3.1. Catalytic Cracking of Vegetable Oil
Table 4 shows recent progresses in the catalytic cracking of vegetable oil to hydrocarbon biofuel. The typical reaction temperature for catalytic cracking of vegetable oil ranged between 380 °C and 525 °C. During preliminary catalytic cracking experiments, vegetable oil was difficult to crack at a low reaction temperature. However, the yield of liquid product was low due to an increase in the formation of gases if the reaction temperature was too high [
8,
70]. During the catalytic cracking of bio-oil over HZSM-5 catalysts, 450 °C was considered as a suitable reaction temperature for maximizing the biofuel yield and minimizing the char/coke yields [
8]. The catalytic cracking of vegetable oil could be carried out at different pressures ranging from 1 atm to 99 atm. It seems a high pressure (e.g., 20–99 atm) could lower the reaction temperature when catalytically cracking sunflower oil to diesel oil-like hydrocarbons [
10]. In addition to reaction temperature and pressure, space velocity and catalyst-to-oil ratio are significant factors for upgrading vegetable oil. Operating conditions and feedstock composition can affect the catalyst deactivation [
71]. The most common reactor used for the catalytic cracking of vegetable oil is a fixed-bed reactor. It is good for continuous vegetable oil feeding. Reactor configuration is significant in the mass transfer of vegetable oil feed and reaction rates. Clogging, entrainment and channeling are major issues in reactors due to irregular distribution of materials [
72]. Shape selective zeolite-based catalysts and metal oxides were used for catalytic cracking of vegetable oil. The cracking and aromatization reactions are usually catalyzed by Brønsted acid sites of zeolites [
73,
74]. Zeolites contain acid sites, and have high surface area and adsorption capacity [
75]. However, some zeolite based catalysts are plagued by catalyst deactivation, short lifetime, and product contamination.
During the catalytic cracking of vegetable oil, several reactions including decarbonylation, decarboxylation, and deoxidation took place to remove the oxygen atoms from the triglyceride molecules [
21]. The hydrocarbon biofuels produced by the catalytic cracking of vegetable oil included organic liquid product, biogasoline, and diesel oil-like hydrocarbons. Similar to bio-oil upgrading, the liquid/coke yields during the catalytic cracking of vegetable oil were highly dependent on the catalyst species and operation conditions [
19]. It was reported that the catalytic cracking of vegetable oil yielded more total hydrocarbon liquid product when compared to catalytic cracking of bio-oil [
8]. Catalytic cracking of bio-oil can be divided into traditional catalytic cracking and a combination of catalytic pyrolysis and catalytic cracking. The combination of catalytic pyrolysis and catalytic cracking refers to a biomass pyrolysis reactor for bio-oil production followed by a subsequent cracking reactor for converting bio-oil to biofuel. It was demonstrated that the combination of catalytic pyrolysis and catalytic cracking technology was superior for improving the biofuel yield and quality compared to traditional catalytic cracking technology [
78].
Table 5 shows the conversion efficiency and mass balance of vegetable oil based feedstock upgrading over various catalysts. Dupain et al. [
79] studied the catalytic cracking of
rapeseed oil over FCC catalyst and obtained at 93% of high conversion of
rapeseed oil. In our previous study on the catalytic cracking of
carinata oil over Zn/Na-ZSM-5 catalyst, the mass balance was 95–96 wt % [
32]. Twaiq et al. [
80] investigated the liquid fuel production from the catalytic cracking of palm oil over zeolite catalysts. Stainless steel reactor was used with a space velocity of 2.5 h
−1. The palm oil conversion obtained over HZSM-5 and MCM-41 was 94%–97%, and 85%–93%, respectively. Nam et al. [
81] studied the biofuel production through the catalytic cracking of vegetable oil sludge over MC-ZSM-5/MCM-41 catalyst in a micro-activity test system. The conversion rate of the vegetable oil sludge was 85%–93%.
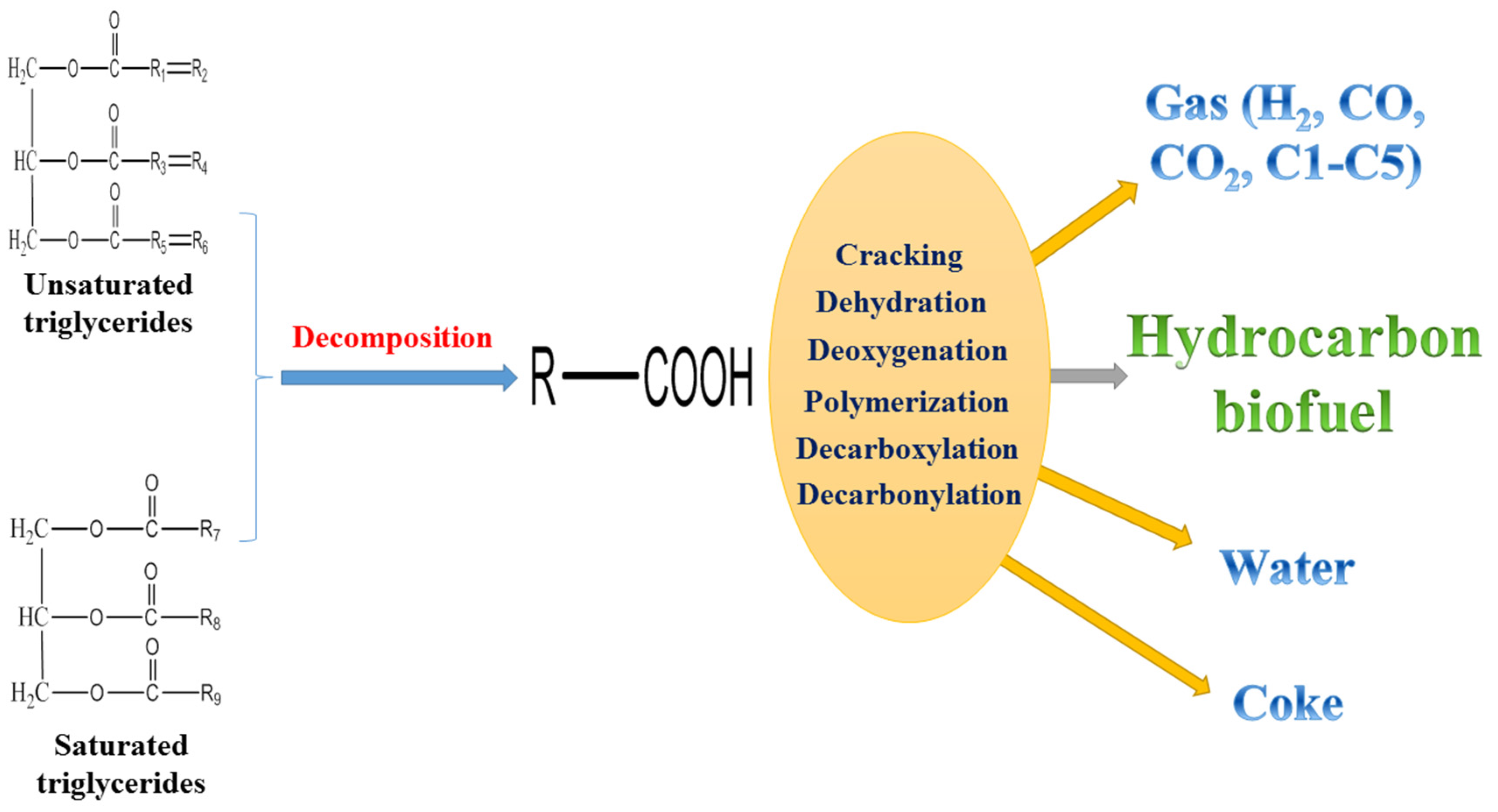
Figure 1 shows a proposed reaction pathway for the catalytic cracking of vegetable oil to hydrocarbon biofuel. Firstly, the unsaturated triglycerides and saturated triglycerides are decomposed to form fatty acids through either a carbonium ion or free-radical mechanism. The decomposition is a relatively fast process depending on the reaction temperature. Secondly, some of the fatty acids are dehydrated to form water. The formation of water required hydrogen atoms, which were donated by the hydrocarbon chains of fatty acids. The gases including H
2, CO, CO
2 and light hydrocarbons (C1–C5) were produced through deoxygenation, dehydrogenation, decarboxylation and decarbonylation. During the catalytic cracking of bio-oil, the formation of CO, CO
2 and H
2O were derived from these oxygen removal reactions [
19]. The hydrocarbon biofuel was obtained through the cracking and deoxygenation reactions. The cracking might take place on the external surface and/or within the internal pore structure of catalysts to produce hydrocarbons and oxygenates. Thirdly, some of the hydrocarbons produced form coke through polymerization. In addition, the direct condensation of vegetable oil forms coke [
21,
79,
89,
90]. The reactions during catalytic cracking of bio-oil include hydrogen transfer, C–C bond cleavage, dehydration, isomerization, decarbonylation and decarboxylation [
91]. The main products of catalytic cracking of bio-oil over zeolite based catalysts were liquid oil, water, coke and gases [
8]. Coke presents a serious challenge for both the catalytic cracking of vegetable oil and bio-oil since it could deactivate the catalysts. Loading metals to zeolites that tune the catalyst selectivity, co-feeding of hydrogen-rich feedstocks, and optimizing the upgrading operation conditions would be helpful for reducing the coke formation [
19]. A certain amount of gas product is also a challenge for the catalytic cracking of vegetable oil to liquid biofuel. Modifying catalysts, optimizing upgrading operation conditions and recycling gases (e.g., tri-reforming of gases to syngas for Fischer–Tropsch synthesis) might be beneficial.
3.2. Hydroprocessing of Vegetable Oil
Table 6 shows the recent progresses in hydroprocessing of vegetable oil to produce hydrocarbon biofuel. Hydrogenation and other reactions crack complicated organic molecules to simpler molecules during the hydroprocessing process [
75]. The typical reaction temperature for hydroprocessing of vegetable oil was between 300 °C and 390 °C. Hydroprocessing of soybean oil was studied over NiMo/ZSM-5 based catalysts at a high reaction temperature of 450 °C [
92]. The reaction temperature used for the hydroprocessing of bio-oil was usually 250–450 °C [
19]. Temperature is a significant factor for the hydroprocessing of vegetable oil since high temperature favors coke formation [
71]. The hydroprocessing of vegetable oil was usually carried out at high pressures, between 19 atm and 79 atm. The pressure used for the hydroprocessing of bio-oil was usually 74–296 atm [
19]. High pressure of H
2 may help slow the coke formation rate [
71]. The catalysts used for the hydroprocessing of vegetable oil were mainly Al
2O
3-based catalysts, which might be due to the high surface area and acidity of Al
2O
3 [
27,
93]. In addition, some zeolites have been investigated for the vegetable oil cracking. The larger mesopores of mesoporous zeolites (e.g., Ni/mesoporous-Y and Ni/mesoporous-HZSM-5) may facilitate the transport of bulky vegetable oil molecules to catalyst active sites [
94]. The vegetable oil upgrading conditions have an effect on catalyst stability and performance [
71]. Hydroprocessing could be used to remove oxygen, sulfur, nitrogen, and metals from vegetable oil and bio-oil. The high oxygen content of many oxygenates, such as acids, esters and phenols, in bio-oil could be removed largely through the introduction of H
2. During the hydroprocessing of vegetable oil, several main reactions including cracking, hydrogenation, decarbonylation, decarboxylation, and hydrodeoxygenation took place to convert the triglyceride molecules to hydrocarbons. Similar reactions occurred during the hydroprocessing of bio-oil. The deoxygenation during the hydroprocessing can result in increased hydrocarbon content [
30]. Vegetable oil can become more saturated through introducing H
2 [
19,
31,
89]. However, minimizing the amount of H
2 is a goal for reducing cost [
71]. The hydrocarbon biofuels produced from the hydroprocessing of vegetable oil mainly include organic product, green diesel, jet fuel and biohydrogenated diesel. The hydrocarbon biofuel yield obtained from the hydroprocessing of vegetable oil can be as high as 94 wt %, and the coke yield was very low or even negligible. The liquid product yield obtained from the hydroprocessing of bio-oil can also be very high, reaching 99 wt % [
19]. Solvent (e.g., methanol or ethanol) plays a significant role in the hydroprocessing of bio-oil since a suitable solvent addition can help resolve the bio-oil feeding issue and improve the overall reaction efficiency [
6]. In our previous study on the hydroprocessing of
carinata oil, Mo-Zn/Al
2O
3 was used as the catalyst. The conversion rate of the
carinata oil was 93%–94% [
31]. Toba et al. [
84] studied the hydrodeoxygenation of waste vegetable oil over sulfide catalysts. An autoclave reactor was used and the conversion rate of waste vegetable oil was 99%. The hydrotreatment of vegetable oil over the Pt/zeolite catalyst was investigated by Wang et al. [
88]. A fixed-bed reactor was utilized with a space velocity of 1 h
−1. The conversion rate of the vegetable oil was 100%. Hydrotreatment of vegetable oil over Ni-Mo based catalysts was studied by Liu et al. [
36]. A fixed-bed reactor was used with a space velocity of 7.6 h
−1. The mass balance was higher than 95% and the vegetable oil conversion rate was 100%.
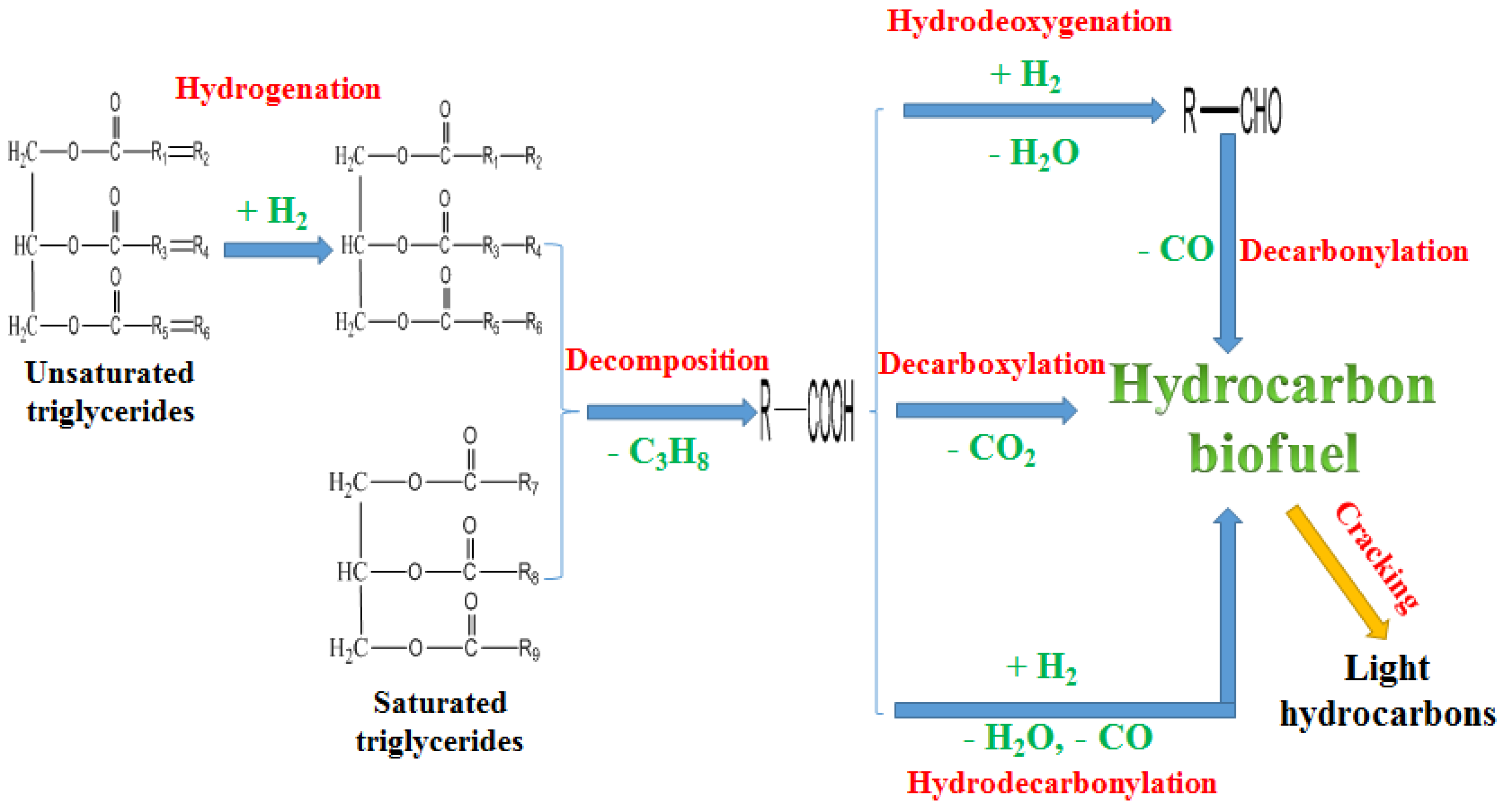
Figure 2 shows the proposed main reactions for hydroprocessing of vegetable oil over a Al
2O
3 based catalyst. The double bonds in unsaturated vegetable oil triglycerides were hydrogenated to saturated triglycerides. The saturated vegetable oil triglycerides were then cleaved to fatty acids. Some of the fatty acids formed oxidants including aldehydes, alcohols, and ketones through the hydrodeoxygenation [
31]. Removing oxygen is the first step for hydroprocessed renewable fuel production. After that, a mixture of straight chain, branched chain, and cyclic paraffinic hydrocarbons are obtained [
63]. Some of the fatty acids yielded CO
2 and hydrocarbons through decarboxylation. Some of the fatty acids produced CO, H
2O and hydrocarbons through hydro-decarbonylation. Some of the hydrocarbons obtained were cracked to light hydrocarbons (C1–C6) [
29,
97,
98]. During the hydroprocessing of bio-oil, multiple reactions such as hydrogenolysis, hydrogenation, hydrodeoxygenation, decarbonylation, decarboxylation, hydrocracking and polymerization took place. These reactions produce a large amount of coke, tar and char, which could cause the catalyst deactivation and reactor clogging [
19,
99]. Besides catalytic cracking and hydroprocessing, emulsification and steam reforming/Fischer–Tropsch are two other technologies for upgrading bio-oil to biofuel [
71]. Developing appropriate catalysts with high stability in harsh conditions and reducing the cost of the H
2 introduction need to be further investigated for hydroprocessing of vegetable oil to hydrocarbon biofuel. In addition, the understanding of the influence of fatty acids present in the vegetable oil on oil upgrading needs to be advanced.
3.4. Catalyst Properties
Table 9 shows some typical properties of catalysts for vegetable oil upgrading process. The surface area, pore volume and acidity are the factors that have the most effect on the catalyst behavior. Zeolite based catalysts and Al
2O
3 based catalysts are main catalysts used to upgrade vegetable oil for hydrocarbon biofuel production. Some other catalysts including Pd/C and Mg/SiO
2 have been used for vegetable oil upgrading. The supports of ZSM-5, Al
2O
3, ZrO
2, SiO
2 and activated carbon are an important part of catalysts for vegetable oil upgrading. The BET surface area of zeolite based catalysts varied from 56 m
2/g to 1126 m
2/g. Various metal elements such as Zn, Ni and Mo were used to modify the activity of zeolites [
101]. Taufiqurrahmi et al. [
9] investigated the cracking of used palm oil over nanocrystalline zeolite beta and nanocrystalline zeolite Y. Compared to microcrystalline zeolites, the nanocrystalline zeolites had a higher surface area (525 m
2/g for nanocrystalline zeolite beta and 1126 m
2/g for nanocrystalline zeolite Y, respectively) and exhibited a higher conversion of used palm oil. Zarchin et al. [
22] studied the hydroprocessing of soybean oil over Ni
2P/HY catalyst. The water vapors generated by hydrodeoxygenation reaction reduced the acidity of the Ni
2P/HY catalyst, thus reducing the hydrocracking activity. Ishihara et al. [
25] investigated the catalytic cracking of soybean oil over hierarchical zeolite containing mesoporous silica-aluminas. Not only the acid site but also the pore size (20 Å) of the zeolite β catalyst was important for obtaining high gasoline yield during the soybean oil upgrading. A high acid site of a zeolite may not be effective if the crystal size is large since the soybean oil molecules may not approach the acid site. Fan et al. [
27] studied the hydrocracking of
Jatropha oil over Ni-H
3PW
12O
40 catalyst and found that the interaction between Ni and H
3PW
12O
40 could increase the acid sites. Wang and Yu [
43] studied the hydrocarbon fuel production from the catalytic cracking of rubber seed oil over the mesoporous molecular sieve (MNC-13) catalyst. The Lewis acid and Brønsted acid sites coexisted on the surface of the MNC-13 catalyst. The relative intensity of Brønsted acid to Lewis acid of the MNC-13 catalyst was 0.9. The results indicate that the Lewis acid site plays a key role in the catalyst activity towards the liquid product yield.
Lovás et al. [
50] studied the catalytic cracking of
rapeseed oil over the fluid catalytic cracking ZSM-5 catalyst. The overall catalytic performance is related to the catalyst acidity, surface area and structure accessibility. A high acidity of the fluid catalytic cracking ZSM-5 catalyst could improve the cracking of olefin intermediates. Yenumala et al. [
57] investigated the hydrodeoxygenation of karanja oil over Ni/ZSM-5 catalyst. The SiO
2 was almost neutral, the Al
2O
3 was weakly acidic, and the HZSM-5 (0.55 mmol of NH
3·g
−1) was strongly acidic. The good catalytic activity of the Ni/ZSM-5 catalyst may result from its high surface area and strong acidity (0.27 mmol of NH
3·g
−1). Twaiq et al. [
74] studied the catalytic conversion of palm oil over various zeolites. The product distribution was found to be different due to the change in the pore size of the different catalysts. A low acidity of the catalyst favored the coke formation on the catalyst surface. Wu et al. [
77] studied jet fuel production from catalytic transformation of triglyceride based oils over zeolites. Compared to HZSM-5 (0.27 mmol of NH
3·g
−1), the HY catalyst had a higher acidity (1.71 mmol of NH
3·g
−1), producing more polycyclic aromatic hydrocarbons, coke and tar. Wang et al. [
102] investigated the hydrotreating of soybean oil over NiMo carbide catalysts. The NiMoC/Al
2O
3 catalyst had the highest surface area (712 m
2/g) and the highest yield of organic liquid product. Pore structure of the catalyst plays a significant role in controlling the diesel selectivity. A larger pore structure could cause less secondary cracking of heavy hydrocarbons, thus resulting in a higher diesel selectivity. Abbasov et al. [
103] studied catalytic cracking of vegetable oils over halloysite nanotubes for biofuel production. Large molecules in vegetable oil are difficult to deeply penetrate the small pores (smaller than 1–2 nm) of catalysts, thus limiting the cracking efficiency of the reaction sites on the catalyst surface. Zeolite based catalysts possess robust catalytic cracking capabilities through reactions of dehydration, decarbonylation and decarboxylation [
104].
The Al
2O
3 based catalysts have a high BET surface area ranging between 148 m
2/g and 358 m
2/g. Compared to pure Al
2O
3, the surface area of modified Al
2O
3 decreased due to the doping of some metals such as Co, Ni, Pd, Pt and Mg. The noble metal catalysts (e.g., Pt, Pd) could activate H
2 and increase catalyst lifetime due to being less susceptible to deactivation [
71]. The pore volume of zeolite based catalysts was between 0.10 cm
3/g and 1.34 cm
3/g. The properties of these catalysts vary significantly. The pore volume of Al
2O
3 based catalysts was between 0.18 cm
3/g and 1.1 cm
3/g. The metal modified Al
2O
3 catalysts had a lower pore volume than the pore volume of pure Al
2O
3 since the introduction of metals may cause partial blockage of the catalyst channels [
105]. Ni is usually used as a promoter to increase the activity of Mo in a traditional catalyst [
72]. The pore size of zeolite based catalysts varied from 3 Å to 64 Å. The medium pore size catalysts were found to be efficient in the cracking process [
74]. The pore size of Al
2O
3 based catalysts was between 32 Å and 90 Å. Catalyst support plays a significant role in dispersing active phases of catalysts and the Al
2O
3 support could partially transform into AlO(OH) in the presence of H
2O [
106]. Bruce et al. [
93] investigated the thermocatalytic cracking kinetics of myristic acid over various catalysts. Compared to HZSM-5 (0.56 mmol of NH
3·g
−1), the silica-alumina catalyst (0.27 mmol of NH
3·g
−1) had lower acidity which favors the short-chain hydrocarbon production.
The pore size of other catalysts ranged from 20 Å to 297 Å. Product selectivity is related to the pore size of catalysts and takes place when parts of products inside the catalyst pores are too large to diffuse out. These large molecules are either transformed to smaller ones or blocking the catalyst pores. The catalyst pores need to have a defined structure for product selectivity [
73].Hwang et al. [
107] studied the biofuel production from
Jatropha oil over Pd/C catalysts. During the catalytic reaction of crude vegetable oils, catalyst deactivated by the accumulation, adsorption and blocking of reactants and products. Asikin-Mijan et al. [
100] investigated the catalytic deoxygenation of
Jatropha curcas oil for green diesel production. The Ni-Co/MWCNT catalyst having a high surface area (109 m
2/g) with large pore volume (1.27 cm
3/g) and pore size (172 Å) was suitable for large vegetable oil molecule reaction. In addition, the Ni-Co/MWCNT catalyst had a high acid site (about 0.76 mmol/g), which favored the pathway of decarboxylation and decarbonylation in the deoxygenation process. The acid site of zeolite based catalysts was between 0.27 mmol/g and 4.55 mmol/g, which was higher than the acid site of Al
2O
3 based catalysts (0.14–0.27 mmol/g). Zeolites contain Lewis acid sites and Brønsted acid sites. The product selectivity during vegetable oil upgrading can be controlled using acid sites’ density distribution and strength [
73]. Acid sites can be classified as strong acid sites, medium acid sites and weak acid sites. A high total acid site is critical for the vegetable oil upgrading to hydrocarbon biofuel [
20]. During the vegetable oil upgrading process, the activity and selectivity of catalysts were affected by factors including acid site and pore size [
74]. The cost of catalysts is a significant expense, but utilizing catalysts derived from waste materials could enhance the reaction process by reducing cost and improving environment friendliness. Red mud is a solid waste formed during the production of alumina from the digestion of bauxite. Red mud catalyst has high pore size and has been used for the transesterification of vegetable oils including soybean oil and Mahua oil. Red mud catalyst exhibits a high activity towards the transesterification of vegetable oils [
108,
109,
110]. The properties of catalysts can be improved under proper conditions, but the commercialization of catalysts is still a challenge due to their large property difference.
Table 10 shows some typical properties of catalysts used in bio-oil upgrading process. Zeolite based catalysts (such as HZSM-5, La/HZSM-5, Mo/HZSM-5, Cu/HZSM-5, β-zeolite and H-mordenite) and Al
2O
3 based catalysts (such as Na
2CO
3/Al
2O
3, Zn/Al
2O
3, Ce/Al
2O
3, Ni-Ce/Al
2O
3, Pt/Al
2O
3, Co-Mo/Al
2O
3 and CoP/Al
2O
3) are usually used to upgrade bio-oil for hydrocarbon biofuel production [
8,
19]. HZSM-5 is an effective catalyst to upgrade bio-oil to hydrocarbons, organic distillates and aromatics [
8]. Fluid catalytic cracking is one important process used in petroleum refinery and is applied to convert crude oil to high value products. Coked fluid catalytic cracking catalysts are periodically burned off in the petroleum field to provide heat for the process and regenerate catalysts [
119]. Mesoporous zeolites such as MFI, Y and beta exhibit high surface area and have been recently used for the upgrading of bio-oil. The mesoporous materials could promote the diffusion of reactants into the meso-pores [
120,
121]. Wei et al. [
122] studied the hydrocarbon production from upgrading rich phenolic compound bio-oil over zeolites. The acid sites of catalysts can donate protons to form hydrocarbon cation. The coke generated via polymerization can reduce both size and area of micropore on ZSM-5. The coke can cause a barrier for guaiacol diffusion, thus blocking the pores of ZSM-5. Lee et al. [
123] investigated the catalytic hydrodeoxygenation of bio-oil model compounds over zeolite catalysts. The cyclohexane yield increased with the increase of acid sites on the Pt/HY catalyst. Compared to Pt/HZSM-5 catalyst, the Pt/HY catalyst had a larger pore size and was expected to improve the guaiacol diffusion toward the acid sites inside the catalyst. Huynh et al. [
124] investigated the upgrading of bio-oil under various catalysts. The low degree of deoxygenation value over the NiCo/ZrO
2 catalyst can be attributed to its lower surface area (40 mm
2/g) and acidity (0.04 mmol/g) compared to other catalysts including NiCo/HBeta, NiCo/HY and NiCo/HZSM-5. The Brønsted acid site concentrations of the catalyst supports followed the order of HY > HZSM-5 > HBeta > ZrO
2. Karnjanakom et al. [
125] studied the catalytic upgrading of bio-oil over Cu/KIT-6 and Cu/MCM-41 catalysts. A higher acidity of the catalyst favored the conversion of oxygenates in the bio-oil to hydrocarbons through the deoxygenation reaction. Jang et al. [
126] investigated the catalytic upgrading of bio-oil model compound over mesoporous solid catalysts. They found the introduction of aluminum to the support (e.g., silica and SBA-15) could generate weak acid sites. Gamliel et al. [
127] studied the catalytic fast pyrolysis of biomass (e.g., cellulose and miscanthus) with tailored mesoporous MFI zeolites. The mesoporous MFI zeolites had a surface area of 342–484 m
2/g, pore volume of 0.25–0.53 cm
3/g, and Brønsted acid site of 0.29–0.55 mmol/g. The mesoporous MFI zeolites were effective at improving the bio-oil yield from catalytic fast pyrolysis of biomass. The carbon balance for catalytic fast pyrolysis of cellulose was 97%–103%. Foster et al. [
128] investigated the catalytic fast pyrolysis of biomass (e.g., glucose and maple wood) over mesoporous and non-mesoporous ZSM-5 catalysts. Incorporating hierarchical mesopores within the zeolite was helpful to increase the production of larger alkylated monoaromatics.
Commercial catalysts commonly used for the hydroprocessing of bio-oil are sulfide Co-Mo/Al
2O
3 and Ni-Mo/Al
2O
3 catalysts. After being sulfided appropriately, the catalysts are thermally stable without rapid coking [
71,
99]. During the upgrading of bio-oil, other types of catalysts have been investigated including ZrO
2/TiO
2, MgO, CaO, Ni/W/TiO
2, Pd/C, Fe/C, Pt/SiO
2, etc. [
19]. Red mud catalyst has also been used for the upgrading of bio-oil. Red mud is a mixture of metal oxides including Al
2O
3, SiO
2 and Fe
2O
3 [
109]. The catalyst type and operation conditions have an influence on the production distributions of bio-oil upgrading [
8]. The BET surface area of zeolite based catalysts varied from 122 m
2/g to 1020 m
2/g. The pore size of zeolite based catalysts varied from 20 Å to 117 Å. The ZSM-5 catalyst has high acidity and shape selectivity, but it has low biofuel yield and short lifetime during the bio-oil upgrading process [
119]. Multifunctional catalysts (e.g., zeolites with doping Zn, Cu, Ni and Mo) are needed for both bio-oil upgrading and vegetable oil upgrading. Sintering of the metal particles could reduce the active surface area of the catalysts. Tuning the catalyst composition through modification of the metal formulation and/or the support material surface could mitigate particle sintering. Nobel metal catalysts have a high hydroprocessing activity, but the application on large scale is hampered by their high cost [
71]. The Al
2O
3 based catalysts have high BET surface area between 135 m
2/g and 381 m
2/g. Al
2O
3 has been widely used in industrial bio-oil upgrading to remove sulfur from the crude oil [
72]. Common metals such as Co, Mo, Ni, and Zn are usually used to modify the activity of Al
2O
3. The instability of the Al
2O
3 support in a high water content environment is a shortcoming during the hydroprocessing of bio-oil [
119]. Understanding the interplay between catalyst surface structure and reaction conditions is significant for the bio-oil upgrading [
71]. The pore volume of zeolite based catalysts was between 0.12 cm
3/g and 1.1 cm
3/g; and the pore volume of Al
2O
3 based catalysts was between 0.08 cm
3/g and 0.72 cm
3/g. The design of catalysts including composition, surface morphology and crystal phase could be modeled to assist synthesizing high activity catalysts [
71]. The pore size of Al
2O
3 based catalysts was between 31 Å and 90 Å. The pore size of other catalysts (e.g., SiO
2) ranged from 32 Å to 289 Å. The supports of Al
2O
3 and SiO
2 have an influence on the product selectivity during the bio-oil upgrading. Support materials such as mesoporous zeolite, silica and activated carbon are more stable in acidic environment than conventional alumina [
71]. The acid site of all catalysts was between 0.03 mmol/g and 1.12 mmol/g. The acidic HZSM-5 catalyst usually causes coke formation through decarboxylation, decarbonylation, dealkylation, cracking and aromatization reactions [
73]. Karnjanakom et al. [
129] studied the bio-oil upgrading over metal-loaded Al
2O
3 catalysts. The large pore size (90 Å) of Al
2O
3 provided the potential for the diffusion of large bio-oil molecules and the reduction of coke formation. The surface area and pore size of the metal-loaded Al
2O
3 catalysts decreased with a higher metal loading. Karnjanakom et al. [
130] investigated the catalytic upgrading of bio-oil to hydrocarbons over metal-doped Al
2O
3 catalysts. The acidity of Zn/Al
2O
3 catalysts increased from 0.29 mmol/g to 0.33 mmol/g with the increase of Zn loading from 1.0 wt % to 2.5 wt %. The loading of metal on Al
2O
3 could promote the electron pair acceptors or Lewis acid sites.
Activated carbon is considered to be a promising support for the hydroprocessing of bio-oil due to its textural properties and thermal stability. The carbon support has a low catalyst deactivation due to its neutral nature. Similar to carbon, the SiO
2 support is often used for hydroprocessing of bio-oil due to its inert character. ZrO
2 and MgO are good basic supports for the hydroprocessing of bio-oil. ZrO
2 is highly coke resistant due to its low acidic and lower affinity towards water [
72]. The activity of the catalyst formulation is affected by many parameters including pH, time, temperature, cations and anions. The presence of non-metal such as P and C on the catalyst surface could create different sites for reaction and adsorption. Ni
2P was reported as a promising catalyst for bio-oil upgrading due to its good stability, high activity and superior selectivity. The acidity of the support materials such as HZSM-5 and Al
2O
3 affects the adsorption properties [
71]. Oh et al. [
131] investigated the bio-oil upgrading over noble metal catalysts of Ru/C and Pt/C. The surface area, pore size, and pore volume of these two catalysts all decreased after three times of reusing. The optimization of metal and supports is important for obtaining catalysts that are economically and technically feasible [
72].
3.5. Catalyst Deactivation and Regeneration
During the vegetable oil upgrading to hydrocarbon biofuel, deactivation of catalysts is a significant factor for the catalytic performance and commercial economic consideration [
151]. During the catalytic cracking process, many side reactions could cause the formation of carbonaceous material known as coke. Coke formation is a major factor in causing the catalyst deactivation. The coke can deposit on the external surface and/or internal pores of catalysts. The coke deposition over the catalyst active sites could affect the catalyst activity; and the coke deposition inside the catalyst pore structures affects the catalyst selectivity. Physical properties of catalysts including pore size, pore shape and crystallite size could strongly influence coke formation [
73]. Other possible reasons for the catalyst deactivation include active metal leaching, and structural changes of catalyst components due to sintering and poisoning [
151,
152,
153]. During the upgrading of bio-oil, the catalyst deactivation results from the coke due to the transformation of bio-oil oxygenates [
19]. The mechanism of catalyst deactivation needs to be further understood.
Regeneration of used catalysts is one of the promising pathways to eliminate the cause of catalyst deactivation [
151].
Table 11 shows some common catalyst regeneration methods. Used catalysts could be calcined in a furnace or reactor in air at high temperatures (500–600 °C) for regeneration. Some researchers used acetone to wash the used catalysts prior to calcination, which may help remove some impurities (e.g., oil) deposited on the used catalysts. The continued regeneration (up to fifth regeneration) of the HZSM-5 zeolite gradually reduced the catalyst activity in converting bio-oil to an aromatic product. The prolonged reaction-regeneration cycles may result in the disappearance of a large amount of acid sites on the catalyst [
9,
26]. Directly regenerating the used catalysts in the reactor with air might be an efficient pathway, resulting in no need to unload and then load the catalysts. The reaction-regeneration cycle (up to third regeneration) gradually reduced the catalyst activity due to the modification of the acidity and texture of the Zn/HZSM-5 catalyst. The Brønsted acid sites and intensity of the Zn/HZSM-5 catalyst decreased with the increase of the reaction-regeneration cycle. During the catalyst regeneration process, a high air flow rate could facilitate the sufficient combustion of coke, thus improving the recovery of the deactivated Zn/HZSM-5 [
79,
152]. Josl et al. studied the regeneration of used zeolite catalysts at 300 °C using a hydrogen pressure of 15 bar in a fixed-bed reactor. The activity of the zeolite catalysts using a hydrogen treatment method could be completely restored under suitable conditions [
154]. Oxidation is also a method for the catalyst regeneration through introducing O
2 or N
2O. N
2O was a more efficient reactivation reagent for the deactivated zeolite catalysts than O
2. The oxidative treatment of used catalyst at high temperatures for regeneration may cause some issues including catalyst destruction, dealumination, and agglomeration of doped metals. These regeneration issues may reduce the catalyst lifetime, resulting in the increased total cost. Oxidation and reduction (with H
2) of deactivated Ni-zeolite catalyst was a successful regeneration process since it decreased the surface area and total pore volume of the catalyst, but it increased the acidity and activity [
151,
155]. Most solid alkylation catalysts seriously deactivate after 3–5 cycles of reaction-regeneration by oxidation regeneration. Petkovic and Ginosar investigated the effect of supercritical isobutane regeneration on the coke deposited on a USY zeolite catalyst and found that the supercritical isobutane regeneration was effective in recovering the surface area and micropore volume of catalysts. A totally deactivated catalyst is difficult to be completely regenerated, but a partially deactivated catalyst could be fully regenerated [
156]. Steam treatment is another method for the catalyst regeneration. This is helpful to tune the acidities of zeolites. During the coke burning of the regeneration process, the introduction of steam may prevent temperature runaway. The steam-treated Ag/HZSM-5 catalyst maintained its initial activity during four reaction-regeneration cycles [
157]. During the process of bio-oil upgrading, used catalysts were usually regenerated using an oxidation treatment through controlling regeneration temperature, regeneration time, and oxygen concentration [
19]. Catalyst regeneration may cause undesirable downtime in a large scale-up commercial process [
71]. Catalysts with high activity, high durability and high renewable capability need to be developed.
Firstly, a suitable regeneration of used catalysts could help reduce the cost of biofuel production from vegetable oil upgrading. Secondly, the vegetable oil feedstock may comprise a high cost due to expensive harvesting/extracting and competition with food resources. In recent years, various vegetable oils including food vegetable oil and non-food vegetable oil were studied. However, utilizing non-food vegetable oil sources such as
camelina oil and waste vegetable oil should receive increased focus in the future in order to reduce the cost and reduce competition with food sources. In addition, a reasonable distribution of the vegetable oil feedstock should be designed to reduce the cost. Thirdly, developing high activity catalysts, optimizing the oil upgrading technologies, and improving the reactor system stability are significant for the cost reduction. Recently, more researches on the catalyst application from waste materials (e.g., red mud) have been reported. The utilization of waste for catalyst use could not only benefit the environment, but also reduce the biofuel production cost. The catalysts should be modified for the maximum effectiveness in the upgrading process with a high space velocity during a scale-up application. Various reactors including fixed-bed reactor, batch reactor, microactivity test reactor, and glass vessel have been studied at a lab-scale. However, in conventional petroleum refineries, most of the continuous biofuel production uses the concept of fluidized-bed reactor [
67,
90,
109,
158,
159,
160]. Recently, the scale-up of vegetable oil/bio-oil upgrading still needs more research targeted towards cheaper feedstock development, high activity catalysts, cost effective processing technology, and stable reactor system.