A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars
Abstract
:1. Introduction
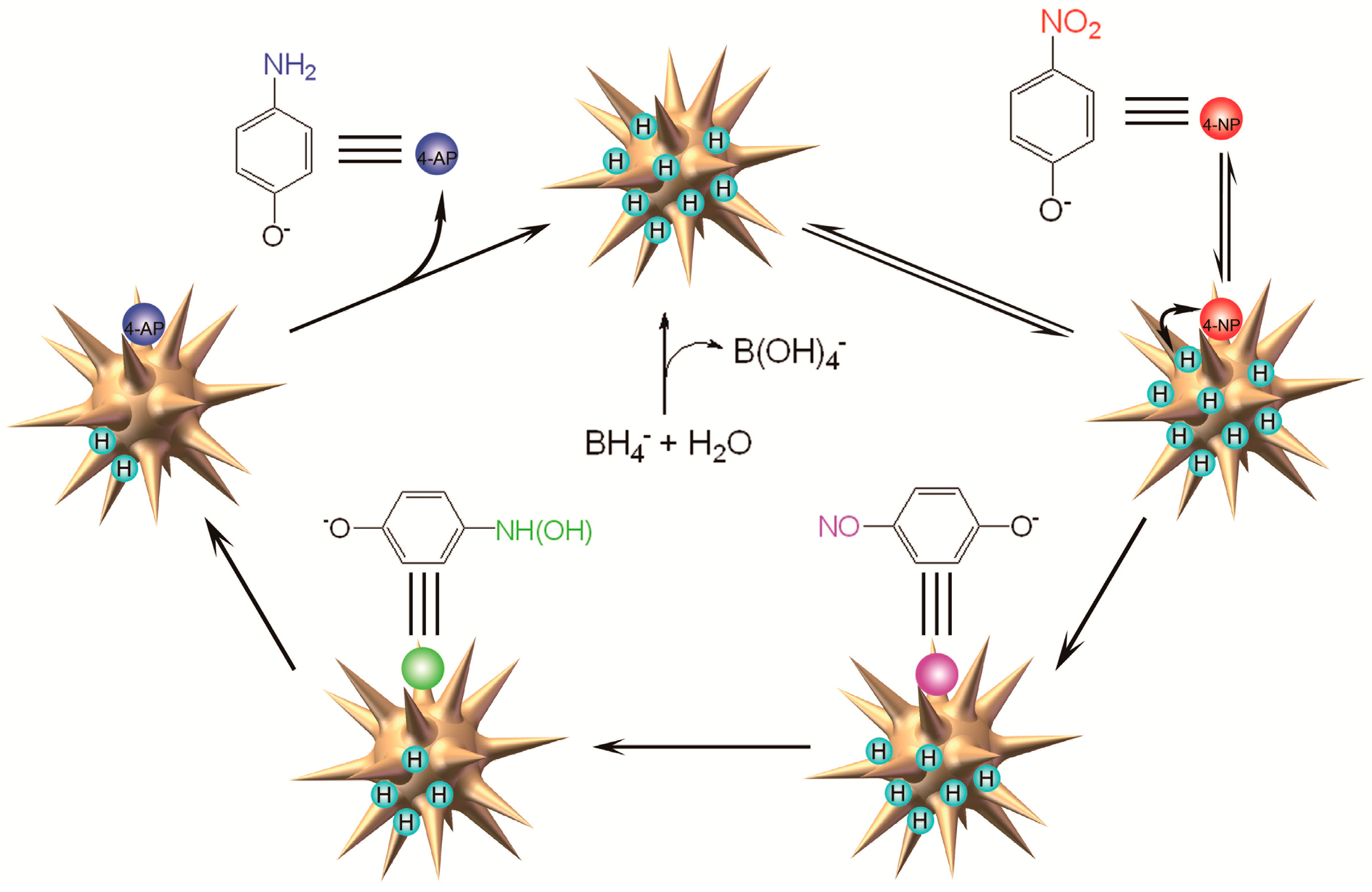
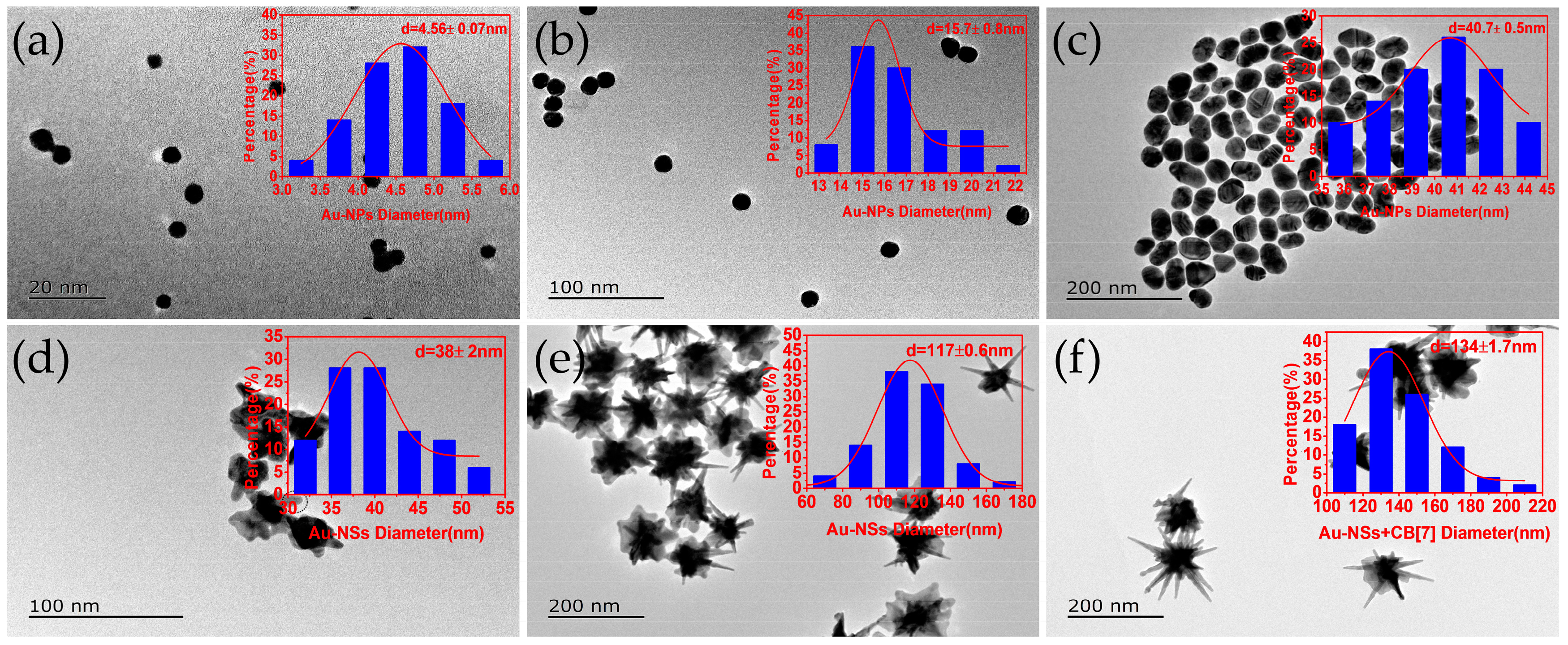
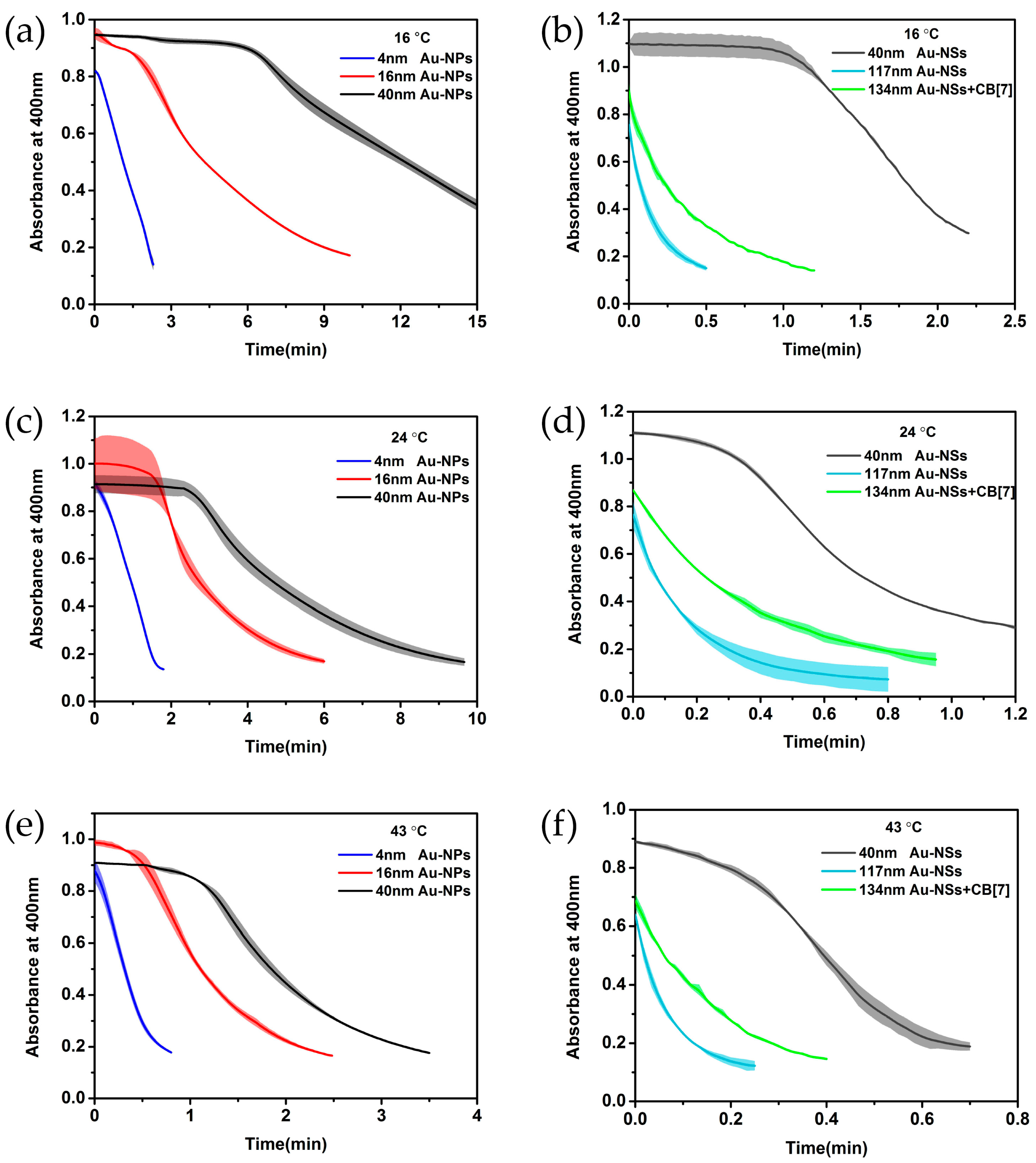
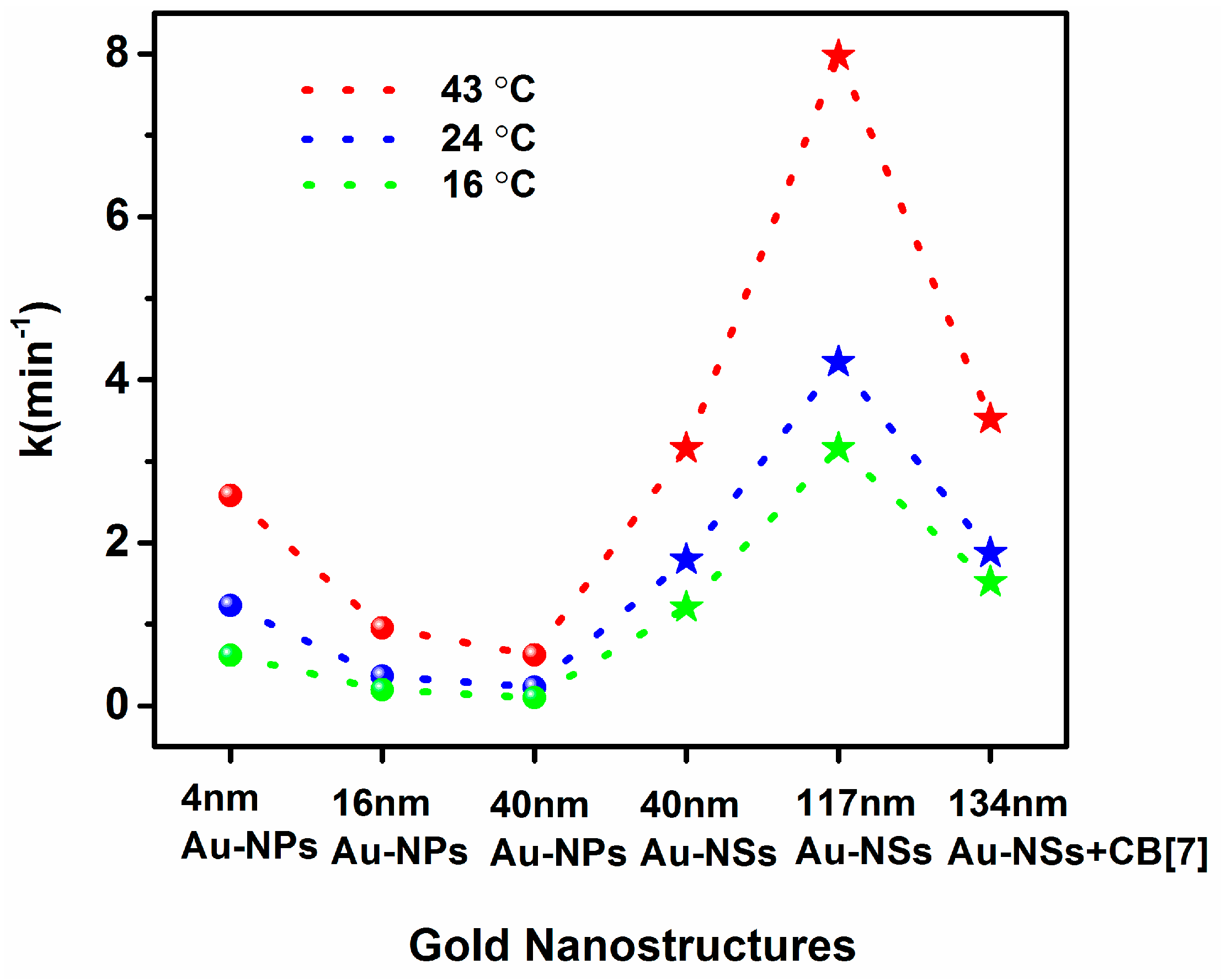
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 40 nm Au-NSs
3.2. Catalysis Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductorparticles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Bedford, E.E.; Boujday, S.; Pradierab, C.-M.; Gu, F.X. Nanostructured and spiky gold in biomolecule detection: Improving binding efficiencies and enhancing optical signals. RSC Adv. 2015, 5, 16461–16475. [Google Scholar] [CrossRef]
- Muench, F.; Rauber, M.; Stegmann, C.; Lauterbach, S.; Kunz, U.; Kleebe, H.-J.; Ensinger, W. Ligand-optimized electroless synthesis of silver nanotubes and their activity in the reduction of 4-nitrophenol. Nanotechnology 2011, 22, 415602. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-Y.; Mei, L.-P.; Chen, W.-Y.; Feng, J.-J.; Chen, J.-Y.; Wang, A.-J. Shaped-controlled electrosynthesis of gold nanodendrites for highlyselective and sensitive SERS detection of formaldehyde. Sens. Actuators B Chem. 2014, 201, 92–99. [Google Scholar] [CrossRef]
- De Oliveira, F.M.; de Araújo Nascimento, L.R.B.; Calado, C.M.S.; Meneghetti, M.R.; da Silva, M.G.A. Aqueous-phase catalytic chemical reduction of p-nitrophenol employing soluble gold nanoparticles with different shapes. Catalysts 2016, 6, 215. [Google Scholar] [CrossRef]
- Thompson, D.T. Catalytically highly active top gold atom on palladium nanocluster. Nano Today 2007, 2, 40–43. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; García, H. Catalytic activity of unsupported gold nanoparticles. Catal. Sci. Technol. 2013, 3, 58–69. [Google Scholar] [CrossRef]
- Scholder, P.; Hafner, M.; Hassel, A.W.; Nischang, I. Gold nanoparticle@polyhedraloligomericsilsesquioxane hybrid scaffolds in microfluidic format—Highly efficient and green catalytic platforms. Eur. J. Inorg. Chem. 2016, 7, 951–955. [Google Scholar] [CrossRef]
- Murzin, D.Y. Nanokinetics for nanocatalysis. Catal. Sci. Technol. 2011, 1, 380–384. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Narayanan, R.; El-Sayed, M.A. Enhancing colloidal metallic nanocatalysis: Sharp edges and corners for solid nanoparticles and cage effect for hollow ones. Acc. Chem. Res. 2013, 46, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Soetan, N.; Zarick, H.F.; Banks, C.; Webb, J.A.; Libson, G.; Coppola, A.; Bardhan, R. Morphology-directed catalysis with branched gold nanoantennas. J. Phys. Chem. C 2016, 120, 10320–10327. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Nanostars shine bright for you: Colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Pradhan, N.; Pal, A.; Pal, T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A 2002, 196, 247–257. [Google Scholar] [CrossRef]
- Esumi, K.; Miyamoto, K.; Yoshimura, T. Comparison of PAMAM–Au and PPI–Au nanocomposites and their catalytic activity for reduction of 4-Nitrophenol. J. Colloid Interface Sci. 2002, 254, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Eo, M.; Baek, J.; Song, H.D.; Lee, S.; Yi, J. Quantification of electron transfer rates of different facets on single gold nanoparticles during catalytic reactions. Chem. Commun. 2013, 49, 5204–5206. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Garlyyev, B.; El-Sayed, M.A. Controlling the catalytic efficiency on the surface of hollow gold nanoparticles by introducing an inner thin layer of Platinum or Palladium. J. Phys. Chem. Lett. 2014, 5, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Chen, B. A review on biomedical applications of single-walled carbon nanotubes. Curr. Med. Chem. 2010, 17, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, F. Application of graphene/grapheneoxide in biomedicine and biotechnology. Curr. Med. Chem. 2014, 21, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Chen, R. Emerging assay platforms based on nanostructures. Curr. Nanosci. 2015, 11, 684. [Google Scholar] [CrossRef]
- Liang, F.; Chen, R. Functional nanomaterials for emerging biological applications. Curr. Nanosci. 2016, 12, 404. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 2012, 11, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Toshima, N. Glucose oxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal. Sci. Technol. 2013, 3, 268–278. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Kawashima, K.; Okumura, M.; Haruta, M.; Toshima, N. Synthesis and catalytic activity of crown jewel-structured (IrPd)/Au trimetallicnanoclusters. Adv. Mater. 2015, 27, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, X.; Liu, Y.; Ai, Q.; Liu, S.; Sun, C.; Liang, F. Supramolecular controlled cargo release via near infrared tunable cucurbit[7]uril-gold nanostars. Sci. Rep. 2016, 6, 22239. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Su, L.; Wang, J.; Lu, X.; Liang, F.; Li, C.; Zhang, X. Value of the debris of reduction sculpture: Thioletching of Au nanoclusters for preparing water-soluble and aggregation-induced emission-active Au(I) complexes as phosphorescent copper ion sensor. Anal. Chem. 2016, 88, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5−40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Pérez-Juste, J.; García de Abajo, F.J.; Liz-Marzán, L.M. Seeded growth of submicron Au colloids with quadrupoleplasmonresonance modes. Langmuir 2006, 22, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Quaresma, P.; Soares, L.; Contar, L.; Miranda, A.; Osorio, I.; Carvalho, P.A.; Franco, R.; Pereira, E. Green photocatalytic synthesis of stable Au and Ag nanoparticles. Green Chem. 2009, 11, 1889–1893. [Google Scholar] [CrossRef]
- Nigra, M.M.; Ha, J.M.; Katz, A. Identification of site requirements for reduction of 4-nitrophenol using gold nanoparticle catalysts. Catal. Sci. Technol. 2013, 3, 2976–2983. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Q.; Chen, J.; Xia, Y. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 2010, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Xia, Y. Metal nanocrystals with highly branched morphologies. Angew. Chem. Int. Ed. 2011, 50, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Briggs, B.D.; Li, Y.; Swihart, M.T.; Knecht, M.R. Reductant and sequence effects on the morphology and catalytic activity of peptide-capped Au nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 8843–8851. [Google Scholar] [CrossRef] [PubMed]
- Copéret, C.; Estes, D.P.; Larmier, K.; Searles, K. Isolated surface hydrides: Formation, structure, and reactivity. Chem. Rev. 2016, 116, 8463–8505. [Google Scholar] [CrossRef] [PubMed]
- Juárez, J.; Cambón, A.; Goy-López, S.; Topete, A.; Taboada, P.; Mosquera, V. Obtention of metallic nanowires by protein biotemplating and their catalytic application. J. Phys. Chem. Lett. 2010, 1, 2680–2687. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yano, H.; Kouchi, H.; Obora, Y.; Arakawa, R.; Kawasaki, H. N,N-Dimethylformamide-stabilized gold nanoclusters as a catalyst for the reduction of 4-nitrophenol. Nanoscale 2012, 4, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticledecorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B 2013, 132, 107–115. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.C.; Song, H. A nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction of p-Nitrophenol. Adv. Mater. 2008, 20, 1523–1528. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.; Kong, W.; Huang, H.; Han, X.; Zhang, X.; Liu, Y.; Kang, Z. Adsorption dominant catalytic activity of a carbon dots stabilized gold nanoparticles system. Dalton Trans. 2014, 43, 10920–10929. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, Y.C.; Wang, Z.; Liu, G.; Zhou, W.; Wen, L.; Li, Q.; Wang, X.; Chen, X.; Zeng, J.; et al. Facile synthesis of pentacle gold–copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 4327. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Meng, F.; Qi, L. Facile Synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced ramanscattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Premkumar, T.; Geckeler, K.E. Cucurbit[7]uril as a tool in the green synthesis of gold nanoparticles. Chem. Asian J. 2010, 5, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-based molecular recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Han, G.; Li, M.; Zhao, S. Deposition of the fractal-like gold particles onto electrospun polymethylmethacrylate fibrous mats and their application in surface-enhanced Raman scattering. Thin Solid Films 2010, 518, 3228–3233. [Google Scholar] [CrossRef]
- Personick, M.L.; Langille, M.R.; Zhang, J.; Harris, N.; Schatz, G.C.; Mirkin, C.A. Synthesis and isolation of {110}-faceted gold bipyramids and rhombic dodecahedra. J. Am. Chem. Soc. 2011, 133, 6170–6173. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | 16 °C | 24 °C | 43 °C | Ea (kJ/mol) | |||
|---|---|---|---|---|---|---|---|
| kapp (min−1) | t0 (min) | kapp (min−1) | t0 (min) | kapp (min−1) | t0 (min) | ||
| 4 nm Au-NPs | 0.621 ± 0.007 | - a | 1.247 ± 0.031 | - | 2.590 ± 0.041 | - | 38.690 ± 7.408 |
| 16 nm Au-NPs | 0.198 ± 0.002 | 2.5 ± 0.3 | 0.363 ± 0.019 | 1.8 ± 0.2 | 0.950 ± 0.013 | 0.5 ± 0.05 | 43.566 ± 1.713 |
| 40 nm Au-NPs | 0.101 ± 0.013 | 6.4 ± 0.42 | 0.234 ± 0.026 | 2.8 ± 0.67 | 0.628 ± 0.040 | 1.1 ± 0.12 | 49.461 ± 7.54 |
| 40 nm Au-NSs | 1.207 ± 0.015 | 1.2 ± 0.17 | 1.776 ± 0.080 | 0.32 ± 0.27 | 3.134 ± 0.107 | 0.15 ± 0.05 | 26.448 ± 0.557 |
| 117 nm Au-NSs | 3.145 ± 0.040 | - | 4.364 ± 0.152 | - | 7.968 ± 0.325 | - | 26.093 ± 0.111 |
| 134 nm Au-NSs + CB[7] | 1.517 ± 0.152 | - | 1.850 ± 0.073 | - | 3.526 ± 0.100 | - | 24.042 ± 1.621 |
| Catalyst (mg) a | Particle Size (nm) | NaBH4/4-NP/Au (mole ratio) | kapp (min−1) | Ref. |
|---|---|---|---|---|
| Au-nanocages/0.08 mg | 50 | 1035/3.45/1 | 2.83 | [33] |
| Au-nanoboxes/0.08 mg | 50 | 1035/3.45/1 | 1.12 | [33] |
| Partially hollow Au-nanoboxes/0.08 mg | 50 | 1035/3.45/1 | 0.59 | [33] |
| Au-solid nanoparticles/0.08 mg | 50 | 1035/3.45/1 | 0.20 | [33] |
| Au-solid nanoparticles /0.08 mg | 5 | 1035/3.45/1 | 0.95 | [33] |
| [Au]/[protein]/0.00532 mg | - b | 18,518/0.463/1 | 0.39 | [37] |
| Dimethylformamide-stabilized Au-NCs | <2 | 200,000/100/1 | 0.18 | [38] |
| Au-NPs/CeO2/10 mg | - | 462/11/1 | 0.13 | [39] |
| Au-NPs@SiO2 | 104 | 750/2.1/1 | 0.84 | [40] |
| Au-NPs-Carbon Dots | 4 | 10.56/0.192/1 | 0.68 | [41] |
| Au-Cu alloy nanocrystals/0.1 mg | 45 | - | 2.56 | [42] |
| Au-Cu nanorod networks/0.1 mg | 100 | - | 0.07 | [42] |
| Au-Cu nanopolyhedrons/0.1 mg | 50 | - | 0.04 | [42] |
| Au nanocrystals/0.1 mg | 5 | - | 1.98 | [42] |
| α-Cyclodextrin-capped Au nanoparticles/0.0118 mg | 11 | 250/5.67/1 | 0.28 | [43] |
| α-Cyclodextrin-capped Au nanoparticles/0.0118 mg | 20 | 250/5.67/1 | 0.21 | [43] |
| α-Cyclodextrin-capped Au nanoparticles/0.0118 mg | 26 | 250/5.67/1 | 0.18 | [43] |
| Calix[6]arene phosphine-modified Au nanoparticles/0.03 mg | 4 | 360/2/1 | 4.3 | [32] |
| Calix[4]arenethiol-modified Au nanoparticles/0.03 mg | 4 | 360/2/1 | 0.92 | [32] |
| CB[7]-protected AuNPs/0.3 mg | 10 | 10/0.23/1 | 0.16 | [44] |
| Au-NPs/0.0056 mg | 4 | 516/1.72/1 | 1.25 | c |
| Au-NPs/0.0056 mg | 16 | 516/1.72/1 | 0.36 | c |
| Au-NPs/0.0056 mg | 40 | 516/1.72/1 | 0.23 | c |
| Au-NSs/0.0056 mg | 40 | 516/1.72/1 | 1.78 | c |
| Au-NSs/0.0056 mg | 117 | 516/1.72/1 | 4.36 | c |
| Au-NPs + CB[7]/0.0056 mg | 134 | 516/1.72/1 | 1.85 | c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars. Catalysts 2017, 7, 38. https://doi.org/10.3390/catal7020038
Ma T, Yang W, Liu S, Zhang H, Liang F. A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars. Catalysts. 2017; 7(2):38. https://doi.org/10.3390/catal7020038
Chicago/Turabian StyleMa, Tao, Wenshuo Yang, Simin Liu, Haijun Zhang, and Feng Liang. 2017. "A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars" Catalysts 7, no. 2: 38. https://doi.org/10.3390/catal7020038






