Highly Selective Catalytic Properties of HZSM-5 Zeolite in the Synthesis of Acetyl Triethyl Citrate by the Acetylation of Triethyl Citrate with Acetic Anhydride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of the Zeolites
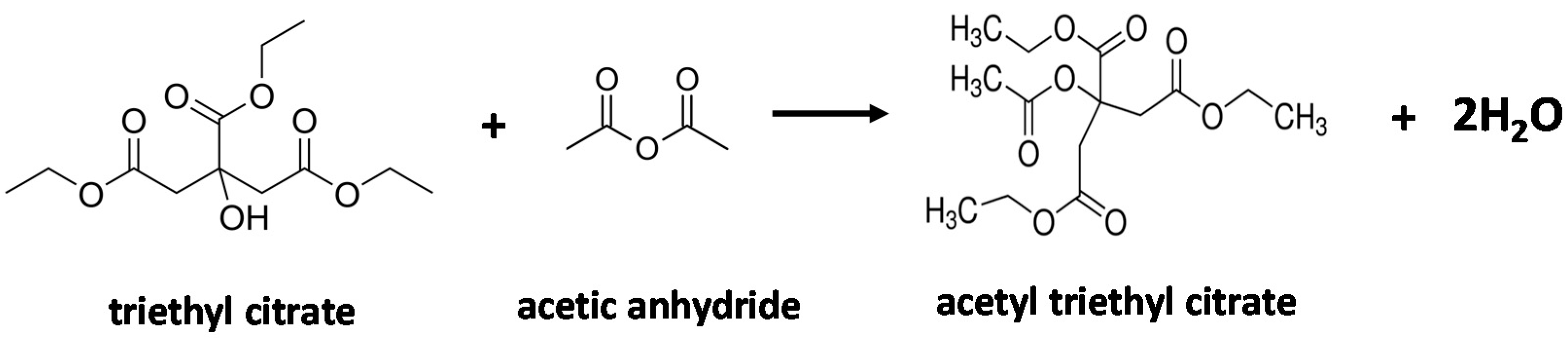
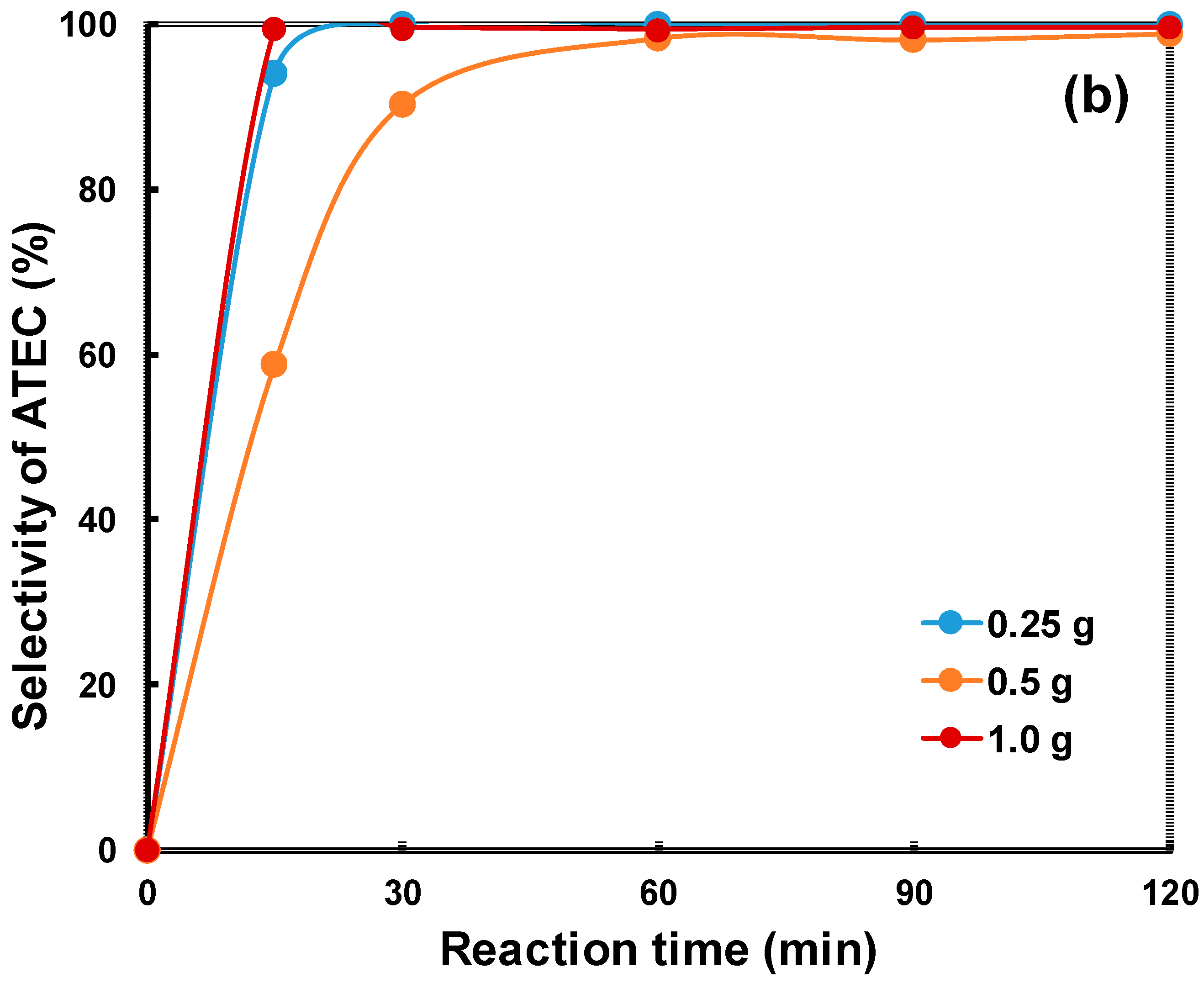
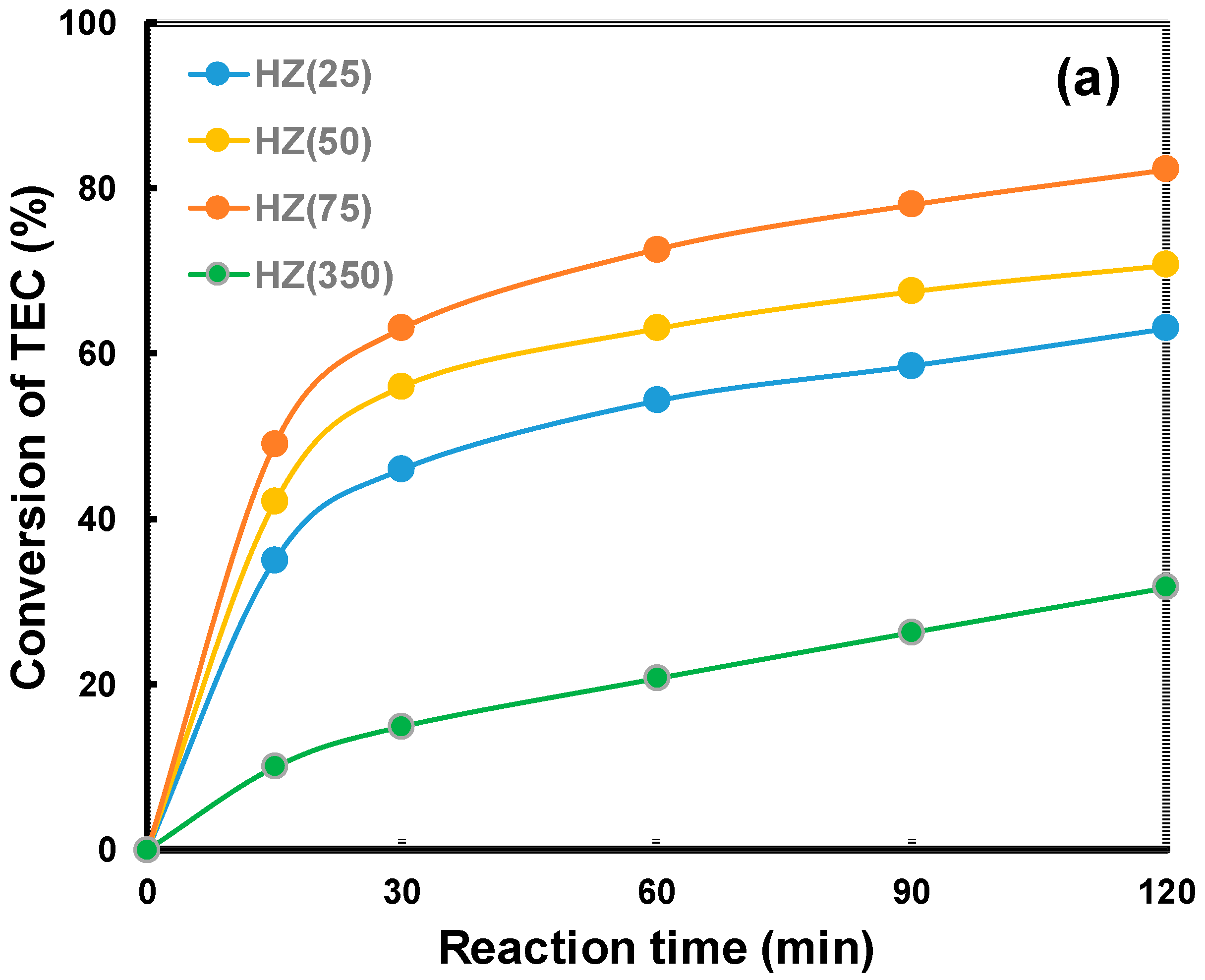
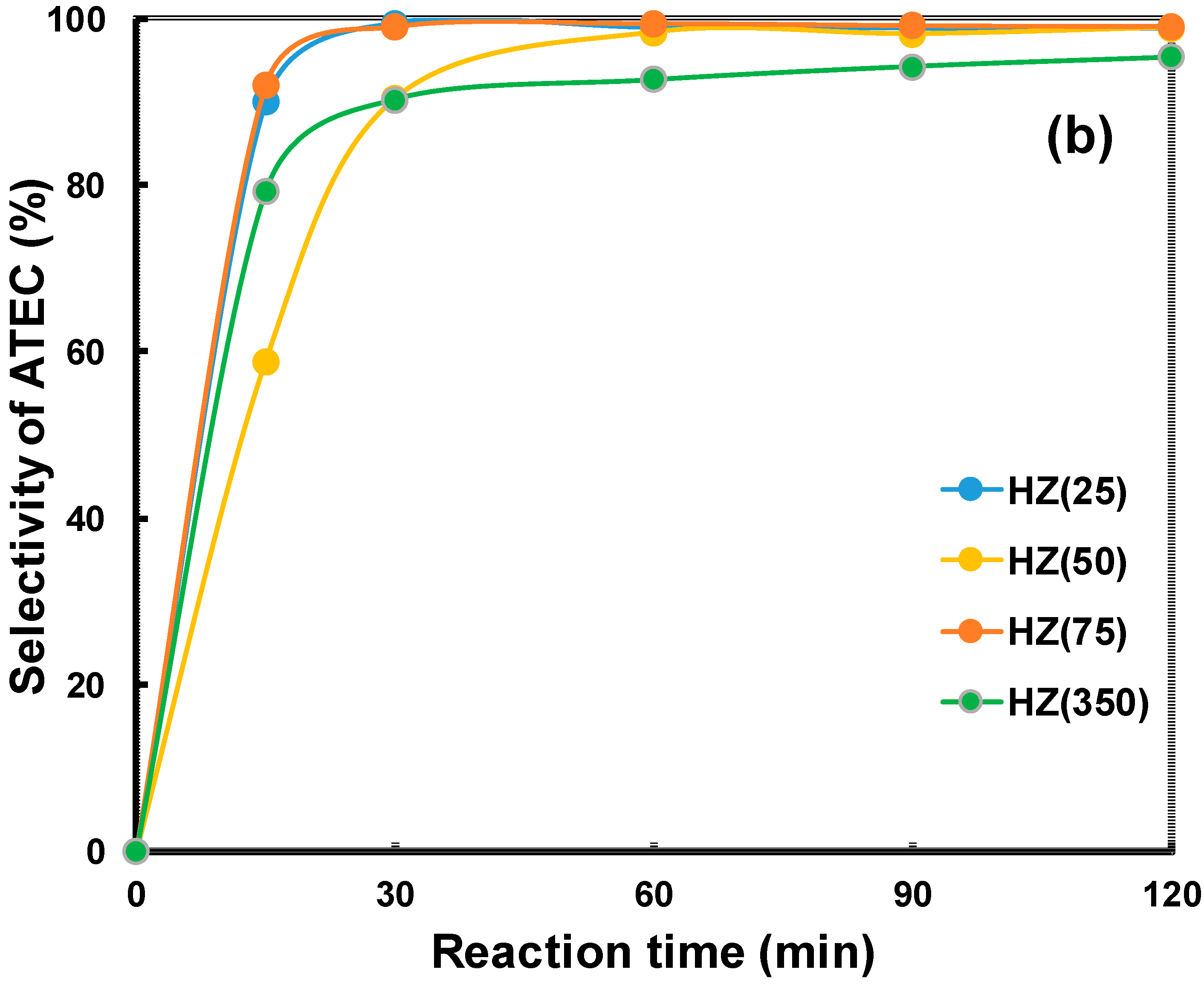
2.2. Catalytic Properties of the Zeolites in the Acetylation
3. Materials and Methods
3.1. Catalysts
3.2. Characterization of Physicochemical Properties of the Zeolite
3.3. Acetylation of TEC with Acetic Anhydride
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sang, B.; Li, Z.; Yu, L.; Li, X.; Zhang, Z. Preparation of zinc hydroxystannate-titanate nanotube flame retardant and evaluation its smoke suppression efficiency for flexible polyvinyl chloride matrix. Mater. Lett. 2017, 204, 133–137. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Wang, Y.; Lin, J.; Peng, N.; Li, J.; Zhao, F. Performance enhancement of polyvinyl chloride ultrafiltration membrane modified with graphene oxide. J. Colloid Interface Sci. 2016, 480, 1–8. [Google Scholar]
- Hunge, Y.M.; Mahadik, M.A.; Moholkar, A.V.; Bhosale, C.H. Photoelectrocatalytic degradation of phthalic acid using spray deposited stratified WO3/ZnO thin films under sunlight illumination. Appl. Surf. Sci. 2017, 420, 764–772. [Google Scholar] [CrossRef]
- Sandhwar, V.K.; Prasad, B. Comparison of phthalic acid removal from aqueous solution by electrochemical methods: Optimization, kinetic and sludge study. J. Environ. Manag. 2017, 203, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Brazel, C. The Plasticizer Market: An Assessment of Traditional Plasticizers and Research Trends to Meet New Challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Wypych, G. Plasticizer Types Handbook of Plasticizers; Chem Tec Publishing: Toronto, ON, USA, 2000; pp. 7–71. [Google Scholar]
- Pan, H.; Hao, Y.; Lang, X.; Zhang, Y.; Wang, Z.; Zhang, H.; Dong, L. Improved mechanical properties, barrier properties and degradation behavior of poly(butylenes adipate-coterephthalate)/poly(propylene carbonate) films. Korean J. Chem. Eng. 2017, 34, 1294–1304. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and Exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.-M.; Laudet-Hesbert, A. Phtalates (II). EMC Toxicol. 2005, 2, 137–150. [Google Scholar] [CrossRef]
- Van Haveren, J.; Oostveen, E.A.; Miccichè, F.; Weijnen, J.G.J. How biobased products contribute to the establishment of sustainable, phthalate free, plasticizers and coatings. In Feedstocks for the Future; Bozell, J.J., Patel, M.K., Eds.; American Chemical Society: Washington, DC, USA, 2006; pp. 99–115. [Google Scholar]
- Wilkinson, C.F.; Lamb, J.C. The potential health effects of phthalate esters in children’s toys: A review and risk assepssment. Regul. Toxicol. Pharmacol. 1999, 30, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Isama, K.; Matsuoka, A. Analysis of phthalic acid diesters, monoester, and other plasticizers in polyvinyl chloride household products in Japan. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2011, 46, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.R.; Portela, M.F.; Bond, G.C. Synthesis of phthalic anhydride: Catalysts, Kinetics, and Reaction Modeling. Catal. Rev. 1997, 39, 169–207. [Google Scholar] [CrossRef]
- Reinecke, H.; Navarro, R.; Pérez, M. Plasticizers. In Handbook of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2011; pp. 1–27. [Google Scholar]
- Kolah, A.K.; Asthana, N.S.; Vu, D.T.; Lira, C.T.; Miller, D.J. Reaction Kinetics of the Catalytic Esterification of Citric Acid with Ethanol. Ind. Eng. Chem. Res. 2007, 46, 3180–3187. [Google Scholar] [CrossRef]
- Arendt, W.D.; Joshi, M. Specialty Plasticizers Handbook of Vinyl Formulating; John Wiley & Sons, Inc.: New York, NY, USA, 2008; pp. 239–286. [Google Scholar]
- Miller, D.J.; Asthana, N.; Kolah, A.; Vu, D.T.; Lira, C.T. Process for Reactive Esterification Distillation. U.S. Patent 7667068 B2, 23 February 2010. [Google Scholar]
- Schröter, J.; Konetzke, G.; Weidemann, F.; Klein, T.; Bohnen, H.; Bergrath, K.; Schmidt, K. Method for Producing Citric Acid Esters. WO2003008369, 30 January 2003. [Google Scholar]
- Bohnen, H.; Bergrath, K.; Klein, T. Citric Esters and a Process for Their Preparation. U.S. Patent 2002/0198402 A1, 8 May 2002. [Google Scholar]
- Kolah, A.K.; Asthana, N.S.; Vu, D.T.; Lira, C.T.; Miller, D.J. Triethyl Citrate Synthesis by Reactive Distillation. Ind. Eng. Chem. Res. 2008, 47, 1017–1025. [Google Scholar] [CrossRef]
- Verhoff, F.H. Citric Acid. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2005; p. 69. [Google Scholar]
- Osorio-Pascuas, O.M.; Santaella, M.A.; Rodriguez, G.; Orjuela, A. Esterification Kinetics of Tributyl Citrate Production Using Homogeneous and Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2015, 54, 12534–12542. [Google Scholar] [CrossRef]
- Jia, P.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. PVC materials without migration obtained by chemical modification of azide-functionalized PVC and triethyl citrate plasticizer. Mater. Chem. Phys. 2017, 190, 25–30. [Google Scholar] [CrossRef]
- Finkelstein, M.; Gold, H. Toxicology of the citric acid esters: Tributyl citrate, acetyl tributyl citrate, triethyl citrate, and acetyl triethyl citrate. Toxicol. Appl. Pharmacol. 1959, 1, 283–298. [Google Scholar] [CrossRef]
- Sawada, S.; Ursino, C.; Galiano, F.; Simone, S.; Figoli, A. Effect of citrate-based non-toxic solvents on poly(vinylidene fluoride) membrane preparation via thermally induced phase separation. J. Membr. Sci. 2015, 493, 232–242. [Google Scholar] [CrossRef]
- Pang, C.; Shanks, R.A.; Daver, F. Characterization of kenaf fiber composites prepared with tributyl citrate plasticized cellulose acetate. Compos. Part A Appl. Sci. Manuf. 2015, 70, 52–58. [Google Scholar] [CrossRef]
- Vertellus. Available online: http://www.vertellus.com (accessed on 24 October 2017).
- Sakakura, A.; Nakagawa, S.; Ishihara, K. Direct ester condensation catalyzed by bulky diarylammonium pentafluorobenzenesulfonates. Nat. Protocol. 2007, 2, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, A.; Kawajiri, K.; Ohkubo, T.; Kosugi, Y.; Ishihara, K. Widely useful DMAP-catalyzed esterification under auxiliary base- and solvent-free conditions. J. Am. Chem. Soc. 2007, 129, 14775–14779. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.M.; Park, J.H.; Baek, J.H.; Hwang, R.H.; Jeon, S.G.; Yi, K.B. Effects of acid treatment of Fe-BEA zeolite on catalytic N2O conversion. Korean J. Chem. Eng. 2017, 34, 81–86. [Google Scholar] [CrossRef]
- Davoodpour, M.; Tafreshi, R.; Khodadadi, A.A.; Mortazavi, Y. Two-stage cracking catalyst of amorphous silica-alumina on Y zeolite for enhanced product selectivity and suppressed coking. Korean J. Chem. Eng. 2017, 34, 681–691. [Google Scholar] [CrossRef]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A Gen. 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Roboson, H.; Lillerud, K.P. Verified Synthesis of Zeolitic Materials, 2nd ed.; Elservier: Amsterdam, The Netherlands, 2001; pp. 225–227. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, J.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kadata, N.; Igi, H.; Kim, J.-H.; Niwa, M. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium. J. Phys. Chem. B 1997, 101, 5969–5977. [Google Scholar] [CrossRef]
- Niwa, M.; Kadata, N.; Sawa, M.; Murakami, Y. Temperature-programmed desorption of ammonia with readsorption based on the derived theoretical equation. J. Phys. Chem. B 1995, 99, 8812–8816. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
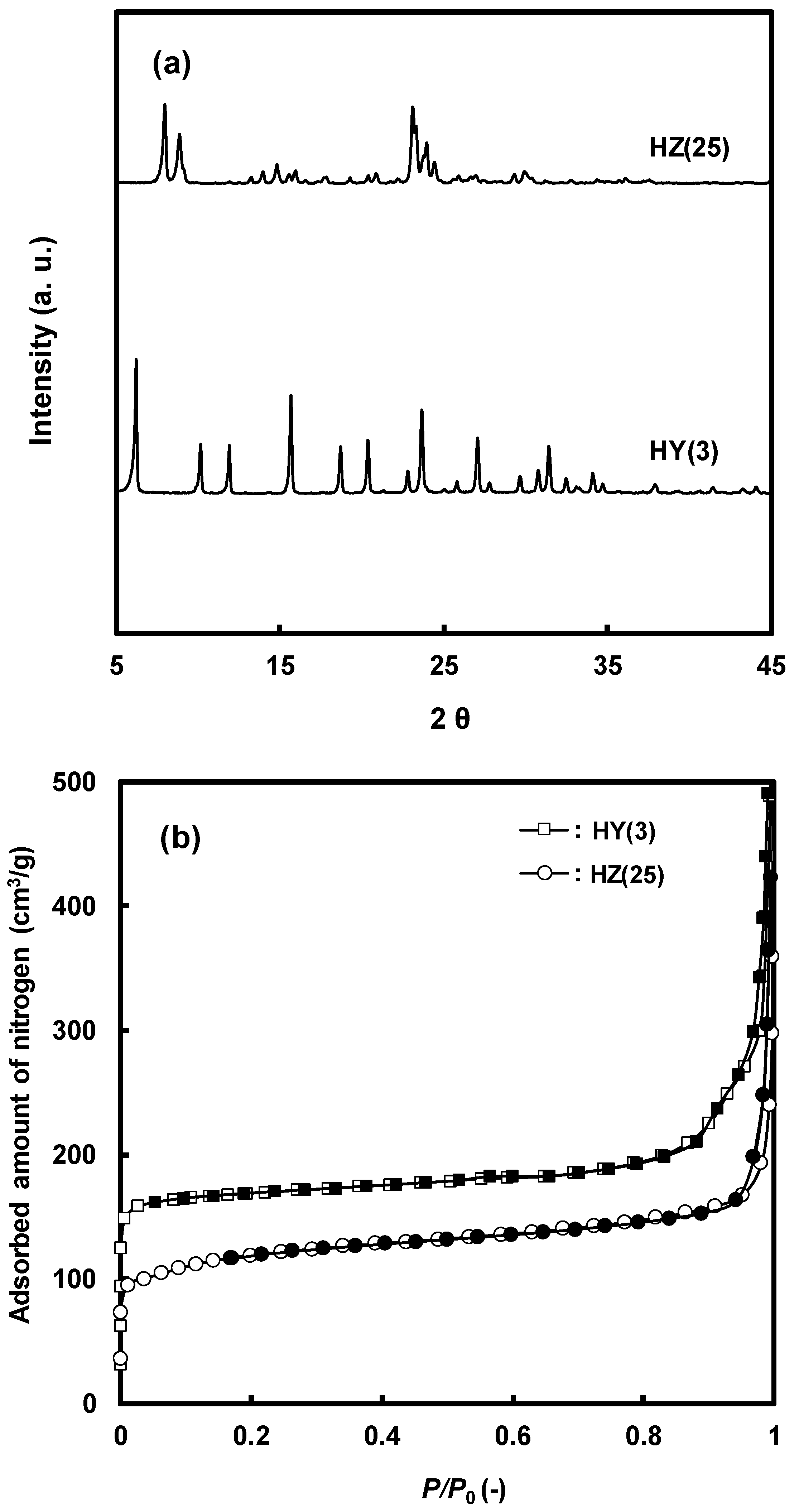
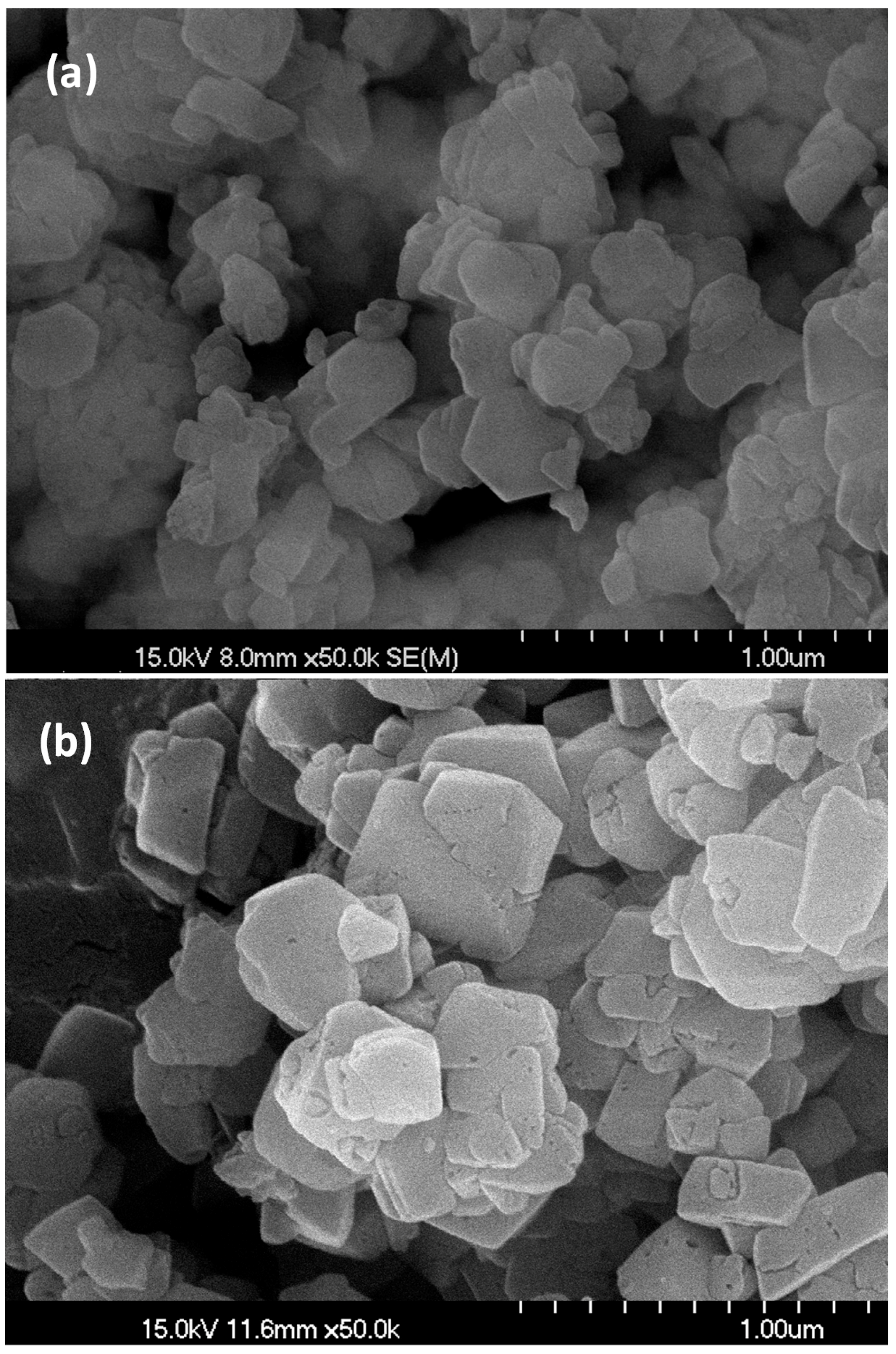
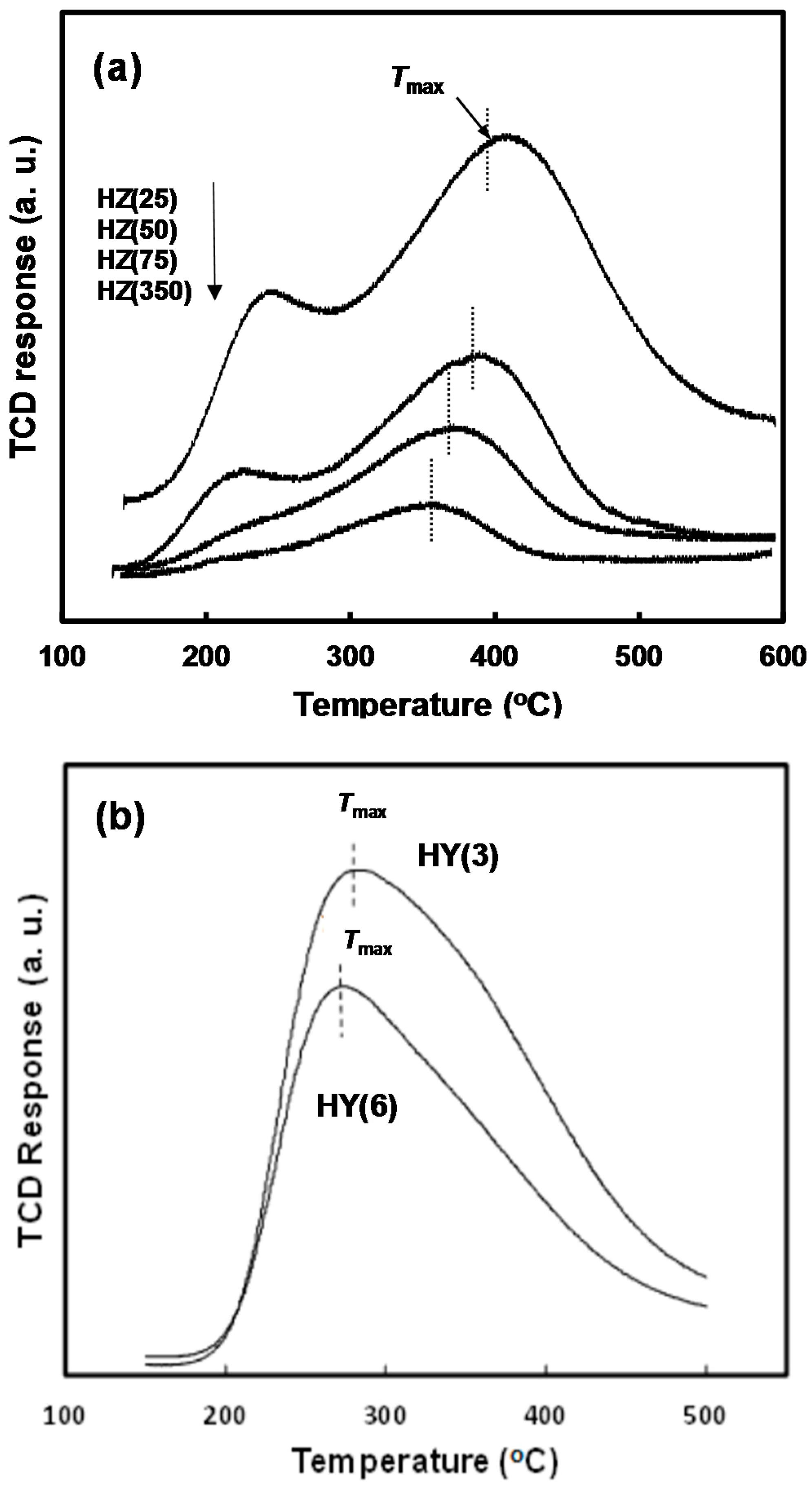
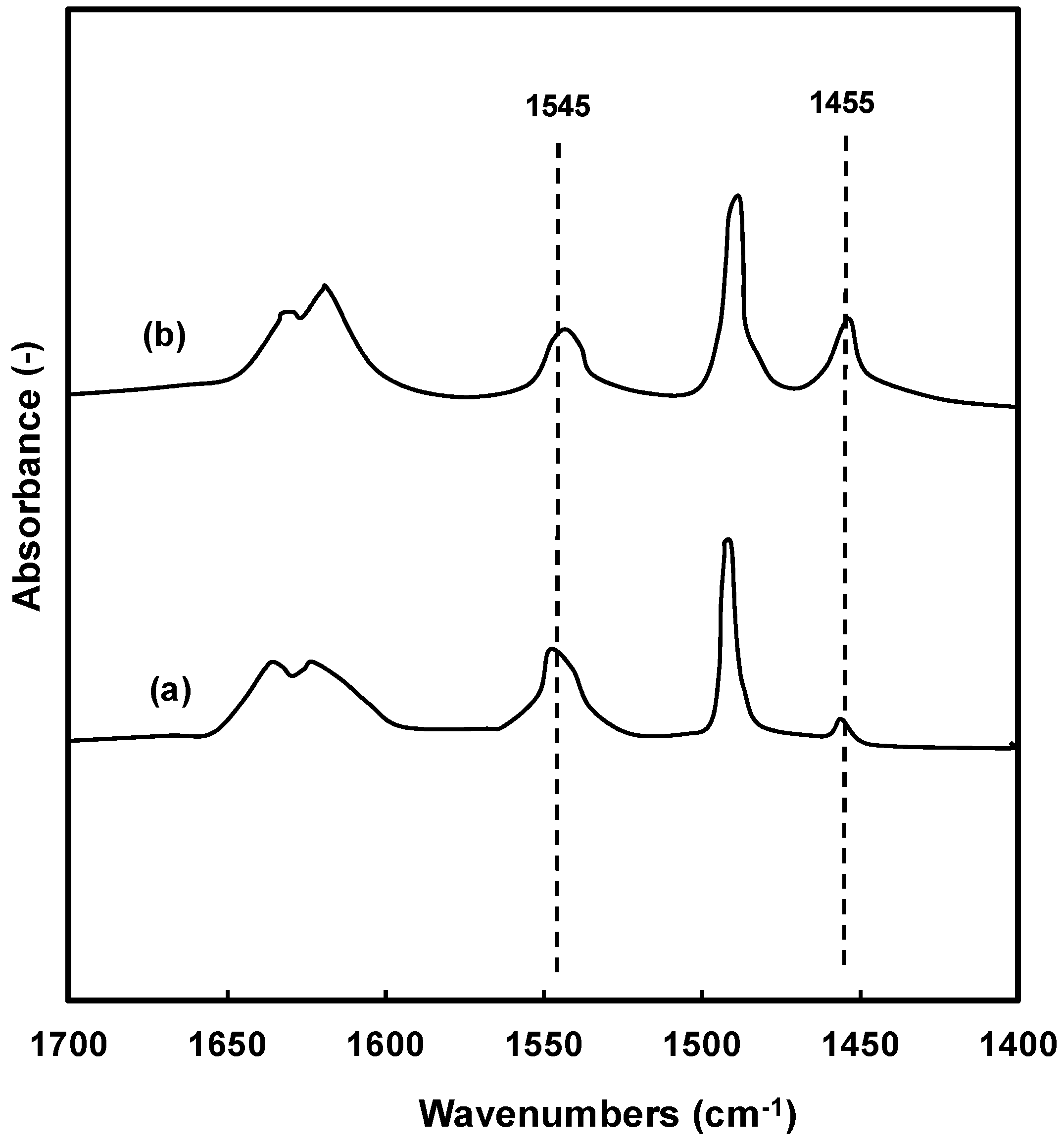

| Zeolite | Pore Topology | Pore Size (Å) | BET Surface Area (m2/g) | Acid Amount a (mmol/g) | Tmax b (°C) |
|---|---|---|---|---|---|
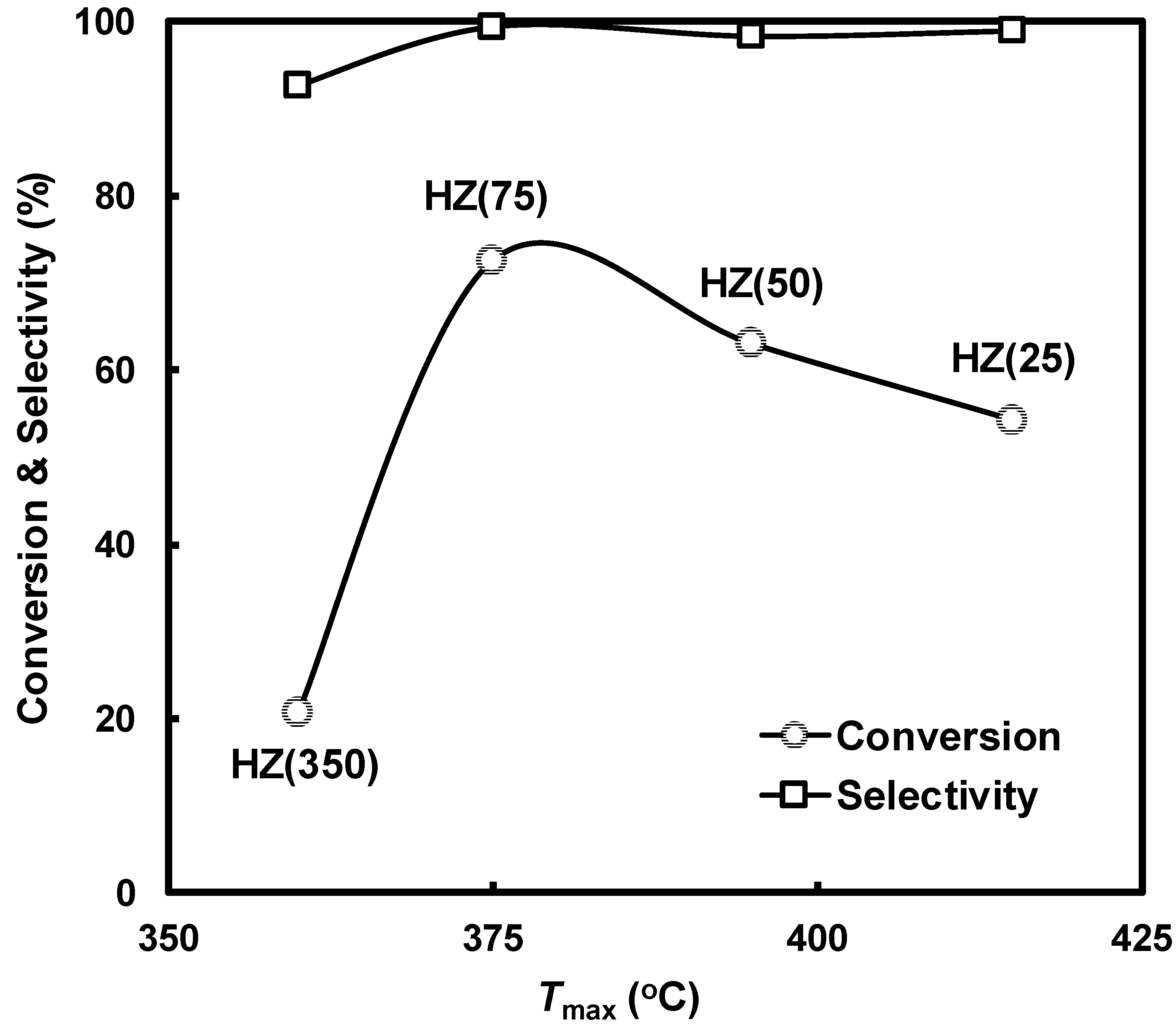
| HZ(25) | 10-oxygen ring | 5.1 × 5.6 [100] 6.3 × 5.6 [010] | 413 | 7.9 × 10−2 | 415 |
| HZ(50) | 405 | 4.5 × 10−2 | 395 | ||
| HZ(75) | 407 | 3.8 × 10−2 | 375 | ||
| HZ(350) | 418 | 0.5 × 10−2 | 360 | ||
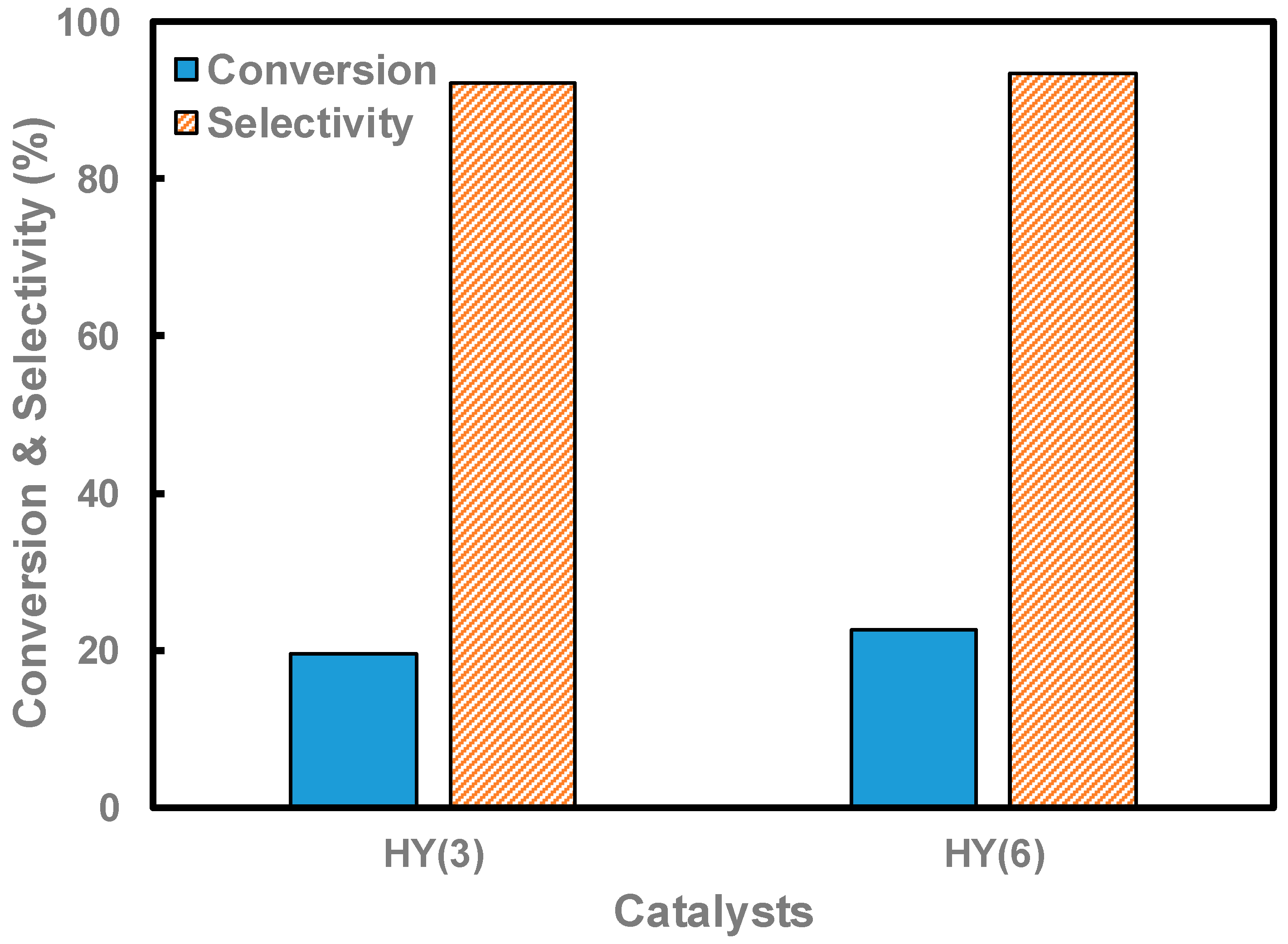
| HY(3) | 12-oxygen ring | 7.4 × 7.4 [111] | 574 | 9.1 × 10−2 | 300 |
| HY(6) | 571 | 8.5 × 10−2 | 280 |
| Catalysts | Conversion of TEC (%) | Selectivity of ATEC (%) |
|---|---|---|
| HZ(25) | 63.0 | 98.8 |
| HZ(50) | 70.7 | 98.9 |
| HZ(75) | 82.2 | 99.1 |
| HZ(350) | 31.7 | 95.4 |
| HY(3) | 19.6 | 92.3 |
| HY(6) | 22.7 | 93.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-H.; Jeong, S.; Kim, H.; Kim, S.-J.; Park, Y.-K.; Jung, S.-C. Highly Selective Catalytic Properties of HZSM-5 Zeolite in the Synthesis of Acetyl Triethyl Citrate by the Acetylation of Triethyl Citrate with Acetic Anhydride. Catalysts 2017, 7, 321. https://doi.org/10.3390/catal7110321
Chung K-H, Jeong S, Kim H, Kim S-J, Park Y-K, Jung S-C. Highly Selective Catalytic Properties of HZSM-5 Zeolite in the Synthesis of Acetyl Triethyl Citrate by the Acetylation of Triethyl Citrate with Acetic Anhydride. Catalysts. 2017; 7(11):321. https://doi.org/10.3390/catal7110321
Chicago/Turabian StyleChung, Kyong-Hwan, Sangmin Jeong, Hangun Kim, Sun-Jae Kim, Young-Kwon Park, and Sang-Chul Jung. 2017. "Highly Selective Catalytic Properties of HZSM-5 Zeolite in the Synthesis of Acetyl Triethyl Citrate by the Acetylation of Triethyl Citrate with Acetic Anhydride" Catalysts 7, no. 11: 321. https://doi.org/10.3390/catal7110321






