Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation
Abstract
:1. Introduction
2. Results and Discussion
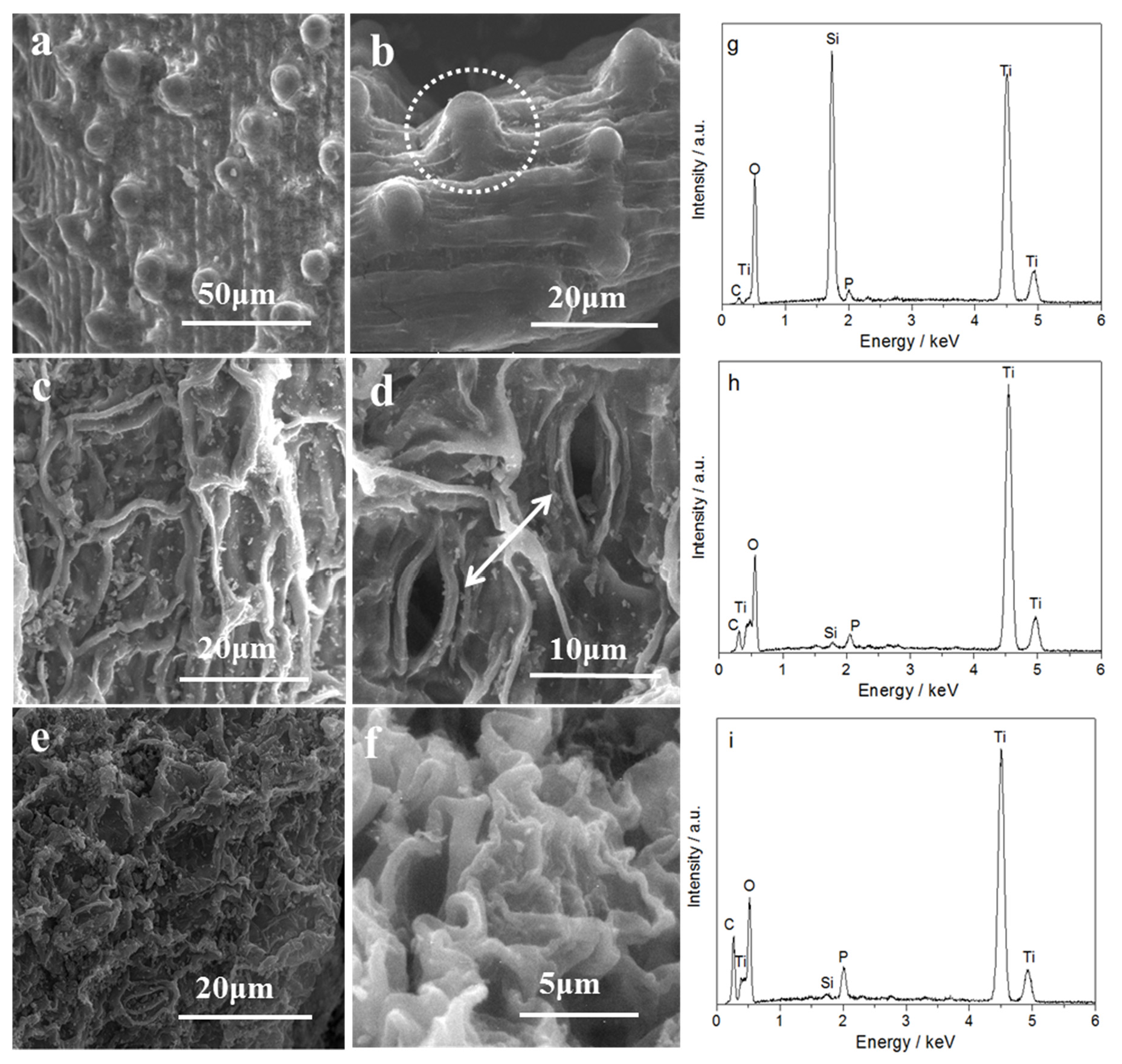
2.1. Components, Structures, and Morphologies of TiO2/SiO2 Photocatalysts
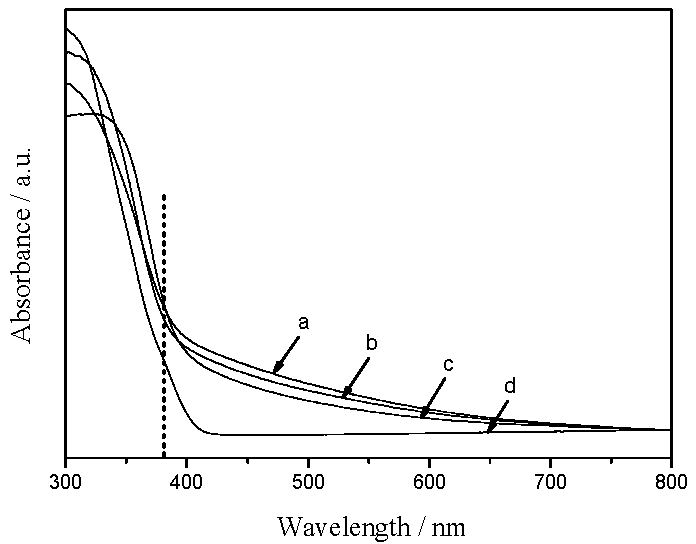
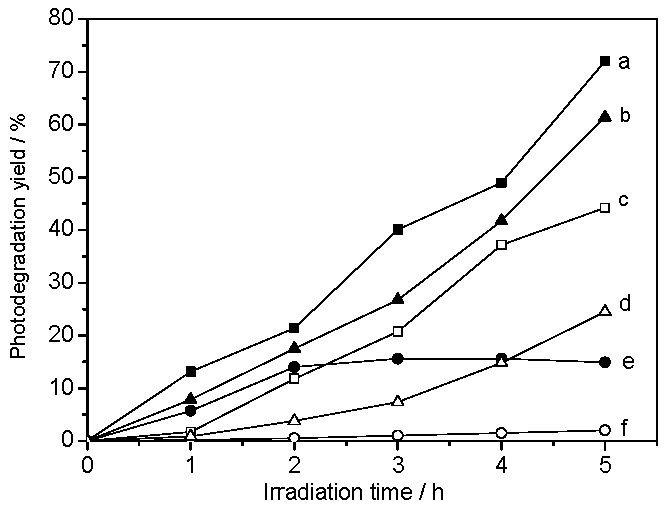
2.2. Studies on Light-Harvesting and Photocatalytic Activities of TiO2/SiO2 Photocatalysts
3. Materials and Methods
3.1. Synthesis
3.2. Characterizations
3.3. Catalytic Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kubacka, A.; Fernandez-Garcia, M. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 155–1614. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zhao, Y. Directed synthesis of hierarchical nanostructured TiO2 catalysts and their morphology-dependent photocatalysis for phenol degradation. Environ. Sci. Technol. 2008, 42, 2342–2348. [Google Scholar] [CrossRef]
- Yu, J.G.; Su, Y.R.; Cheng, B. Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Funct. Mater. 2007, 62, 35–50. [Google Scholar] [CrossRef]
- Todorova, N.; Vaimakis, T.; Petrakis, D.; Hishita, S.; Boukos, N. N and N, S-doped TiO2 photocatalysts and their activity in NOx oxidation. Catal. Today 2013, 209, 41–46. [Google Scholar]
- Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Jafry, H.R.; Liga, M.V.; Li, Q.; Barron, A.R. Simple route to enhanced photocatalytic activity of P25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2011, 45, 1563–1568. [Google Scholar] [CrossRef]
- Jafry, H.R.; Liga, M.V.; Li, Q.; Barron, A.R. Single walled carbon nanotubes (SWNTs) as templates for the growth of TiO2: The effect of silicon in coverage and the positive and negative synergies for the photocatalytic degradation of Congo red dye. New J. Chem. 2011, 35, 400–406. [Google Scholar] [CrossRef]
- Zhang, C.; Mcadams, D.A., II; Grunlan, J.C. Nano/micro-manufacturing of bioinspired materials: A review of methods to mimic natural structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar]
- Han, T.; Fan, T.X.; Chow, S.K.; Zhang, D. Biogenic N-P-codoped TiO2: Synthesis, characterization and photocatalytic properties. Bioresour. Technol. 2010, 101, 6829–6835. [Google Scholar] [CrossRef]
- Li, X.; Fan, T.; Zhou, H.; Chow, S.K.; Zhang, W.; Zhang, D.; Guo, Q.; Ogawa, H. Enhanced light-harvesting and photocatalytic properties in morph-TiO2 from green-leaf biotemplates. Adv. Funct. Mater. 2009, 19, 45–56. [Google Scholar] [CrossRef]
- Dorian, A.H.H.; Sorrell, C.C. Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar]
- Kibombo, H.S.; Zhao, D.; Gonshorowski, A.; Budhi, S.; Koppang, M.D.; Koodali, R.T. Cosolvent-induced gelation and the hydrothermal enhancement of the crystallinity of titania-silica mixed oxides for the photocatalytic remediation of organic pollutants. J. Phys. Chem. C 2011, 115, 6126–6135. [Google Scholar] [CrossRef]
- Yang, D.L.; Fan, T.X.; Zhou, H.; Ding, J.; Zhang, D. Biogenic hierarchical TiO2/SiO2 derived from rice husk and enhanced photocatalytic properties for dye degradation. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, W.W.; Zhu, S.W.; Ma, Q.; Fan, Y.S.; Cheng, B.J. Biotemplated hierarchical TiO2-SiO2 composites derived from Zea mays Linn. for efficient dye photodegradation. J. Porous Mater. 2013, 20, 1205–1215. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Yang, W.; Li, M.; Song, Y. Aquatic plant inspired hierarchical artificial leaves for highly efficient photocatalysis. J. Mater. Chem. 2013, 1, 7760–7766. [Google Scholar] [CrossRef]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A.; Mackenzie, K.J.D. Effect of silica additive on the anatase-to-rutile phase transition. J. Am. Ceram. Soc. 2004, 84, 1591–1596. [Google Scholar] [CrossRef]
- Dirken, P.J.; Smith, M.E.; Whitfield, H.J. 17O and 29Si solid state NMR study of atomic scale structure in sol-gel-prepared TiO2-SiO2 materials. J. Phys. Chem. 1995, 99, 395–401. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Liu, S.; Xing, Y.; Xie, K. Silica-titania mixed oxides: Si–O–Ti connectivity, coordination of titanium, and surface acidic properties. Catal. Lett. 2008, 124, 185–194. [Google Scholar] [CrossRef]
- Li, Y.Z.; Kim, S.J. Synthesis and characterization of nano titania particles embedded in mesoporous silica with both high photocatalytic activity and adsorption capability. J. Phys. Chem. B 2005, 109, 12309–12315. [Google Scholar] [CrossRef] [PubMed]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Yu, X.X.; Yu, J.G.; Cheng, B.; Jaroniec, M. Synthesis of hierarchical flower-like AlOOH and TiO2/AlOOH superstructures and their enhanced photocatalytic properties. J. Phys. Chem. C 2009, 113, 17527–17535. [Google Scholar] [CrossRef]
- Poulson, M.E.; Vogelmann, T.C. Epidermal focussing and effects upon photosynthetic light-harvesting in leaves of Oxalis. Plant Cell Environ. 1990, 13, 803–811. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Fan, T.; Osterloh, F.E.; Ding, J.; Sabio, E.M.; Zhang, D.; Guo, Q. Artificial inorganic leafs for efficient photochemical hydrogen production inspired by natural photosynthesis. Adv. Mater. 2010, 22, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Walendziewski, J.; Robert, D.; Weber, J.V.; Stolarski, M. The study of photocatalytic activities of titania and titania silica aerogels. Appl. Catal. B 2003, 46, 441–451. [Google Scholar] [CrossRef]
- Chen, X.F.; Wang, X.C.; Fu, X.Z. Hierarchical macro/mesoporous TiO2/SiO2 and TiO2/ZrO2 nanocomposites for environmental photocatalysis. Energy Environ. Sci. 2009, 2, 872–877. [Google Scholar] [CrossRef]
- Dong, W.Y.; Lee, C.W.; Lu, X.C.; Sun, Y.J.; Hua, W.M.; Zhuang, G.S.; Zhang, S.C.; Chen, J.M.; Hou, H.Q.; Zhao, D.Y. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2-SiO2 nanocomposites generating excellent degradation activity of RhB dye. Appl. Catal. B 2009, 95, 197–207. [Google Scholar] [CrossRef]
- Tao, J.; Gong, W.; Yan, Z.; Duan, D.; Zeng, Y.; Wang, J. UV/visible-light photodegradation for organic dyes over mesoporous titania prepared by using 2,4,5-triphenylimidazole as template. Mater. Sci. Semicond. Process. 2014, 27, 452–460. [Google Scholar] [CrossRef]




| Samples | Ti a (%) | O a (%) | C a (%) | Si a (%) | dXRD b (nm) | SBET c (m2·g−1) |
|---|---|---|---|---|---|---|
| R-TiO2/SiO2 | 27.14 | 57.20 | - | 15.66 | 4.2 | 235 |
| WH-TiO2/SiO2 | 45.26 | 50.52 | - | 4.22 | 7.7 | 127 |
| D-TiO2/SiO2 | 44.84 | 50.53 | 3.98 | 0.65 | 3.5 | 139 |
| Samples | Eg (eV) |
|---|---|
| P25 | 3.16 |
| R-TiO2/SiO2 | 3.25 |
| WH-TiO2/SiO2 | 3.22 |
| D-TiO2/SiO2 | 3.21 |
| Samples | Adsorption Yields (%) | Degradation Yields (%) |
|---|---|---|
| R-TiO2/SiO2 | 62.4 | 72.0 |
| WH-TiO2/SiO2 | 51.5 | 61.4 |
| D-TiO2/SiO2 | 43.0 | 11.7 |
| P25 | 7.4 | 42.4 |
| TiO2-p | 20.6 | 24.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; He, J.; Guo, L.; Li, Y.; Duan, D.; Chen, Y.; Li, J.; Yuan, F.; Wang , J. Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation. Catalysts 2017, 7, 82. https://doi.org/10.3390/catal7030082
Yan Z, He J, Guo L, Li Y, Duan D, Chen Y, Li J, Yuan F, Wang J. Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation. Catalysts. 2017; 7(3):82. https://doi.org/10.3390/catal7030082
Chicago/Turabian StyleYan, Zhiying, Jiao He, Lei Guo, Yueting Li, Deliang Duan, Yongjuan Chen, Junjie Li, Fagui Yuan, and Jiaqiang Wang . 2017. "Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation" Catalysts 7, no. 3: 82. https://doi.org/10.3390/catal7030082
APA StyleYan, Z., He, J., Guo, L., Li, Y., Duan, D., Chen, Y., Li, J., Yuan, F., & Wang , J. (2017). Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation. Catalysts, 7(3), 82. https://doi.org/10.3390/catal7030082






