Preparation of Poly(Butadiene–Styrene–Vinyl Pyridine)/Poly(Acrylonitrile–Butadiene) Core–Shell Nanoparticles by Intermittent Seeded Emulsion Polymerization and Their Catalytic Latex Hydrogenation
Abstract
:1. Introduction
2. Results
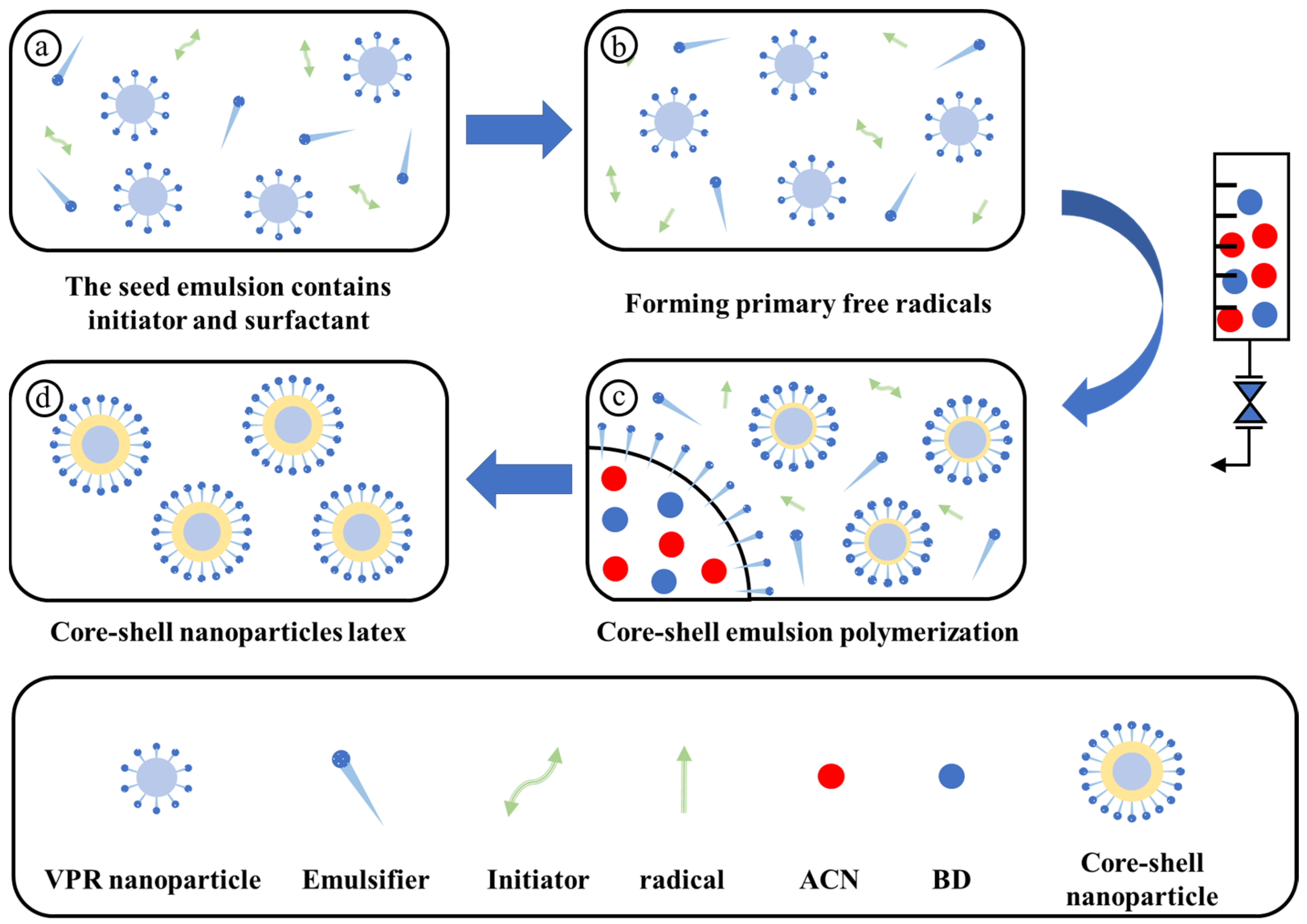
2.1. Particle Size of Core–Shell Polymer Latex Nanoparticles
2.2. Morphology of Core–Shell Latex Nanoparticles
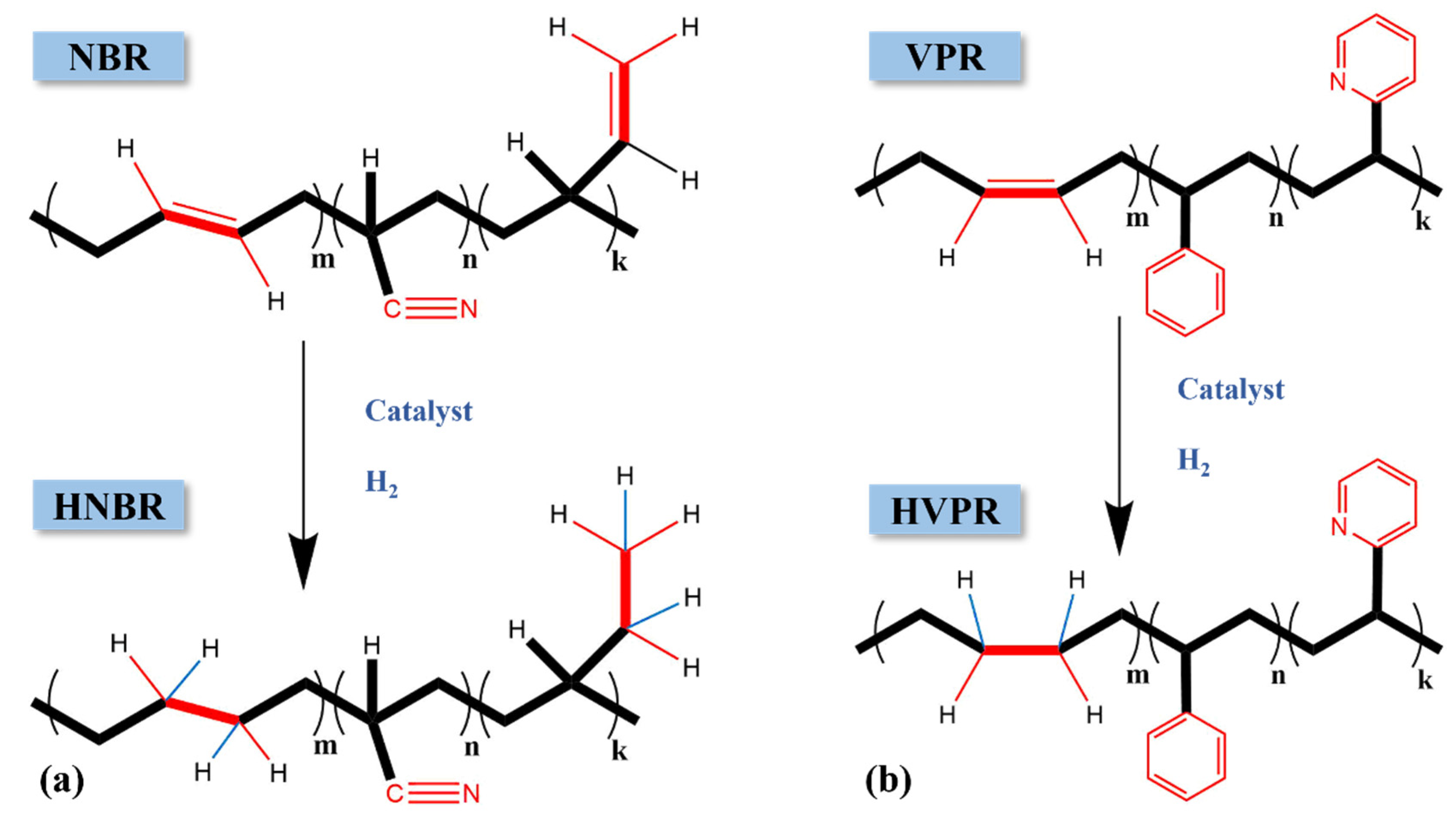
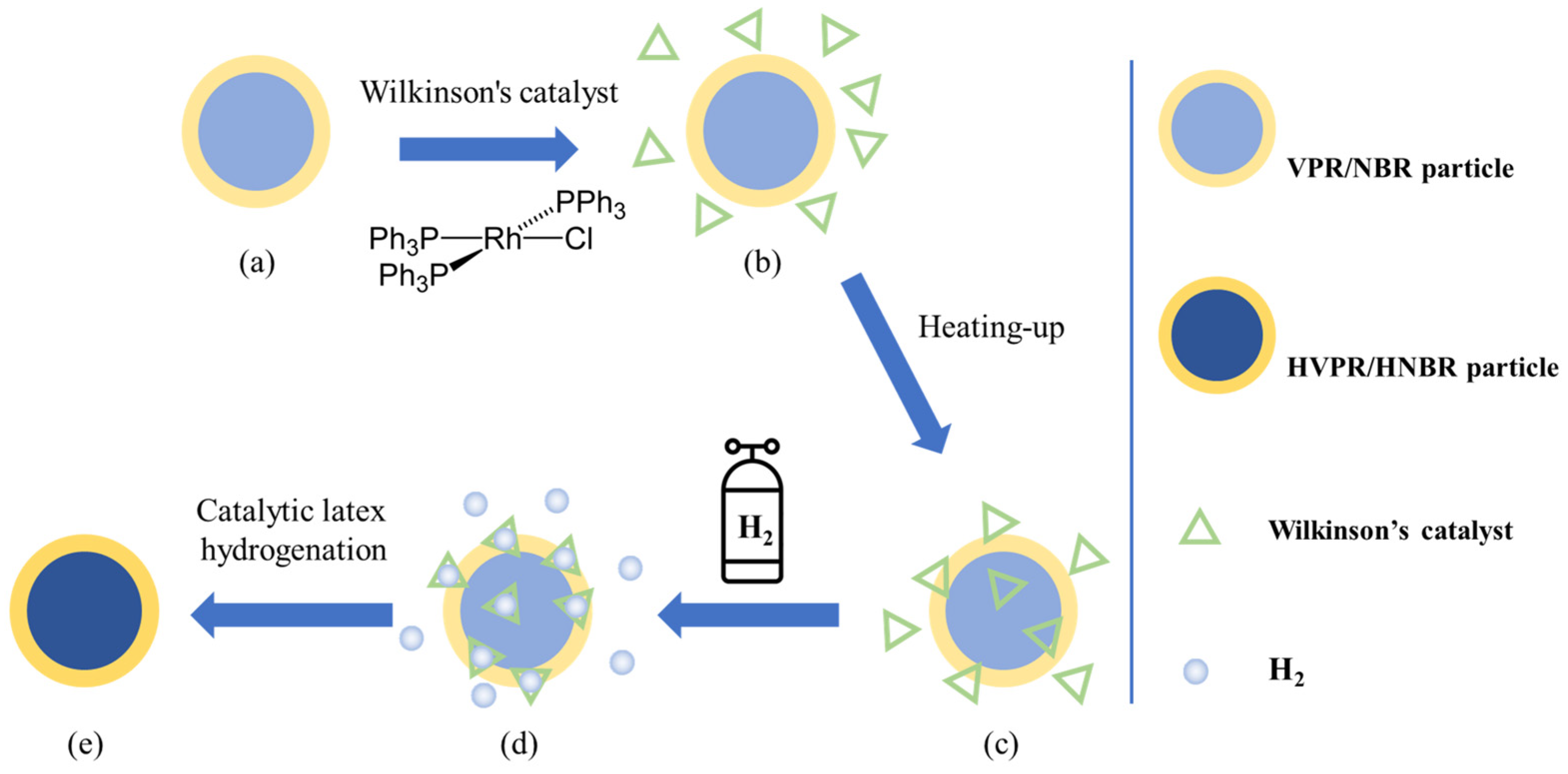
2.3. Catalytic Latex Hydrogenation of NBR Latex, VPR Latex, and VPR/NBR Core–Shell Nanoparticles Latex
2.4. Effect of Hydrogenation Process on Particle Size and Particle Size Distribution
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Polymerization
- VPR/NBR Seeded Emulsion Polymerization
- NBR Emulsion Polymerization
3.2.2. Post-Processing of Polymer Latex
3.2.3. Hydrogenation
- VPR/NBR Core–shell Latex Hydrogenation
- NBR Latex Hydrogenation without Seed
- VPR Latex Hydrogenation without Shell
3.3. Characterization
3.3.1. Particle Size
3.3.2. Transmission Electron Microscope (TEM)
3.3.3. Fourier Transform Infrared Spectrometer (FT–IR) and Attenuated Total Reflectance (ATR)
3.3.4. Conversion Rate of Polymerization
3.3.5. Hydrogenation Degree
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lovell, P.A.; Schork, F.J. Fundamentals of Emulsion Polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, Z.; Liu, S.; Cui, L.; Dai, Q.; He, J.; Dong, W.; Bai, C. Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers. Catalysts 2019, 9, 97. [Google Scholar] [CrossRef]
- Visseaux, M. Catalysts for the Controlled Polymerization of Conjugated Dienes. Catalysts 2018, 8, 442. [Google Scholar] [CrossRef]
- Hlalele, L.; D’hooge, D.R.; Dürr, C.J.; Kaiser, A.; Brandau, S.; Barner–Kowollik, C. RAFT Mediated ab Initio Emulsion Copolymerization of 1,3–Butadiene with Acrylonitrile. Macromolecules 2014, 47, 2820–2829. [Google Scholar] [CrossRef]
- Kausar, A. Emulsion polymer derived nanocomposite: A review on design and tailored attributes. Polym. Plast. Technol. Mater. 2022, 59, 1737–1750. [Google Scholar] [CrossRef]
- Chern, C.S. Emulsion polymerization mechanisms and kinetics. Prog. Polym. Sci. 2006, 31, 443–486. [Google Scholar] [CrossRef]
- Chen, L.; Hong, L.; Lin, J.; Meyers, G.; Harris, J.; Radler, M. Epoxy-acrylic core-shell particles by seeded emulsion polymerization. J. Colloid Interface Sci. 2016, 473, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ion, F.; Petrescu, T.; Liu, J.; Li, H.; Shi, G. Synthesis of Dimpled Particles by Seeded Emulsion Polymerization and Their Application in Superhydrophobic Coatings. Membranes 2022, 12, 876. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Q.; Hammond, M.; Rempel, G.L. Preparation of Poly(methyl methacrylate)–Poly(acrylonitrile–co–butadiene) Core–Shell Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 736–749. [Google Scholar] [CrossRef]
- Sheng, X.; Xie, D.; Yang, W.; Zhang, X.; Zhong, L. High solid content poly(butyl acrylate)/poly(methyl methacrylate) core/shell nanosized spheres synthesised by microemulsion polymerization. Micro Nano Lett. 2016, 11, 164–168. [Google Scholar] [CrossRef]
- Mun, H.; Hwang, K.; Kim, W. Synthesis of emulsion styrene butadiene rubber by reversible addition–fragmentation chain transfer polymerization and its properties. J. Appl. Polym. Sci. 2019, 136, 47069. [Google Scholar] [CrossRef]
- Giurginca, M.; Zaharescu, T. Thermal and radiation behaviour of HNBR and CSPE blends. Polymer 2000, 41, 7583–7587. [Google Scholar] [CrossRef]
- Tong, W.; Chen, H.; Zhang, Y.; Zhang, Z.; Fu, Y.; Qi, H.; Zhou, D.; Li, Y.; Wang, H. Selective hydrogenation of nitrile butadiene rubber latex using Catmetium®RF 4 catalyst. Catal. Today 2023, 407, 80–88. [Google Scholar] [CrossRef]
- Guerriero, A.; Peruzzini, M.; Gonsalvi, L. Ruthenium (II)-Arene Complexes of the Water-Soluble Ligand CAP as Catalysts for Homogeneous Transfer Hydrogenations in Aqueous Phase. Catalysts 2018, 8, 88. [Google Scholar] [CrossRef]
- Schulz, G.A.S.; Comin, E.; de Souza, R.F. Catalytic hydrogenation of nitrile rubber using palladium and ruthenium complexes. J. Appl. Polym. Sci. 2007, 106, 659–663. [Google Scholar] [CrossRef]
- Yue, D.M.; Shen, Z.M.; Xu, R.Q.; Wei, Y.K. A New Bimetallic Complex Catalyst for NBR Hydrogenation and Properties of Hydrogenated NBR. J. Elastomers. Plast. 2016, 34, 225–237. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Wang, C.; Zong, C. Diimide hydrogenation of NBR latex using different zinc ions catalytic system. Colloid. Polym. Sci. 2022, 300, 661–674. [Google Scholar] [CrossRef]
- Samran, J.; Phinyocheep, P.; Daniel, P.; Kittipoom, S. Hydrogenation of unsaturated rubbers using diimide as a reducing agent. J. Appl. Polym. Sci. 2005, 95, 16–27. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Rempel, G.L. Homogeneous Hydrogenation Art of Nitrile Butadiene Rubber: A Review. Polym. Rev. 2013, 53, 192–239. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Zhou, D.; Chen, H.; Li, Y.; Song, J.; Zhang, S.; Wang, H. Hydrogenation of Carboxyl Nitrile Butadiene Rubber Latex Using a Ruthenium–Based Catalyst. Catalysts 2022, 12, 97. [Google Scholar] [CrossRef]
- Cao, P.; Huang, C.; Zhang, L.; Yue, D. One-step fabrication of RGO/HNBR composites via selective hydrogenation of NBR with graphene-based catalyst. RSC Adv. 2015, 5, 41098–41102. [Google Scholar] [CrossRef]
- Lin, X.; Pan, Q.; Rempel, G.L. Hydrogenation of nitrile–butadiene rubber latex with diimide. Appl. Catal. A Gen. 2004, 276, 123–128. [Google Scholar] [CrossRef]
- Ai, C.; Gong, G.; Zhao, X.; Liu, P. Selectively Catalytic Hydrogenation of Nitrile–Butadiene Rubber Using Grubbs II Catalyst. Macromol. Res. 2017, 25, 461–465. [Google Scholar] [CrossRef]
- Wang, H.; Pan, Q.; Rempel, G.L. Diene–Based Polymer Nanoparticles: Preparation and Direct Catalytic Latex Hydrogenation. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2098–2110. [Google Scholar] [CrossRef]
- Lamb, D.J.; Fellows, C.M.; Gilbert, R.G. Radical entry mechanisms in redox–initiated emulsion polymerizations. Polymer 2005, 46, 7874–7895. [Google Scholar] [CrossRef]
- Thickett, S.C.; Gilbert, R.G. Emulsion polymerization: State of the art in kinetics and mechanisms. Polymer 2007, 48, 6965–6991. [Google Scholar] [CrossRef]
- Joseph Schork, F. Monomer transport in emulsion polymerization. Can. J. Chem. Eng. 2022, 100, 645–653. [Google Scholar] [CrossRef]
- Wang, H.; Rempel, G.L. Organic solvent–free catalytic hydrogenation of diene–based polymer nanoparticles in latex form: Mass transfer of hydrogen in a semibatch process. J. Ind. Eng. Chem. 2015, 25, 29–34. [Google Scholar] [CrossRef]
- de Arbina, L.L.; Barandiaran, M.J.; Gugliotta, L.M.; Asuat, J.M. Emulsion polymerization: Particle growth kinetics. Polymer 1996, 37, 5907–5916. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, F.; Fu, Y.; Chen, H.; Zhang, Y.; Wang, C.; Li, Y.; Wang, H. Preparation of Diene–based Nanoparticles by Semibatch Microemulsion Polymerization and Their Catalytic Hydrogenation. Catal. Today 2023, 407, 156–164. [Google Scholar] [CrossRef]
- Kuwano, N.; Kaur, J.; Rahmah, S. Electron microscopy determination of crystallographic polarity of aluminum nitride thin films. Micron 2019, 116, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wu, J.; Pan, Q.; Rempel, G.L. Direct Catalytic Hydrogenation of an Acrylonitrile–Butadiene Rubber Latex Using Wilkinson’s Catalyst. Macromol. Rapid Commun. 2005, 26, 1768–1772. [Google Scholar] [CrossRef]
- Nelson, D.; Li, R. Christopher Brammer. Using Correlations to Compare Additions to Alkenes: Homogeneous Hydrogenation by Using Wilkinson’s Catalyst. J. Org. Chem. 2005, 70, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Takahashi, R.; Asai, S. ONIOM Study of the Mechanism of Olefin Hydrogenation by the Wilkinson’s Catalyst: Reaction Paths and Energy Surfaces oftrans- andcis-Forms. Bull. Chem. Soc. Jpn. 2013, 86, 243–254. [Google Scholar] [CrossRef]
- Brück, D. IR spectrometric determination of the proportions of acrylonitrile, butadiene and hydrogenated butadiene in hydrogenated acrylonitrile–butadiene rubbers. Part 1. Principles. Kautsch. Gummi Kunstst. 1989, 42, 107–110. [Google Scholar]
- Brück, D. IR spectrometric determination of the proportions of acrylonitrile, butadiene and hydrogenated butadiene in hydrogenated acrylonitrile–butadiene rubbers. Part 2. Residual double bonds in commercial HNBR products. Kautsch. Gummi Kunstst. 1989, 42, 194–197. [Google Scholar]
- Wang, H.; Chan, E.; Bregu, S.; Rempel, G.L. Preparation of Poly(styrene–co–butadiene) Fine Latex via a Gemini Surfactant Induced Low Temperature Initiation Semibatch Emulsion Polymerization System. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1669–1678. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Shang, J.; Mao, Z.; Yang, C. Measurement methods of particle size distribution in emulsion polymerization. Chin. J. Chem. Eng. 2021, 39, 1–15. [Google Scholar] [CrossRef]
- Chen, S.A.; Lee, S.T. Seeded latex polymerizations: Studies on the particle growth mechanism of latex particles. Polymer 1992, 33, 1437–1444. [Google Scholar] [CrossRef]
- SH/T 1763-2008; Nitrile Rubber. Determination of Residual Unsaturation in Hydrogenated Nitrile Rubber (HNBR) by Iodine Value. The Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
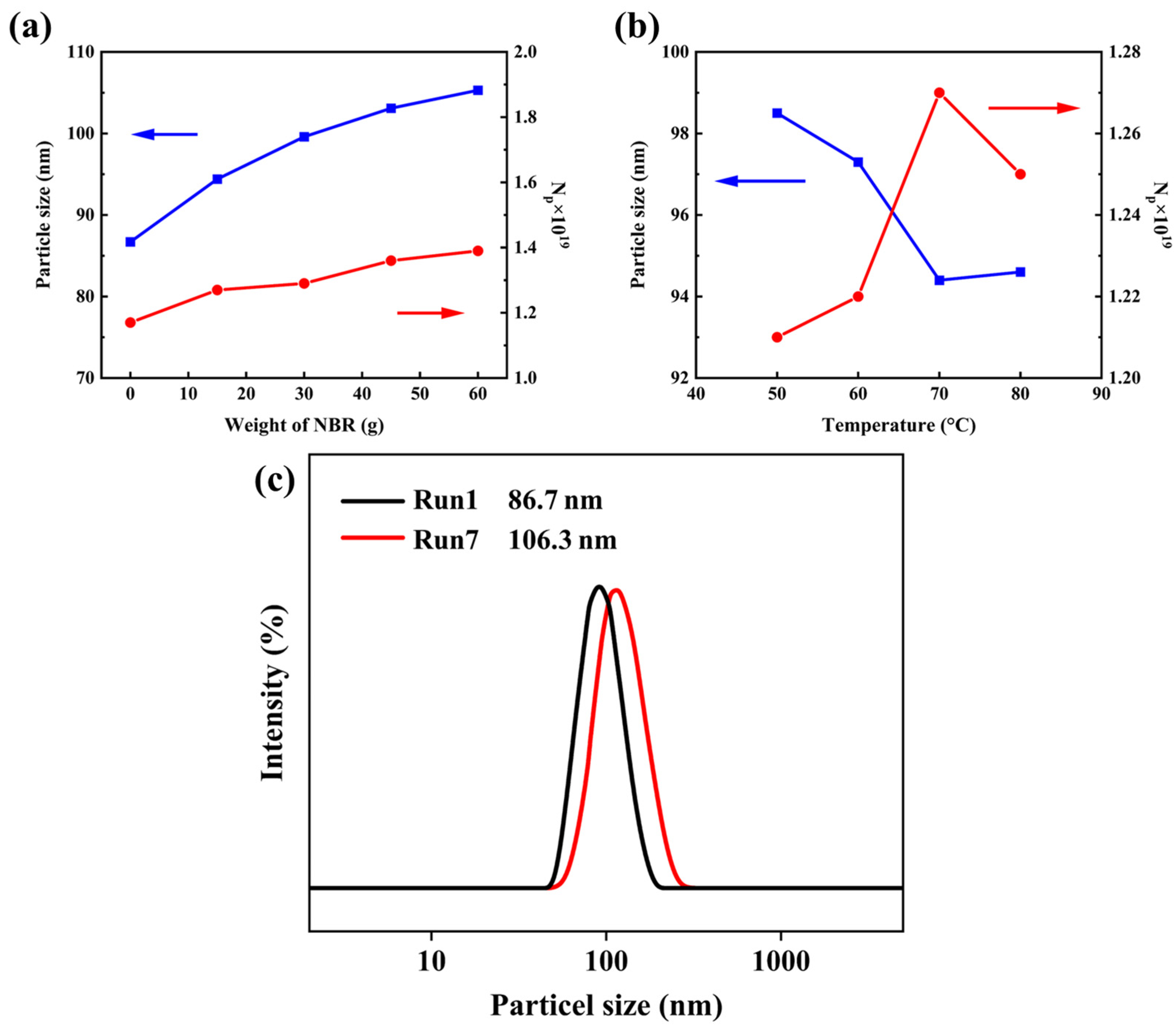
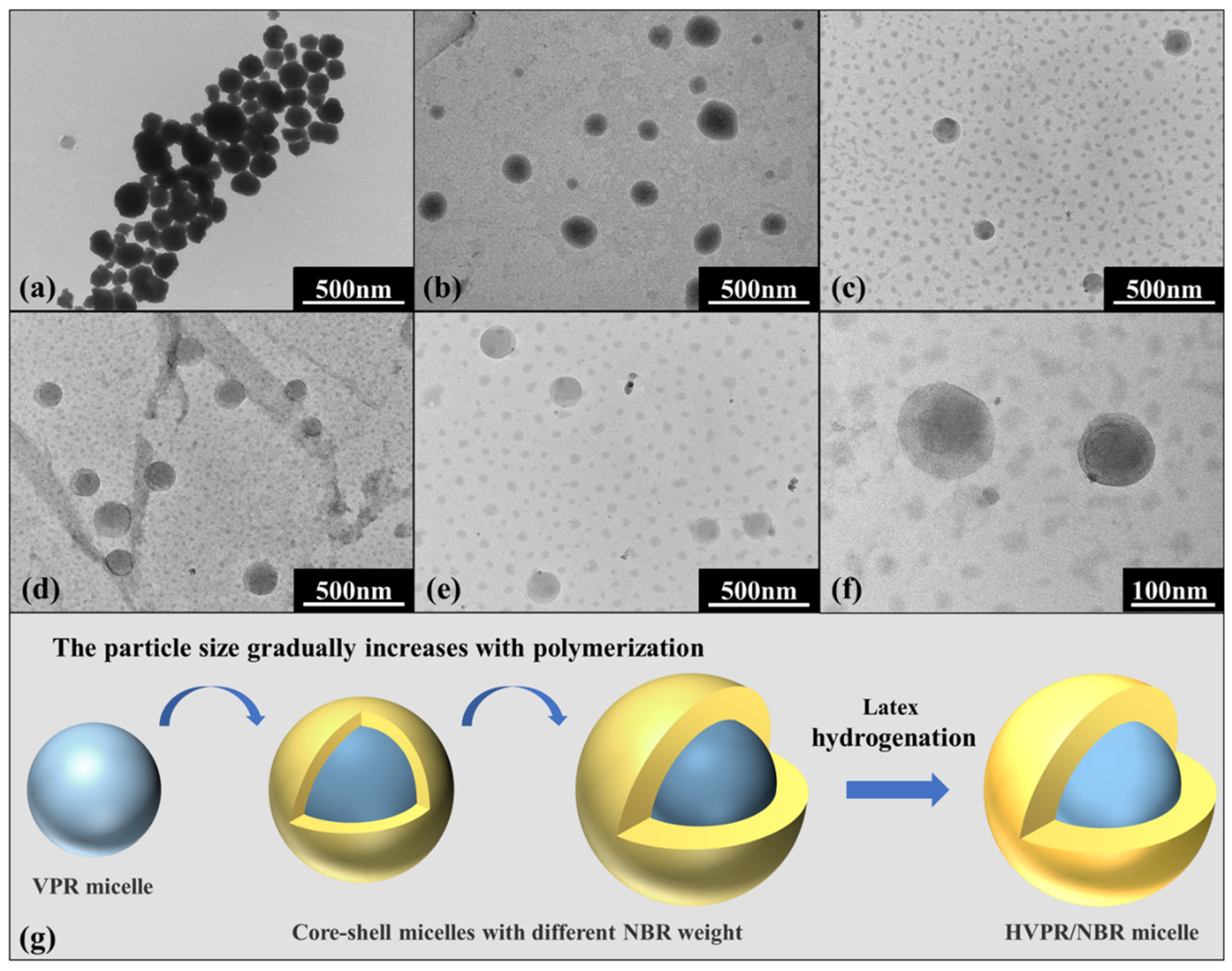
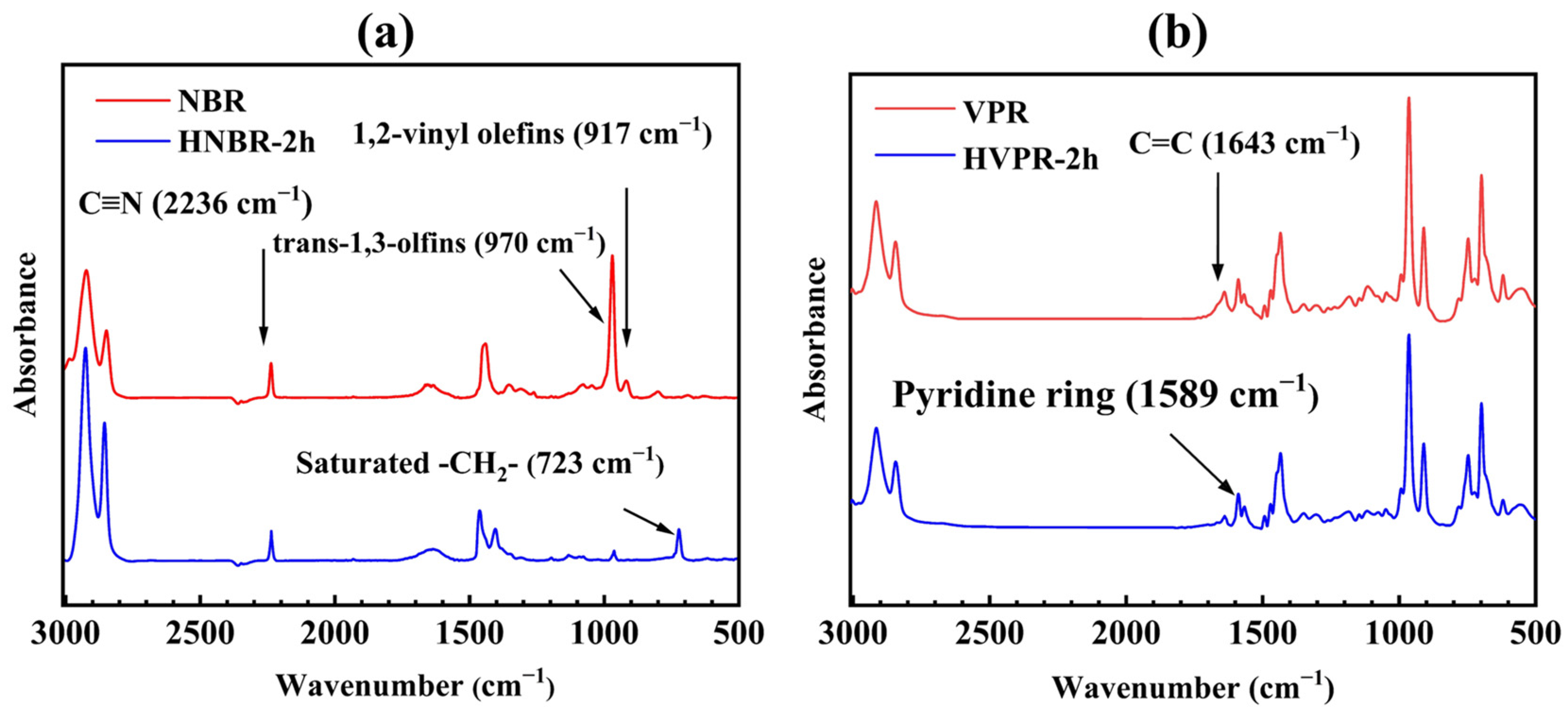
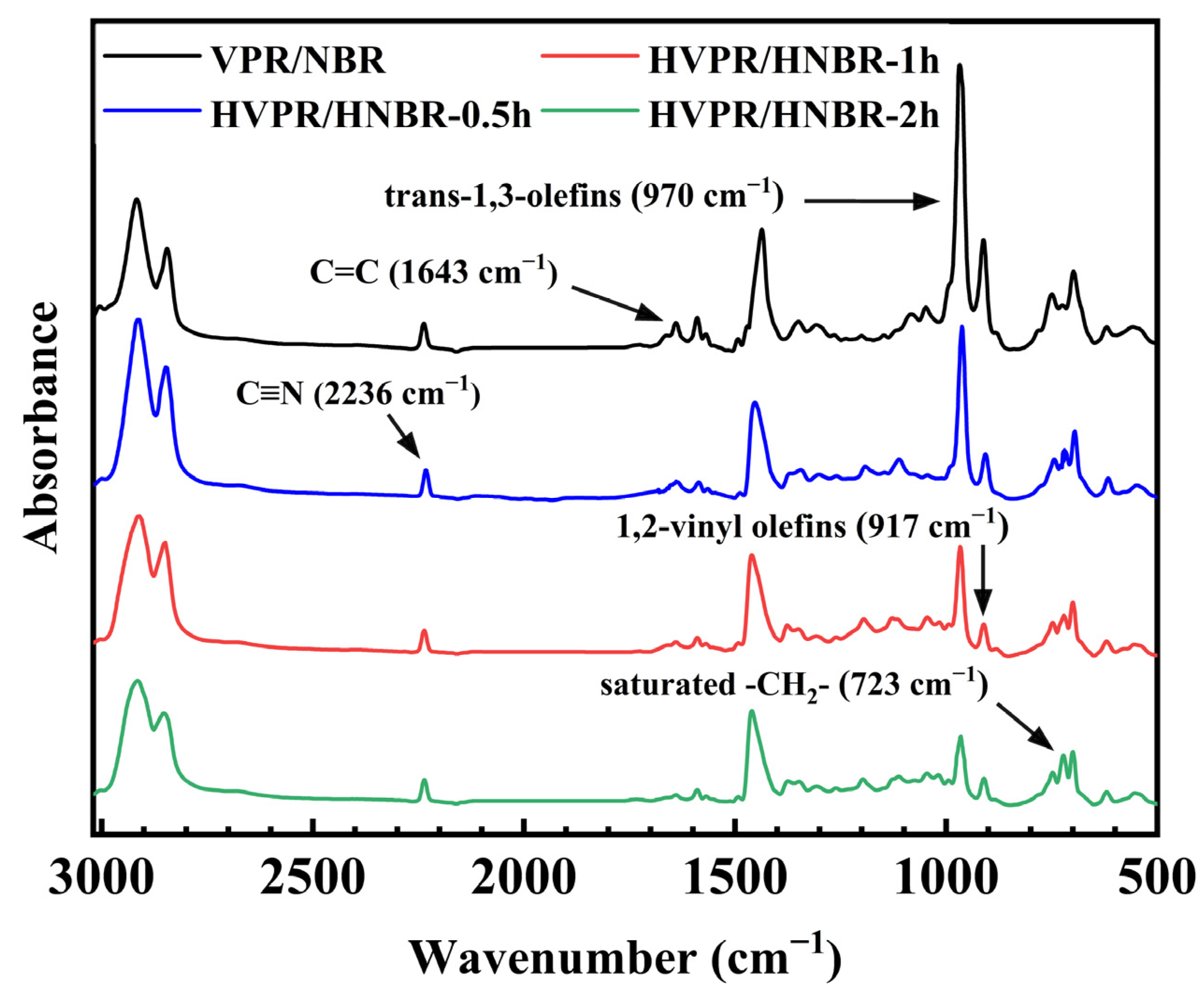

| Run (a) | Water (b) (wt%) | Weight of VPR (g) | Weight of NBR (c) (g) | Temperature (°C) | Dz (nm) | Np × 1019 (d) | PDI |
|---|---|---|---|---|---|---|---|
| 1 | – | 60 | 0 | – | 86.7 | 1.17 | 0.045 |
| 2 | 46.3 | 30 | 15 | 50 | 98.5 | 1.21 | 0.043 |
| 3 | 46.3 | 30 | 15 | 60 | 97.3 | 1.22 | 0.046 |
| 4 | 46.3 | 30 | 15 | 70 | 94.4 | 1.27 | 0.047 |
| 5 | 81.3 | 30 | 30 | 70 | 99.6 | 1.29 | 0.042 |
| 6 | 116.3 | 30 | 45 | 70 | 103.1 | 1.36 | 0.039 |
| 7 | 151.3 | 30 | 60 | 70 | 106.3 | 1.39 | 0.041 |
| 8 | 46.3 | 30 | 15 | 80 | 94.6 | 1.25 | 0.045 |
| 9 | 140 | 0 | 60 | 70 | 91.3 | 1.13 | 0.40 |
| Run | Latex | Wilkinson’s Catalyst (wt%) | SDS (wt%) | Time (h) | Temperature (°C) | Pressure (MPa) | Hydrogenation Degree (%) |
|---|---|---|---|---|---|---|---|
| 10 | NBR | 0.025 | 3 | 2 | 120 | 8.6 | 92.8 |
| 11 | VPR | 0.025 | 3 | 2 | 120 | 8.6 | 88.6 |
| 12 | VPR/NBR | 0.04 | 3 | 0.5 | 120 | 8.6 | 28.6 |
| 13 | VPR/NBR | 0.04 | 3 | 1 | 120 | 8.6 | 58.5 |
| 14 | VPR/NBR | 0.04 | 3 | 2 | 120 | 8.6 | 85.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, F.; Li, X.; Dou, J.; Zhang, B.; Song, X.; Li, L.; Liu, J.; Li, Y.; Jiang, Y.; Wang, H. Preparation of Poly(Butadiene–Styrene–Vinyl Pyridine)/Poly(Acrylonitrile–Butadiene) Core–Shell Nanoparticles by Intermittent Seeded Emulsion Polymerization and Their Catalytic Latex Hydrogenation. Catalysts 2024, 14, 277. https://doi.org/10.3390/catal14040277
Yuan F, Li X, Dou J, Zhang B, Song X, Li L, Liu J, Li Y, Jiang Y, Wang H. Preparation of Poly(Butadiene–Styrene–Vinyl Pyridine)/Poly(Acrylonitrile–Butadiene) Core–Shell Nanoparticles by Intermittent Seeded Emulsion Polymerization and Their Catalytic Latex Hydrogenation. Catalysts. 2024; 14(4):277. https://doi.org/10.3390/catal14040277
Chicago/Turabian StyleYuan, Fei, Xudong Li, Jianying Dou, Baojia Zhang, Xueling Song, Lin Li, Junjie Liu, Yanyan Li, Yigao Jiang, and Hui Wang. 2024. "Preparation of Poly(Butadiene–Styrene–Vinyl Pyridine)/Poly(Acrylonitrile–Butadiene) Core–Shell Nanoparticles by Intermittent Seeded Emulsion Polymerization and Their Catalytic Latex Hydrogenation" Catalysts 14, no. 4: 277. https://doi.org/10.3390/catal14040277






