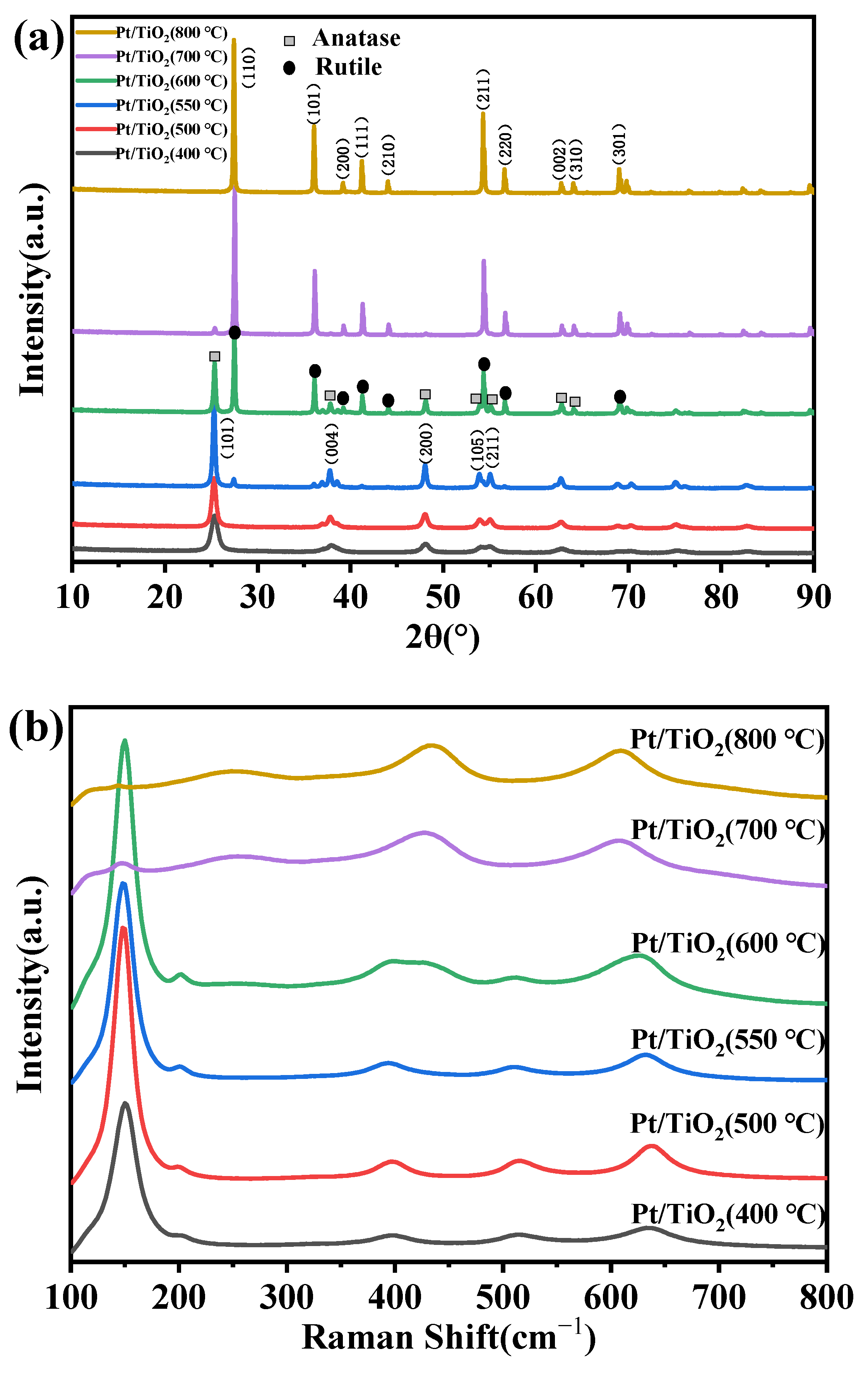
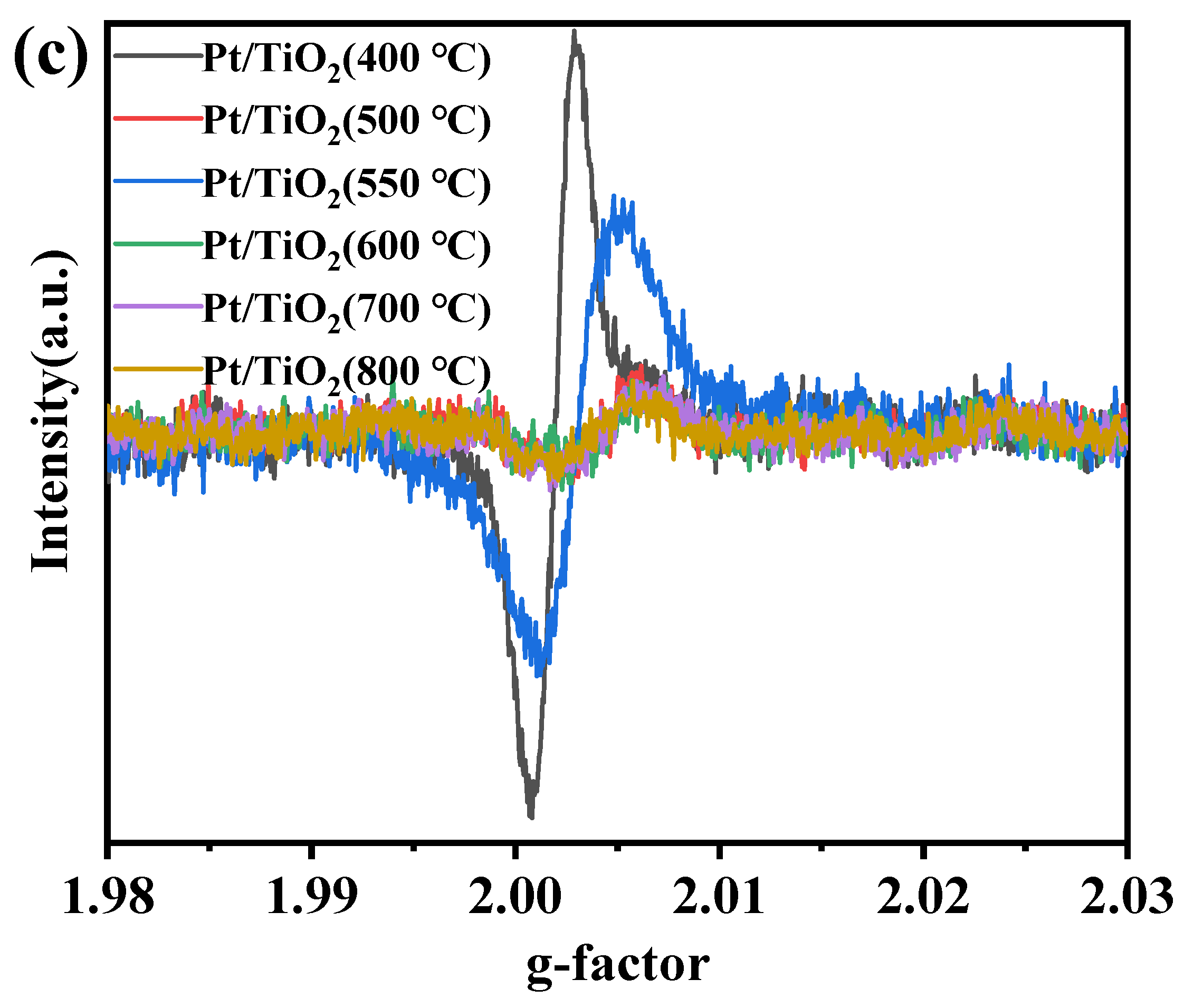
2.1. Structural Properties of the Catalysts
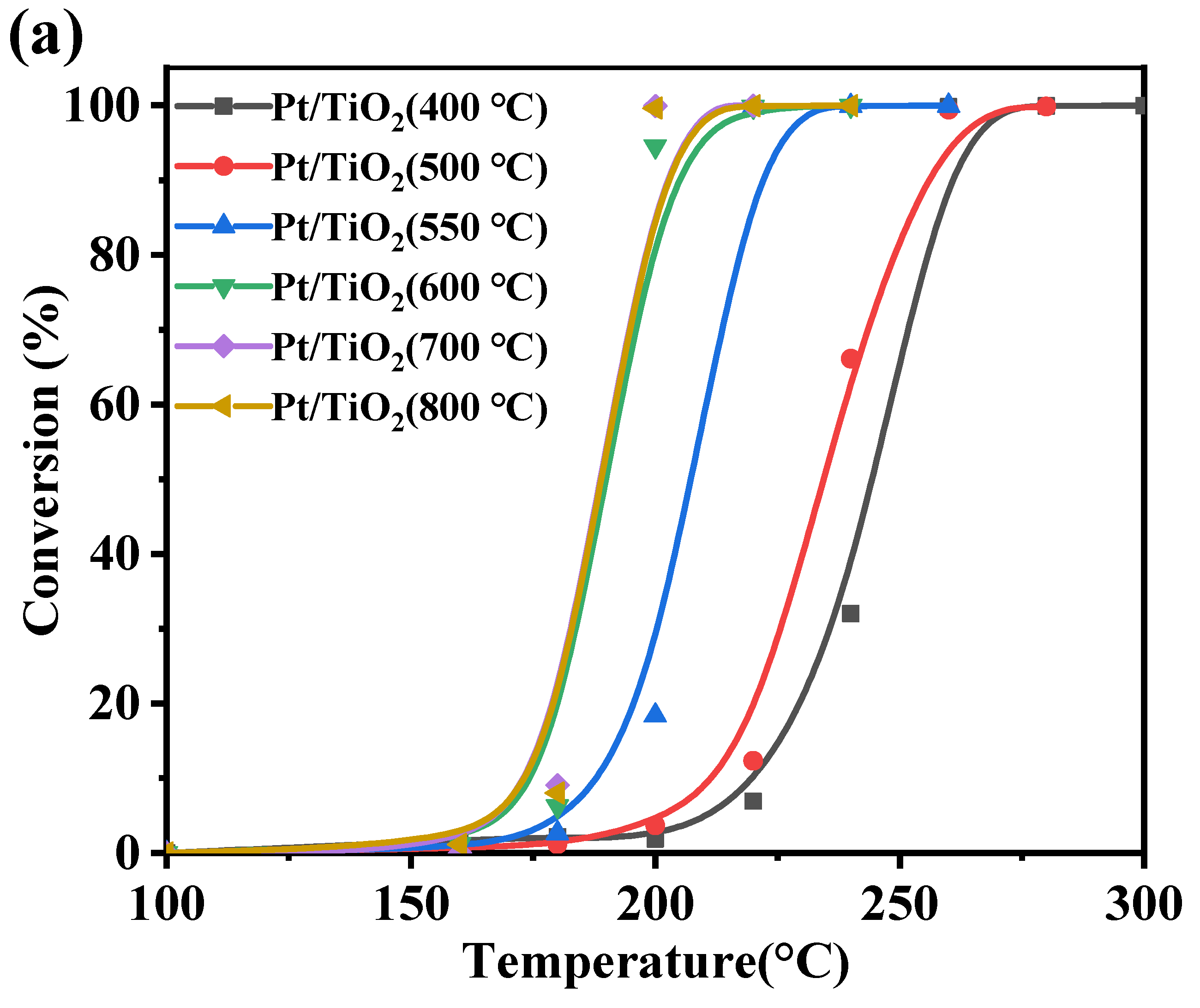
2.2. Benzene Catalytic Performance
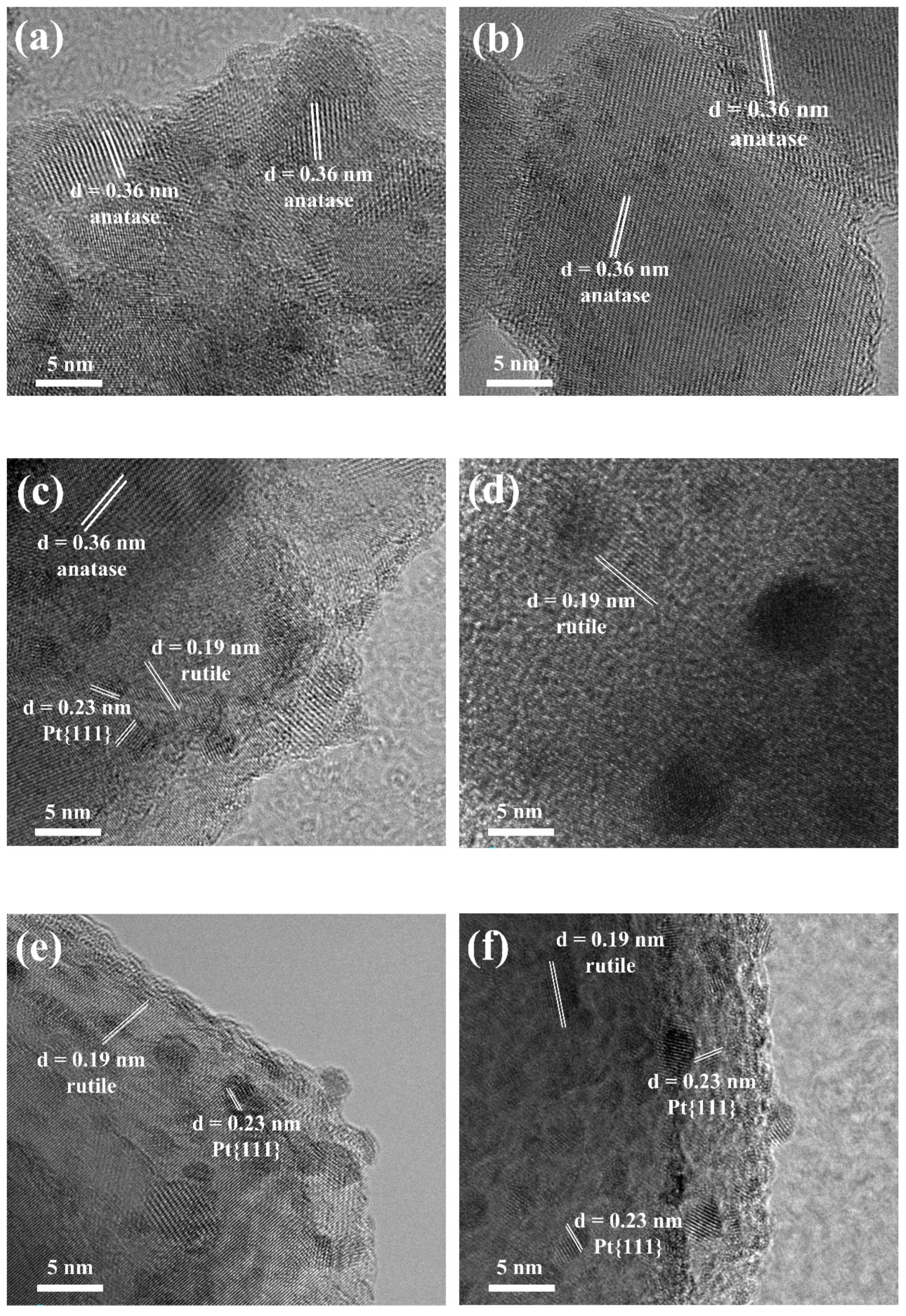
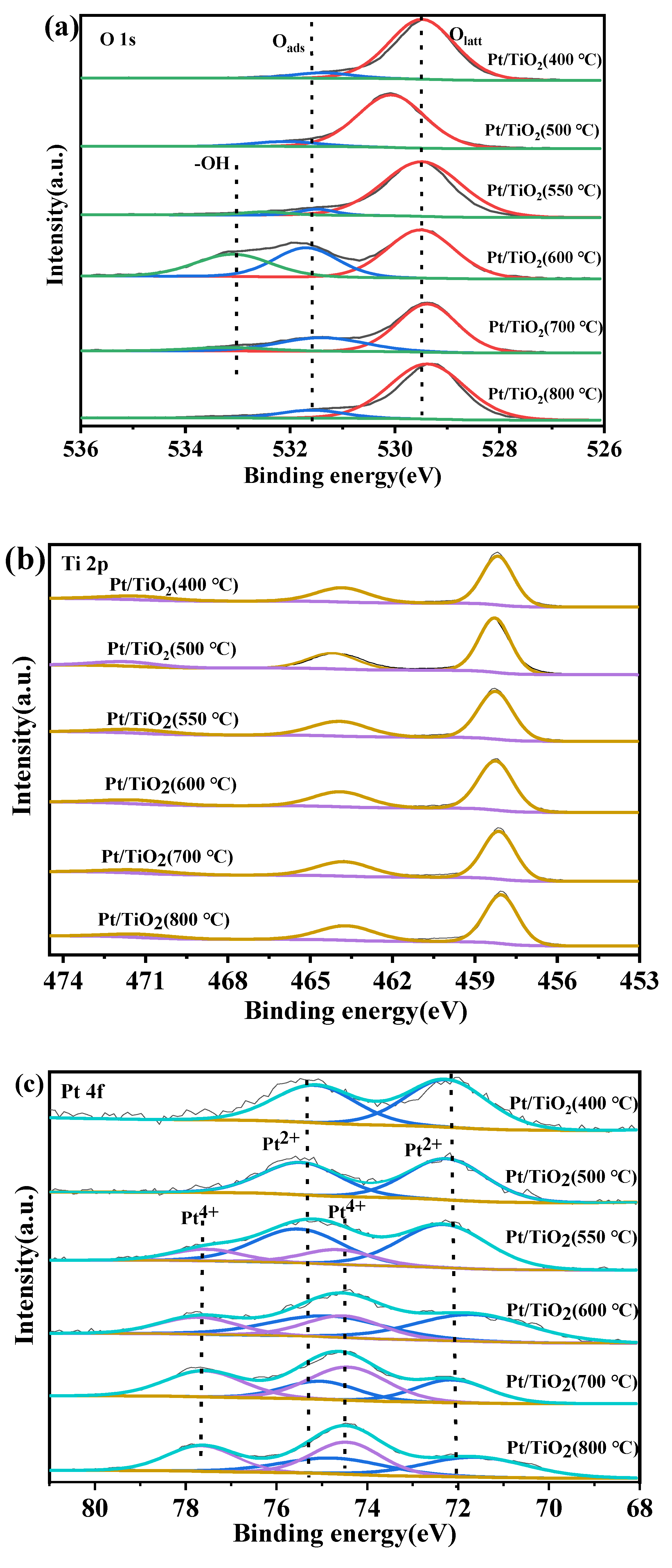
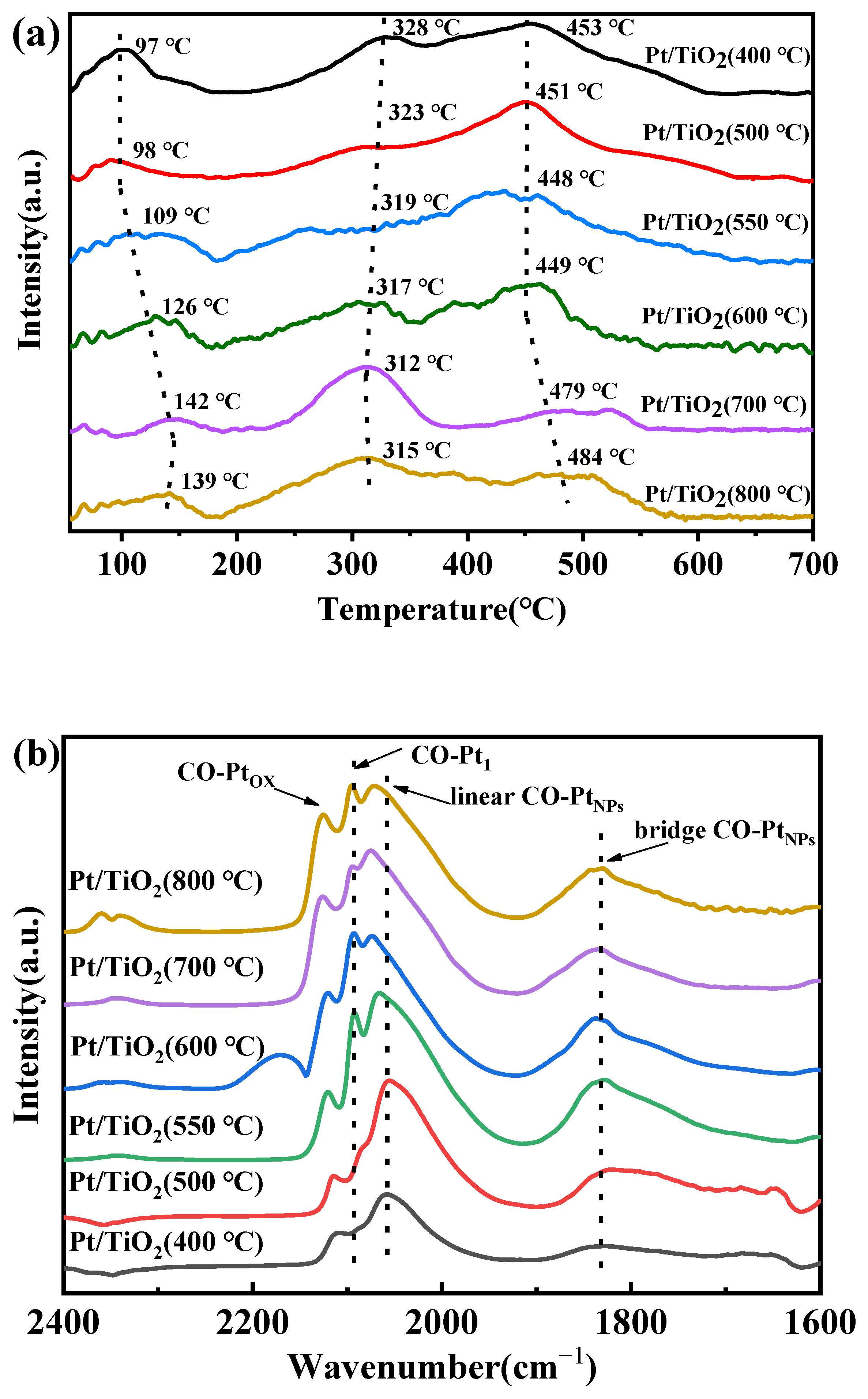
2.3. Analysis of Catalyst Morphology and Interaction Mechanisms
3. Experimental Section
3.1. Materials
3.2. Preparation of Catalysts
3.2.1. Preparation of TiO2
3.2.2. Preparation of Pt/TiO2
3.3. Catalyst Characterization
3.4. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)-A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Dumanoglu, Y.; Kara, M.; Altiok, H.; Odabasi, M.; Elbir, T.; Bayram, A. Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmos. Environ. 2014, 98, 168–178. [Google Scholar] [CrossRef]
- Liang, X.; Chen, X.; Zhang, J.; Shi, T.; Sun, X.; Fan, L.; Wang, L.; Ye, D. Reactivity-based industrial volatile organic compounds emission inventory and its implications for ozone control strategies in China. Atmos. Environ. 2017, 162, 115–126. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, X.; Li, H.; Chen, B.; Ye, S.; Zhang, N.; Yu, Z.; Zheng, J.; Chen, B. Enhancing electronic metal support interaction (EMSI) over Pt/TiO2 for efficient catalytic wet air oxidation of phenol in wastewater. J. Hazard. Mater. 2022, 426, 128088. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Vikrant, K.; Kim, T.; Kim, K.-H.; Dong, F. Thermocatalytic oxidation of gaseous benzene by a titanium dioxide supported platinum catalyst. Chem. Eng. J. 2022, 428, 131090. [Google Scholar] [CrossRef]
- Hao, H.; Jin, B.F.; Liu, W.; Wu, X.; Yin, F.; Liu, S. Robust Pt@TiOx/TiO2 Catalysts for Hydrocarbon Combustion: Effects of Pt-TiOx Interaction and Sulfates. ACS Catal. 2020, 10, 13543–13548. [Google Scholar] [CrossRef]
- Liu, H. Catalyst design of Pt/TiO2 microsphere for benzene oxidation under microwave irradiation. Catal. Today 2021, 376, 285–291. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt Particle Size and Valence State on the Performance of Pt/TiO2 Catalysts for CO Oxidation at Room Temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
- Rui, Z.; Wu, S.; Peng, C.; Ji, H. Comparison of TiO2 Degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem. Eng. J. 2014, 243, 254–264. [Google Scholar] [CrossRef]
- Serna, P.; Rodriguez-Fernandez, A.; Yacob, S.; Kliewer, C.; Moliner, M.; Corma, A. Single-Site vs. Cluster Catalysis in High Temperature Oxidations. Angew. Chem.-Int. Ed. 2021, 60, 15954–15962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.P.; Mao, S.J.; Lu, B.; Chen, Y.Z.; Zhang, X.; Chen, Z.R.; Wang, Y. Decoupling the electronic and geometric effects of Pt catalysts in selective hydrogenation reaction. Nat. Commun. 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Pei, W.; Li, G.; Liu, W.; Du, P.; Wang, Z.; Qin, Z.; Qi, H.; Liu, X.; et al. Crystal-Phase-Mediated Restructuring of Pt on TiO2 with Tunable Reactivity: Redispersion versus Reshaping. ACS Catal. 2022, 12, 3634–3643. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Y.; Shangguan, W.F.; Jiang, Z. Influence of Support and Metal Precursor on the State and CO Catalytic Oxidation Activity of Platinum Supported on TiO2. J. Phys. Chem. C 2012, 116, 19396–19404. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Yang, H.; Xu, B.; Feng, L.; Yang, M.; Chen, Y. Strong metal-support interaction and catalytic properties of anatase and rutile supported palladium catalyst Pd/TiO2. Chem. Phys. Lett. 2003, 372, 160–165. [Google Scholar] [CrossRef]
- Jung, K.Y.; Park, S.B.; Anpo, M. Photoluminescence and photoactivity of titania particles prepared by the sol–gel technique: Effect of roasting temperature. J. Photochem. Photobiol. A Chem. 2005, 170, 247–252. [Google Scholar] [CrossRef]
- Dai, J.; Yang, J.; Wang, X.; Zhang, L.; Li, Y. Enhanced visible-light photocatalytic activity for selective oxidation of amines into imines over TiO2(B)/anatase mixed-phase nanowires. Appl. Surf. Sci. 2015, 349, 343–352. [Google Scholar] [CrossRef]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2(B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatments. J. Solid State Chem. 2005, 178, 2179–2185. [Google Scholar] [CrossRef]
- DeRita, L.; Dai, S.; Lopez-Zepeda, K.; Pham, N.; Graham, G.W.; Pan, X.; Christopher, P. Catalyst Architecture for Stable Single Atom Dispersion Enables Site-Specific Spectroscopic and Reactivity Measurements of CO Adsorbed to Pt Atoms, Oxidized Pt Clusters, and Metallic Pt Clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, Y.; Li, X.; Wu, X.; Li, Z. The double peaks and symmetric path phenomena in the catalytic activity of Pd/Al2O3-TiO2 catalysts with different TiO2 contents. J. Solid State Chem. 2018, 262, 335–342. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Guo, Y.; Wang, Y.; Guo, Y. Effect of TiO2 crystal structure on the catalytic performance of Co3O4/TiO2 catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 1268–1275. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zeng, J.; Zhu, T. Catalytic oxidation of trichloroethylene over TiO2 supported ruthenium catalysts. Catal. Commun. 2016, 76, 13–18. [Google Scholar] [CrossRef]
- Li, Z.; Yang, K.; Liu, G.; Deng, G.; Li, J.; Li, G.; Yue, R.; Yang, J.; Chen, Y. Effect of Reduction Treatment on Structural Properties of TiO2 Supported Pt Nanoparticles and Their Catalytic Activity for Benzene Oxidation. Catal. Lett. 2014, 144, 1080–1087. [Google Scholar] [CrossRef]
- Ertl, G. Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Padayachee, D.; Mahomed, A.S.; Singh, S.; Friedrich, H.B. Effect of the TiO2 Anatase/Rutile Ratio and Interface for the Oxidative Activation of n-Octane. ACS Catal. 2020, 10, 2211–2220. [Google Scholar] [CrossRef]
- Garetto, T.F.; Apesteguía, C.R. Structure sensitivity and in situ activation of benzene combustion on Pt/Al2O3 catalysts. Appl. Catal. B Environ. 2001, 32, 83–94. [Google Scholar] [CrossRef]
- McCabe, R. The Passivating Oxidation of Platinum. J. Catal. 1988, 114, 354–367. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, S.; Feng, R.; Ma, Z.; Gao, Y.; Xing, S. Comparative study for low-temperature catalytic oxidation of o-xylene over doped OMS-2 catalysts: Role of Ag and Cu. Mol. Catal. 2017, 442, 164–172. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wu, X.; Ye, Q.; Xu, X.; Li, B.; Wang, F. Novel synthesis and shape-dependent catalytic performance of Cu-Mn oxides for CO oxidation. Appl. Surf. Sci. 2017, 403, 335–341. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Schweitzer, N.M.; Schaidle, J.A.; Ezekoye, O.K.; Pan, X.; Linic, S.; Thompson, L.T. High activity carbide supported catalysts for water gas shift. J. Am. Chem. Soc. 2011, 133, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Fu, Q.; Jiang, Z.; Mei, B.; Bao, X. Carbide-Supported Au Catalysts for Water-Gas Shift Reactions: A New Territory for the Strong Metal-Support Interaction Effect. J. Am. Chem. Soc. 2018, 140, 13808–13816. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Miao, S.; Li, Y.; Kuld, S.; Sehested, J.; Liu, J.; Aoki, T.; Hong, S.; et al. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Uner, D.; Tapan, N.A.; Özen, İ.; Üner, M. Oxygen adsorption on Pt/TiO2 catalysts. Appl. Catal. A Gen. 2003, 251, 225–234. [Google Scholar] [CrossRef]
- Xue, T.; Wu, C.; Ding, X.; Sun, J. Dissociative adsorption of O2 on strained Pt(111). Phys. Chem. Chem. Phys. 2018, 20, 17927–17933. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Li, X.; Kusakabe, K.; Abudula, A. Embedded structure catalyst: A new perspective from noble metal supported on molybdenum carbide. RSC Adv. 2015, 5, 15002–15005. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at surfaces: From atoms to complexity (Nobel Lecture). Angew. Chem. Int. Ed. 2008, 47, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. A Structural Investigation of Titanium Dioxide Photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Yan, M.; Chen, F.; Zhang, J.; Anpo, M. Preparation of Controllable Crystalline Titania and Study on the Photocatalytic Properties. J. Phys. Chem. B 2005, 109, 8673–8678. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, W.; Ma, Y.; Ye, X.; Yao, J. Photocatalytic activity of pure rutile particles derived from a photo-assisted sol-gel method. New J. Chem. 2003, 27, 529–532. [Google Scholar] [CrossRef]
- Weaver, J.F. Surface chemistry of late transition metal oxides. Chem. Rev. 2013, 113, 4164–4215. [Google Scholar] [CrossRef] [PubMed]
- Venezia, A.M.; Di Carlo, G.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B Environ. 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Zepeda, T.A. Comparison and performance of different sulphided Ti-loaded mesostructured silica-supported CoMo catalysts in deep HDS. Appl. Catal. A Gen. 2008, 347, 148–161. [Google Scholar] [CrossRef]
- Santacesaria, E.; Cozzolino, M.; Di Serio, M.; Venezia, A.M.; Tesser, R. Vanadium based catalysts prepared by grafting: Preparation, properties and performances in the ODH of butane. Appl. Catal. A Gen. 2004, 270, 177–192. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Mejía, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Zhang, S.; Plessow, P.N.; Willis, J.J.; Dai, S.; Xu, M.; Graham, G.W.; Cargnello, M.; Abild-Pedersen, F.; Pan, X. Dynamical Observation and Detailed Description of Catalysts under Strong Metal-Support Interaction. Nano Lett. 2016, 16, 4528–4534. [Google Scholar] [CrossRef] [PubMed]
- Thang, H.V.; Pacchioni, G.; DeRita, L.; Christopher, P. Nature of stable single atom Pt catalysts dispersed on anatase TiO2. J. Catal. 2018, 367, 104–114. [Google Scholar] [CrossRef]
- Han, B.; Guo, Y.; Huang, Y.; Xi, W.; Xu, J.; Luo, J.; Qi, H.; Ren, Y.; Liu, X.; Qiao, B.; et al. Strong Metal-Support Interactions between Pt Single Atoms and TiO2. Angew. Chem. Int. Ed. 2020, 59, 11824–11829. [Google Scholar] [CrossRef] [PubMed]


| Catalysts | Specific Surface (m2g−1) | Catalytic Activity | H2-TPR | XPS a | Pt Particle Size | ||
|---|---|---|---|---|---|---|---|
| T90 (°C) | Initial Reduction Temperature (°C) | Pt4+/Pt2+ | O2−/O2− | Ti2p3/2 | nm | ||
| Pt/TiO2 (400 °C) | 82.7 | 261 | 97 | 0 | 12.9 | 458.26 | 1.29 |
| Pt/TiO2 (500 °C) | 20.3 | 255 | 98 | 0 | 18.9 | 458.26 | 1.36 |
| Pt/TiO2 (550 °C) | 24.6 | 222 | 109 | 0.35 | 20.4 | 458.25 | 2.01 |
| Pt/TiO2 (600 °C) | 10.5 | 204 | 126 | 0.54 | 1.75 | 458.24 | 5.91 |
| Pt/TiO2 (700 °C) | 8.3 | 201 | 142 | 1.53 | 2.46 | 458.12 | 2.33 |
| Pt/TiO2 (800 °C) | 8.1 | 202 | 139 | 1.05 | 8.14 | 458.11 | 1.72 |
| Catalyst | Catalyst Activation | Benzene Concentration (ppm) | Space Velocity | T90 (°C) | Reference |
|---|---|---|---|---|---|
| 1% Pt/TiO2 | H2 pretreatment | 100 | 8696 mLg−1h−1 | 167 | [6] |
| 1% Pt/TiO2 | Impregnation method | 1000 | 60,000 mLg−1h−1 | >225 | [23] |
| 1% Pt/TiO2 | H2 pretreatment and Impregnation method | 1000 | 60,000 mLg−1h−1 | 180 | [23] |
| 1% Pt/TMS | H2 pretreatment | 400 | 60,000 mLg−1h−1 | 178 | [8] |
| 1% Pt/TiO2 (700 °C) | Impregnation method | 1000 | 60,000 mLg−1h−1 | 201 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Hu, J.; Wu, D.; Bao, W.; Hou, C.; Lv, X.; Chang, L.; Wang, J. Effect of Interaction between Pt and Different Crystalline Phases of TiO2 on Benzene Oxidation. Catalysts 2024, 14, 234. https://doi.org/10.3390/catal14040234
Cheng H, Hu J, Wu D, Bao W, Hou C, Lv X, Chang L, Wang J. Effect of Interaction between Pt and Different Crystalline Phases of TiO2 on Benzene Oxidation. Catalysts. 2024; 14(4):234. https://doi.org/10.3390/catal14040234
Chicago/Turabian StyleCheng, Hang, Jiangliang Hu, Dongxia Wu, Weiren Bao, Changming Hou, Xianyan Lv, Liping Chang, and Jiancheng Wang. 2024. "Effect of Interaction between Pt and Different Crystalline Phases of TiO2 on Benzene Oxidation" Catalysts 14, no. 4: 234. https://doi.org/10.3390/catal14040234