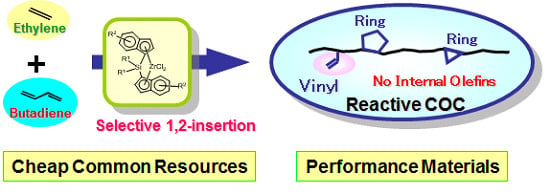
Synthesis of Ethylene or Propylene/1,3-Butadiene Copolymers Possessing Pendant Vinyl Groups with Virtually No Internal Olefins
Abstract
:1. Introduction

2. Results and Discussion
2.1. Ethylene/Butadiene Copolymerization by rac-[SiMe2(Indenyl’)2]ZrCl2
Influence of Substituent on Insertion Mode of Butadiene in Ethylene/Butadiene Copolymerization


| Entry | Cat | A b | Mw c (103) | Mw/Mn | BD in Polym. d (mol %) | Mole Fraction e | Quantity per 1000 Carbon f | Tg (°C) | Tm (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,2-addition units | Internal olefins g | Vinyl groups h | Internal olefins g | ||||||||
| fv/fcycPro/fcycPen | |||||||||||
| 1 | 1 | 371.2 | 91.9 | 2.0 | 2 | 11/0/89 | 0 | 1 | 0.2 | - | 113 |
| 2 | 2 | 44.9 | 156.6 | 3.2 | 4 | 5/29/66 | 0 | 1 | 0.2 | - | 100 |
| 3 | 3 | 14.1 | 89.2 | 2.4 | 7 | 28/29/43 | 0 | 5 | 0.1 | −33 | 71/117 |
| 4 | 4 | 6.2 | 37.7 | 2.4 | 9 | 44/21/35 | 0 | 7 | 0.3 | −39 | 52/114 |
| 5 | 5 | 6.8 | 71.1 | 2.0 | 8 | 46/21/33 | 0 | 11 | 0.3 | −32 | 55 |
| 6 | 6 | 5.3 | 50.5 | 2.1 | 9 | 53/15/32 | 0 | 13 | 0.3 | −33 | 45 |
| 7 | 7 | 2.4 | 77.9 | 3.8 | 9 | 47/26/28 | 0 | 15 | 0.1 | −32 | 52/113 |
| 8 | 8 | 3.6 | 77.9 | 2.9 | 12 | 61/25/14 | 0 | 18 | 0.3 | −33 | 33/105 |
| 9 i | 8 | 4.4 | 114.0 | 2.8 | 10 | 49/27/23 | 0 | 18 | 0.1 | −31 | 37 |
| 10 j | 8 | 4.5 | 132.5 | 2.9 | 12 | 72/14/14 | 0 | 41 | 0.0 | −23 | - |
| 11 k | 8 | 1.9 | 35.7 | 3.1 | 10 | 79/5/16 | 0 | 49 | 0.2 | −14 | - |
| 12 l | 8 | 1.3 | 39.3 | 3.9 | 16 | 95/0/5 | 0 | 76 | 1.3 | −10 | - |


2.2. Propylene/Butadiene Copolymerization by Complexes 3 and 8
| Entry | Cat | A b | Mw c (103) | Mw /Mn | BD in Polym. d (mol %) | Mole Fraction e | Quantity per 1000 Carbon f | Tg (°C) | Tm (°C) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,2-addition units | Internal olefins g | fH-1,4 | Vinyl groups h | Internal olefins g | ||||||||
| fv/fcycPro/fcycPen | ||||||||||||
| 1 | 3 | 16.6 | 99.6 | 2.4 | 5 | 38/2/28 | 0 | 32 | 6 | 0 | 6 | 122 |
| 2 | 8 | 25.4 | 257.0 | 3.0 | 9 | 60/5/25 | 0 | 10 | 17 | 0 | −15.3/3.9 | 102 |
| 3 i | 8 | 0.7 | 37.6 | 2.6 | 37 | 58/13/26 | 0 | 3 | 86 | 3.7 | –24 | - |


3. Experimental Section
3.1. General
3.2. Materials
3.3. Polymerization Procedure
3.3.1. Ethylene/Butadiene Copolymerization (Table 1)
3.3.2. Propylene/Butadiene Copolymerization (Table 2)
3.4. Polymer Characterization
3.4.1. Microstructure Analysis of Ethylene/Butadiene Copolymer by 13C-NMR
- A:
- (Integrated value per one carbon of the vinyl groups) = integrated value of the peak at 113.8 ppm
- B:
- (Integrated value per one carbon of the cyclopropane skeleton) = [(integrated value of the peak at 17.2 ppm (cis CH in Chemical Formula (α))) + (integrated value of the peak at 19.2 ppm (trance CH in Chemical Formula (α)))]/2
- C:
- (Integrated value per one carbon of the cyclopentane skeleton) = [(integrated value of the peak at 43.1 ppm (cis CH in Chemical Formula (β))) + (integrated value of the peak at 46.3 ppm (trans CH in Chemical Formula (β)))]/2
- D:
- (Integrated value per one carbon of the 1,4-addition units) = [integrated value of the peaks at 131–130 ppm
- E:
- (Integrated value per one carbon of the 1,3-addition units) = [integrated value of the peak at 137.3 ppm
3.4.2. Microstructure Analysis of Ethylene/Butadiene Copolymers by 1H-NMR
- F:
- (Integrated value per one proton of the pendant vinyl groups) = integrated value of the peak at 5.4–5.6 ppm
- G:
- (Integrated value per one proton of the terminal vinyl groups) = integrated value of the peak at 5.6–5.8 ppm
- H:
- (Integrated value per one proton of the internal olefins) = [integrated value of the peaks at 5.2–5.4 ppm
3.4.3. Microstructure Analysis of Propylene/Butadiene Copolymer by 13C-NMR
- I:
- (Integrated value per one carbon of the vinyl groups) = integrated value of the peak at 113.2 ppm
- J:
- (Integrated value per one carbon of the cyclopropane skeleton) = [(integrated value of the peak at 12.9 ppm (CH2 in Chemical Formula (α'))
- K:
- (Integrated value per one carbon of the methylcyclopentane skeleton) = integrated value of the peak at 32.5 ppm (CH in Chemical Formula (β'))
- L:
- (Integrated value per one carbon of the 1,4-addition units) = [integrated value of the peaks at 128–133 ppm
- M:
- (Integrated value per one carbon of the 1,3-addition units) = [integrated value of the peak at 134–136 ppm
- N:
- (Integrated value per one carbon of the hydrogenated 1,4-addition units) = [integrated value of the peaks at 37.1 ppm (CH2 in Chemical Formula (γ'))]/2
3.4.4. Microstructure Analysis of Propylene/Butadiene Copolymer by 1H-NMR
- O:
- (Integrated value per one proton of the vinyl groups) = [integrated value of the peak at 4.7–5.0 ppm + integrated value of the peak at 5.4–5.8 ppm
- P:
- (Integrated value per one proton of the internal olefins) = [integrated value of the peaks at 5.2–5.4 ppm
3.4.5. GPC Analysis
3.4.6. DSC Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Franssen, N.M.G.; Reek, J.N.H.; de Bruin, B. Synthesis of functional “polyolefins”: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Welborn, H.C., Jr. Copolymers of Ethylene and 1,3-butadiene. EP0275676, 27 July 1988. [Google Scholar]
- Galimberti, M.; Albizzati, E.; Abis, L.; Bacchilega, G. 13C-NMR analysis of α-olefins copolymers with 1,3-butadiene obtained with zirconocenes/methylalumoxane catalysts. Makromol. Chem. 1991, 192, 2591–2601. [Google Scholar] [CrossRef]
- Morizono, K.; Okada, K.; Yamaguchi, M. Unsaturated Copolymers, Processes for Preparing the Same, and Compositions Containing the Same. US6310164, 30 October 2001. [Google Scholar]
- Morizono, K.; Okada, K.; Yamaguchi, M. Unsaturated Copolymer, Preparation Thereof and Composition Containing Said Copolymer. Patent JPH1180269, 26 March 1999. [Google Scholar]
- Pragliola, S.; Milano, G.; Guerra, G.; Longo, P. Stereoselective Cyclopropanation by Cyclocopolymerization of Butadiene. J. Am. Chem. Soc. 2002, 124, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
- Longo, P.; Pragliola, S.; Milano, G.; Guerra, G. E Stereoregular 1,1 and 1,3 Constitutional Units from 1,3-Butadiene in Copolymerizations Catalyzed by a Highly Hindered C2 Symmetric Metallocene. J. Am. Chem. Soc. 2003, 125, 4799–4803. [Google Scholar] [CrossRef] [PubMed]
- Longo, P.; Napoli, M.; Pragliola, S.; Costabile, C.; Milano, G.; Guerra, G. Butadiene Insertion and Constitutional Units in Ethene Copolymerizations by C2-Symmetric Metallocenes. Macromolecules 2003, 36, 9067–9074. [Google Scholar] [CrossRef]
- Pragliola, S.; Costabile, C.; Magrino, M.; Napoli, M.; Longo, P. Ethene/1,3-Butadiene Copolymerization in the Presence of rac-(CH2-(3-tert-butyl-1-indenyl)2)ZrCl2/MAO Catalytic System: Study of the Polymerization Mechanism by Using 13C-Labeled 1,3-Butadiene. Macromolecules 2004, 37, 238–240. [Google Scholar] [CrossRef]
- Ishihara, T.; Shiono, T. Hydrogenated 1,4-Insertion of Butadiene in the Copolymerization with Propylene Using an Isospecific Zirconocene Catalyst. J. Am. Chem. Soc. 2005, 127, 5774–5775. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Ban, H.T.; Hagihara, H.; Shiono, T. Additiveeffectsofalkylaluminiumcompoundsonpr opylene-1,3-butadiene copolymerization using isospecific zirconocenecatalysts. J. Organomet. Chem. 2010, 695, 1694–1699. [Google Scholar] [CrossRef]
- Michiue, K.; Ishii, S.; Mitani, M.; Karino, T. Copolymer of Olefin and Conjugated Diene, and Process for Producing Same. Patent WO2010113975, 7 October 2010. [Google Scholar]
- Partial pressure of butadiene was increased by increasing polymerization temperature from 60 °C to 70 °C.
- Spaleck, W.; Kuber, F.; Winter, A.; Rohrmann, J.; Bachmann, B.; Antberg, M.; Dolle, V.; Paulus, E.F. The Influence of Aromatic Substituents on the Polymerization Behavior of Bridged Zirconocene Catalysts. Organometallics 1994, 13, 954–963. [Google Scholar] [CrossRef]
- Fukuoka, O.; Tashiro, K.; Kawai, K.; Saito, J.; Imuda, J.; Fujita, T.; Nitahara, M.; Ueda, T.; Kiso, Y.; Yoshida, M. New Transition Metal Compound, Olefin Polymerization Catalyst Component Comprising the Same, Olefin Polymerization Catalyst Containing this Component and Polymerization of Olefin. Patent JPH07286005, 31 October 1995. [Google Scholar]
- Fukuoka, O.; Kawai, K.; Nitahara, M. Catalyst for Olefin Polymerization and Polymerization Method of Olefin. Patent JPH1087716, 7 April 1998. [Google Scholar]
- Hagihara, H.; Shiono, T.; Ikeda, T. Living Polymerization of Propene and 1-Hexene with the [t-BuNSiMe2Flu]TiMe2/B(C6F5)3 Catalyst. Macromolecules 1998, 31, 3184–3188. [Google Scholar] [CrossRef]
- Choo, T.N.; Waymouth, R.M. The Dual-Site Alternating Cyclocopolymerization of 1,3-Butadiene with Ethylene. J. Am. Chem. Soc. 2003, 125, 8970–8971. [Google Scholar] [CrossRef] [PubMed]
- The assignment of signals in 13C-NMR spectra for the methylcyclopentane skeleton unit in the propylene/butadiene copolymer was made together with the results by Distortion-less Enhancement by Polarization Transfer (DEPT) NMR experiments.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michiue, K.; Mitani, M.; Fujita, T. Synthesis of Ethylene or Propylene/1,3-Butadiene Copolymers Possessing Pendant Vinyl Groups with Virtually No Internal Olefins. Catalysts 2015, 5, 2001-2017. https://doi.org/10.3390/catal5042001
Michiue K, Mitani M, Fujita T. Synthesis of Ethylene or Propylene/1,3-Butadiene Copolymers Possessing Pendant Vinyl Groups with Virtually No Internal Olefins. Catalysts. 2015; 5(4):2001-2017. https://doi.org/10.3390/catal5042001
Chicago/Turabian StyleMichiue, Kenji, Makoto Mitani, and Terunori Fujita. 2015. "Synthesis of Ethylene or Propylene/1,3-Butadiene Copolymers Possessing Pendant Vinyl Groups with Virtually No Internal Olefins" Catalysts 5, no. 4: 2001-2017. https://doi.org/10.3390/catal5042001