Catalysts Promoted with Niobium Oxide for Air Pollution Abatement
Abstract
:1. Introduction
2. Result and Discussion
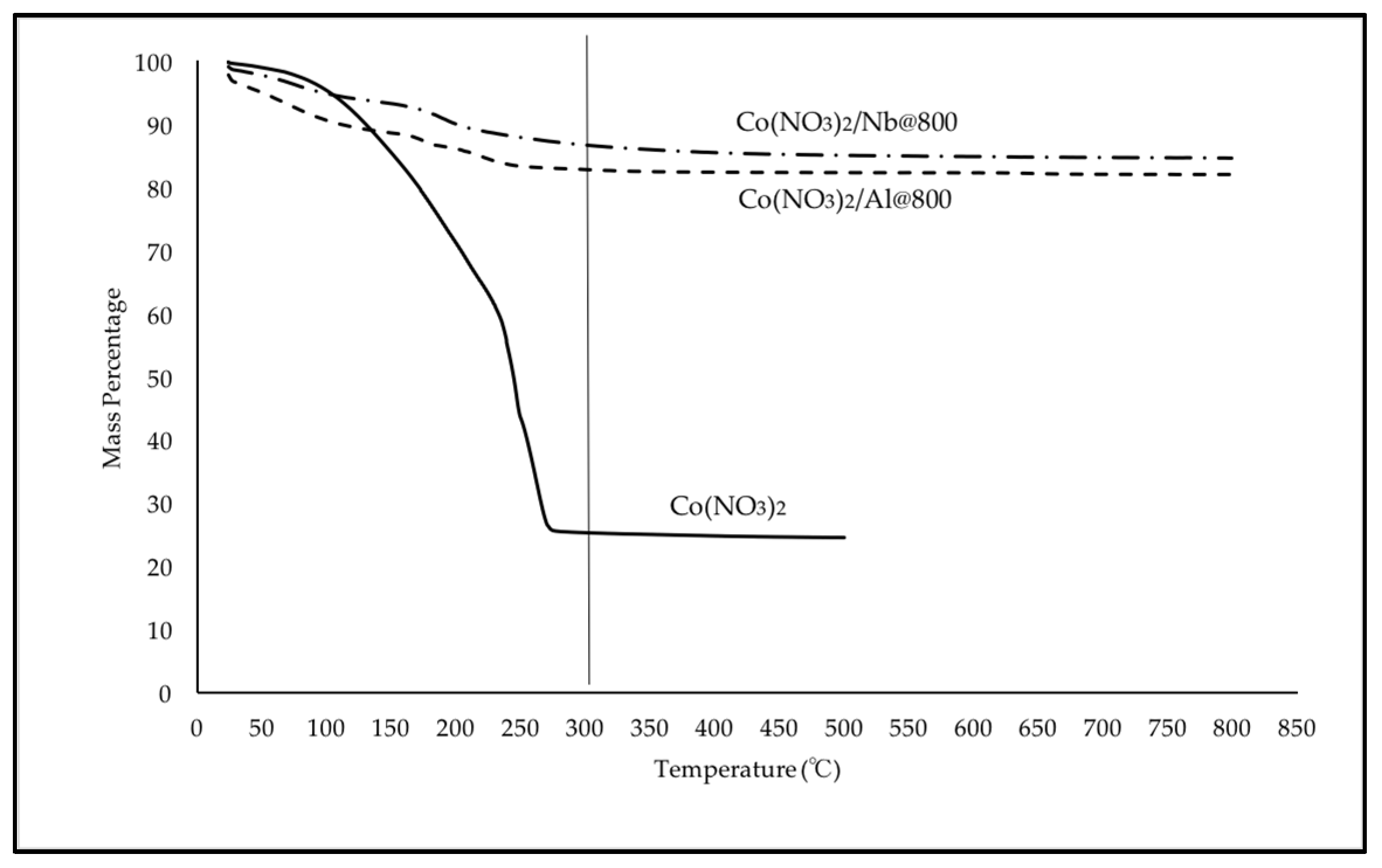
2.1. TGA Results for Precursor of Cobalt (Co)
2.2. Preparative Details of Co/Nb and Co/Al
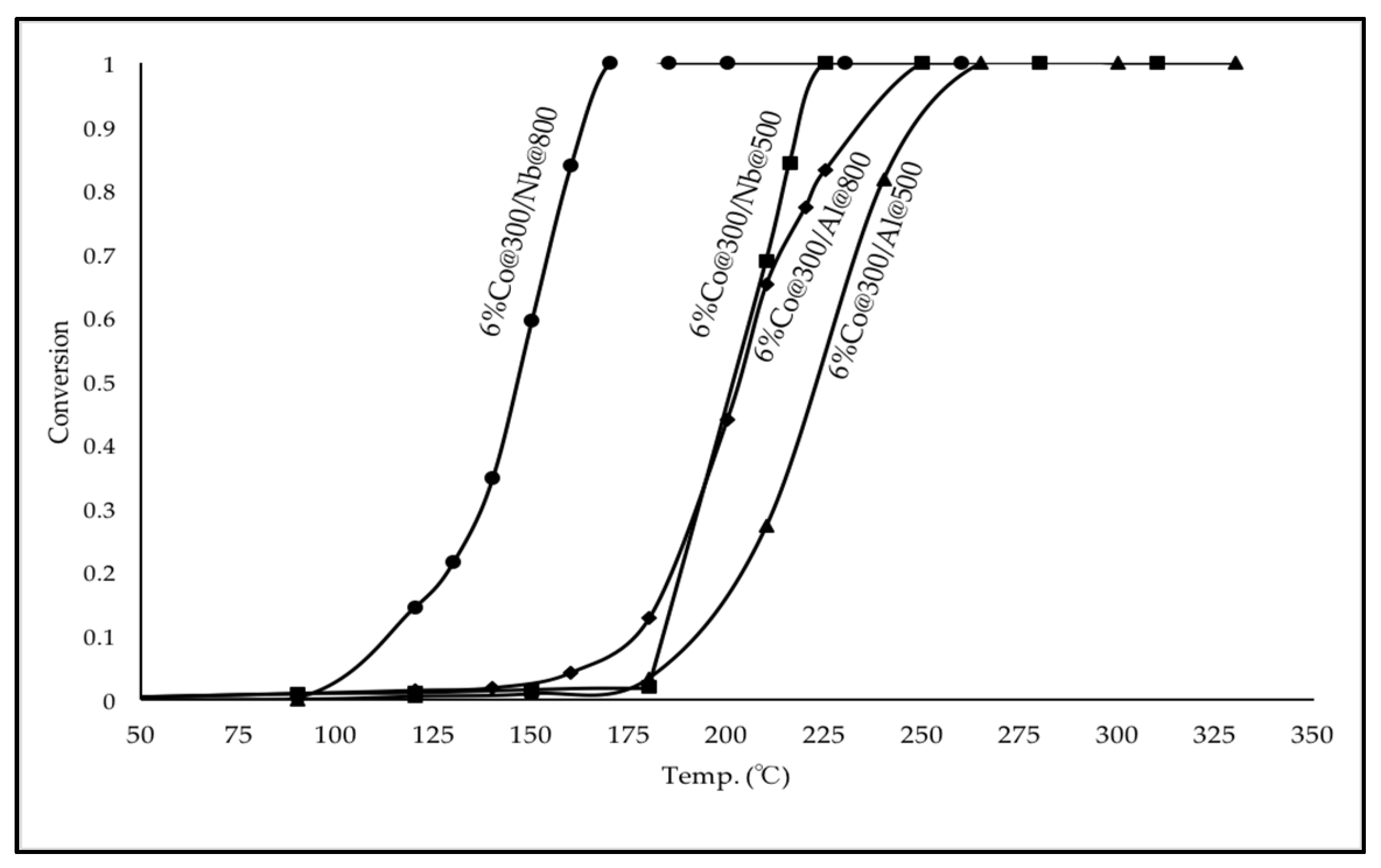
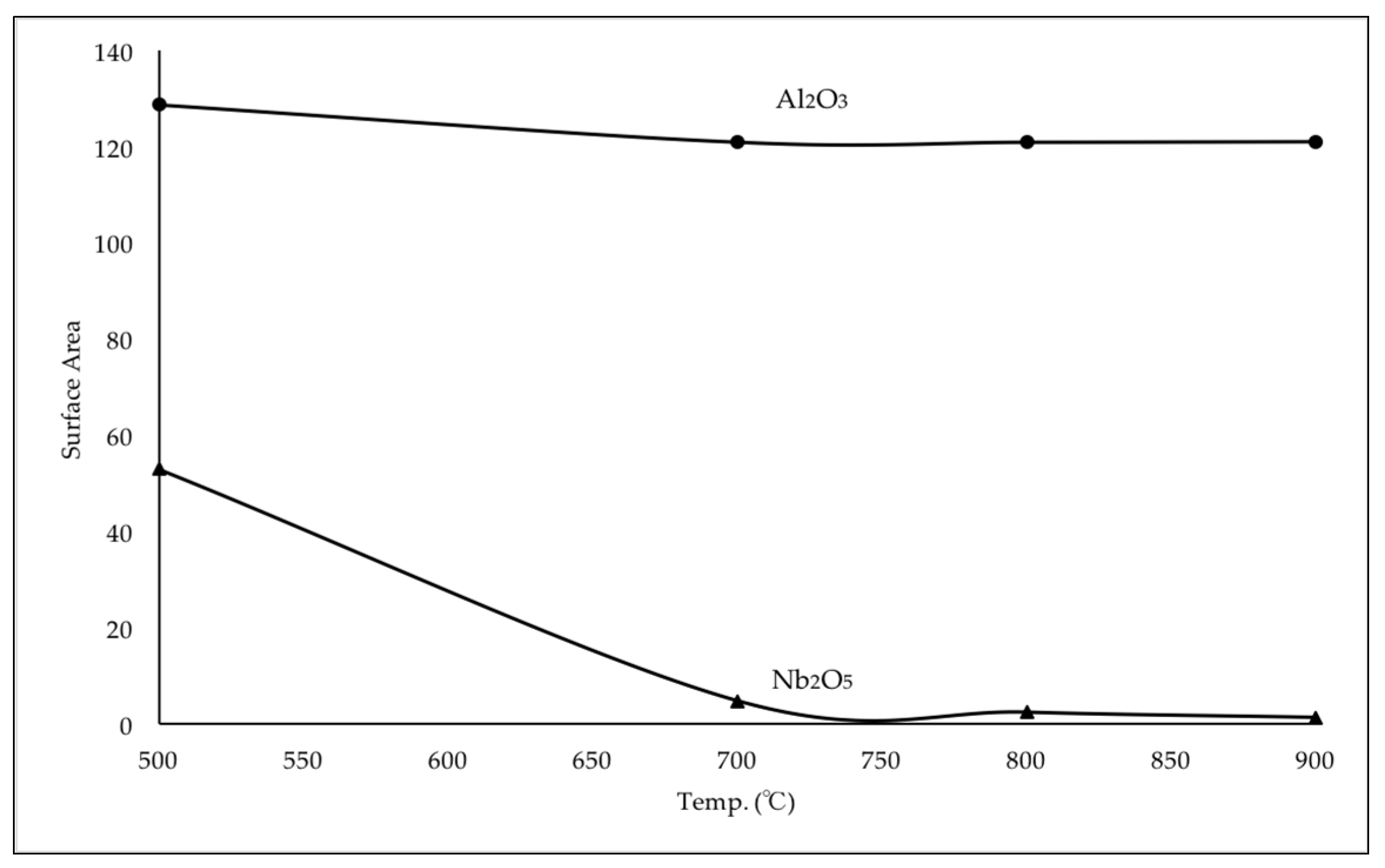
2.2.1. Pre-Calcination Temperature of Carriers Nb2O5 and Al2O3
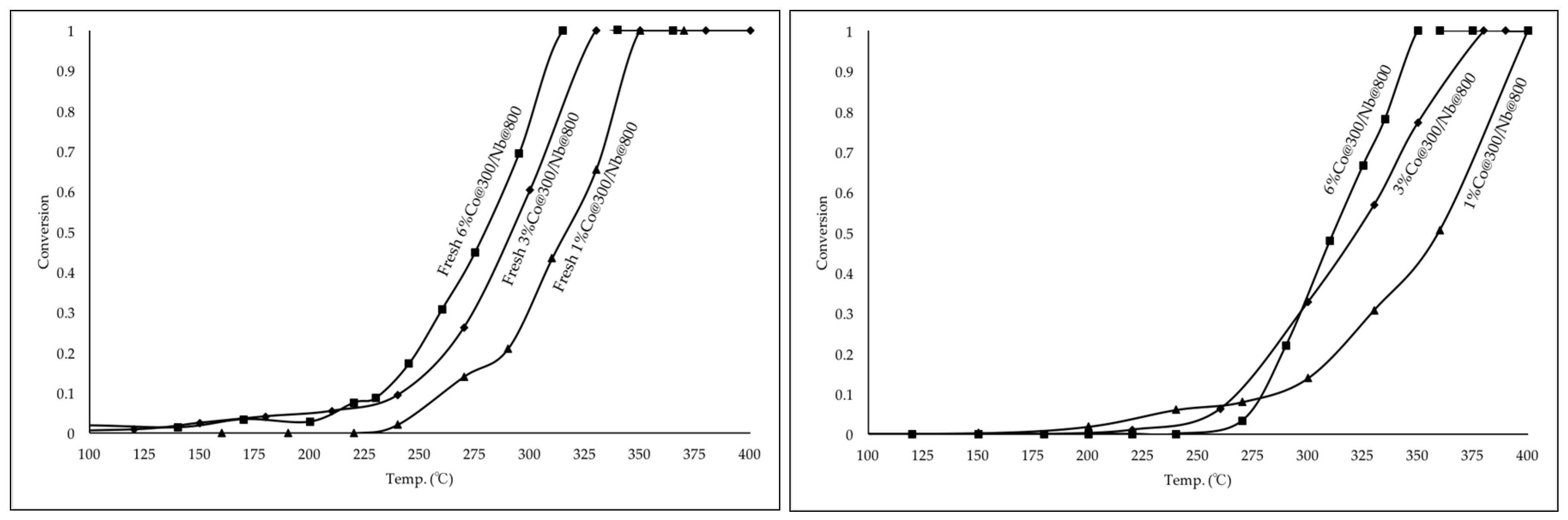
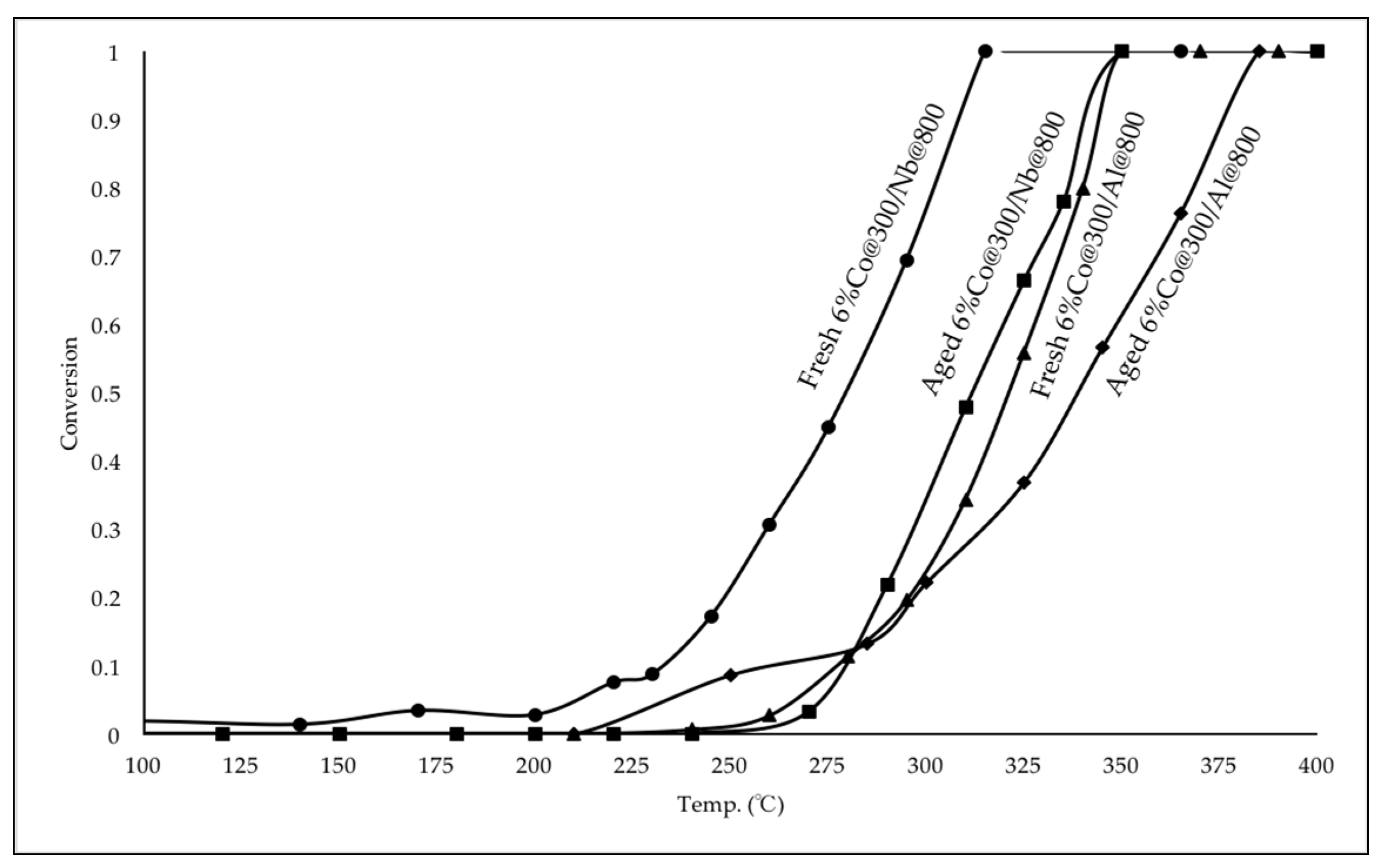
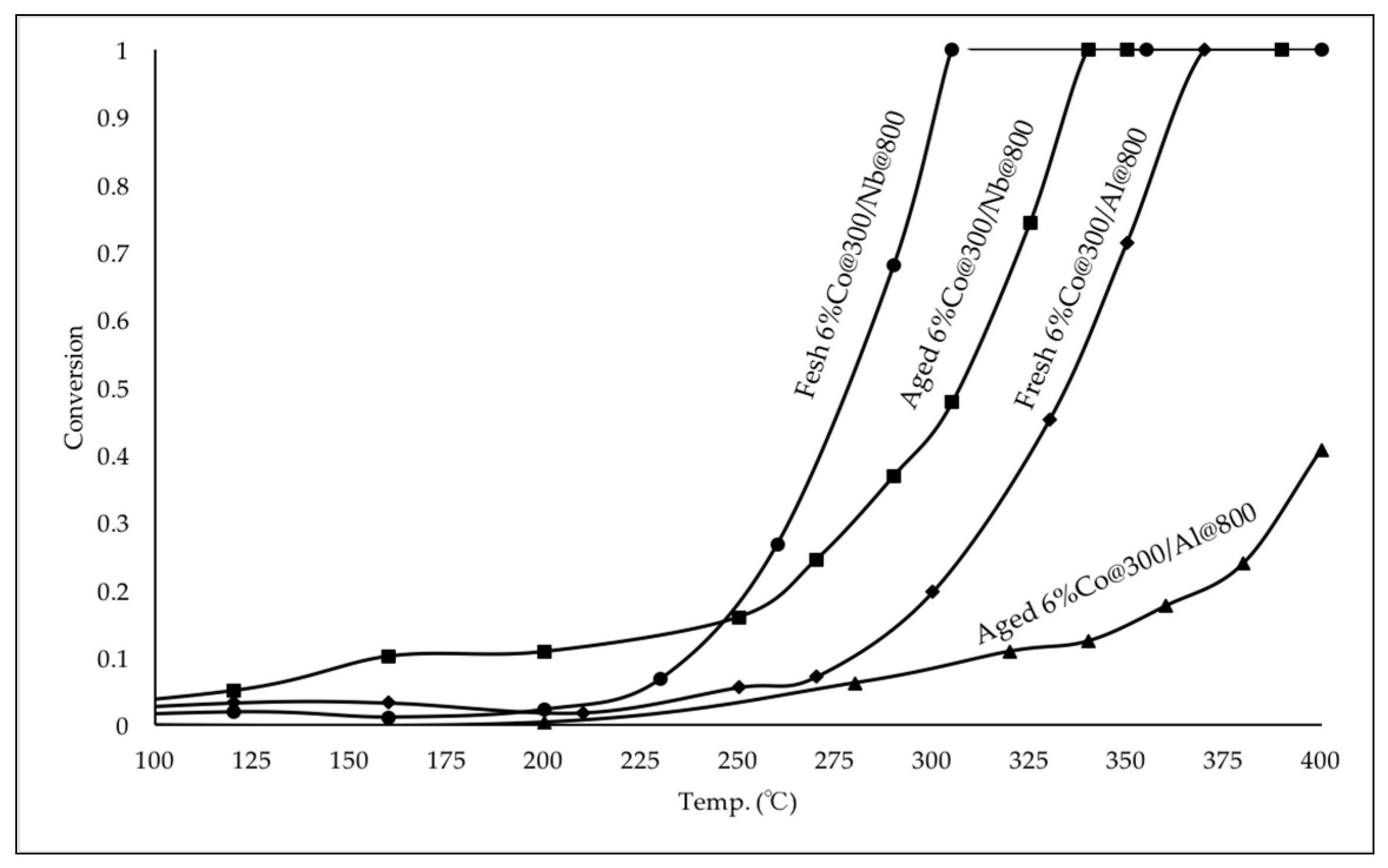
2.2.2. Catalytic Performance of Fresh and Aged Co Catalysts for CO Oxidation
2.3. Various Loadings of Co3O4 Supported on Nb2O5
2.3.1. Catalysts with Various Co3O4 Loadings: Propane Oxidation
2.3.2. Propane Oxidation with and without Moisture
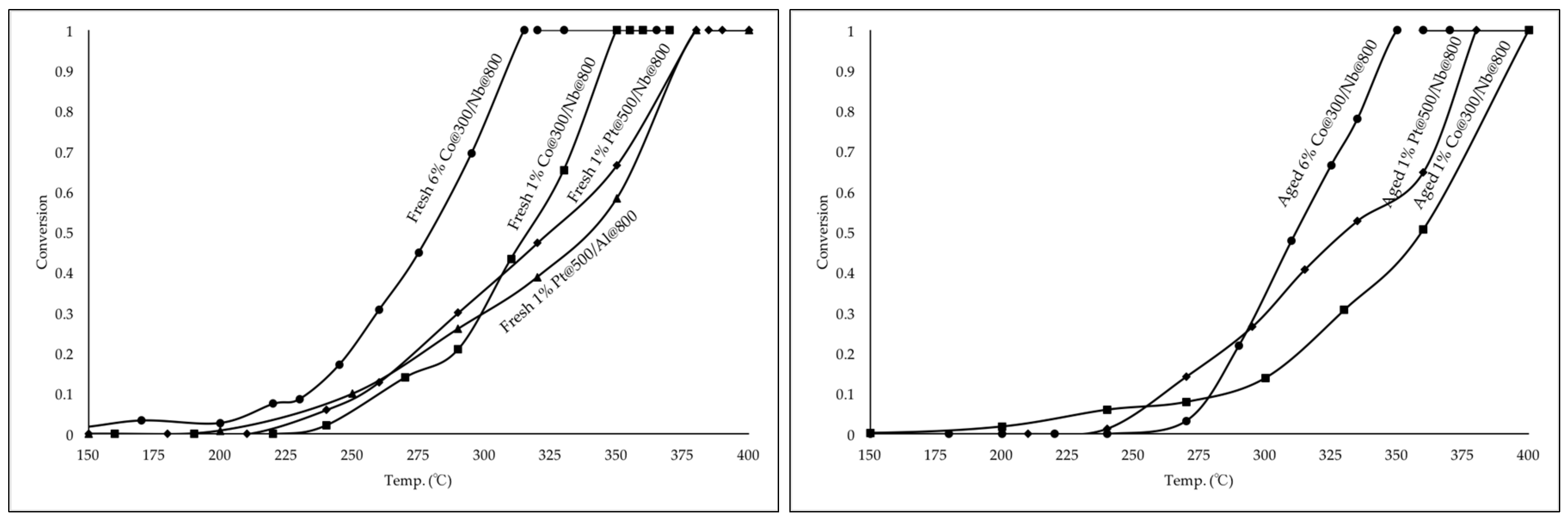
2.4. Comparison of Co3O4 and Pt for Propane Oxidation
2.5. Other Base Metal Oxides for Propane Oxidation
2.6. Cobalt Oxide Catalyst Characterization
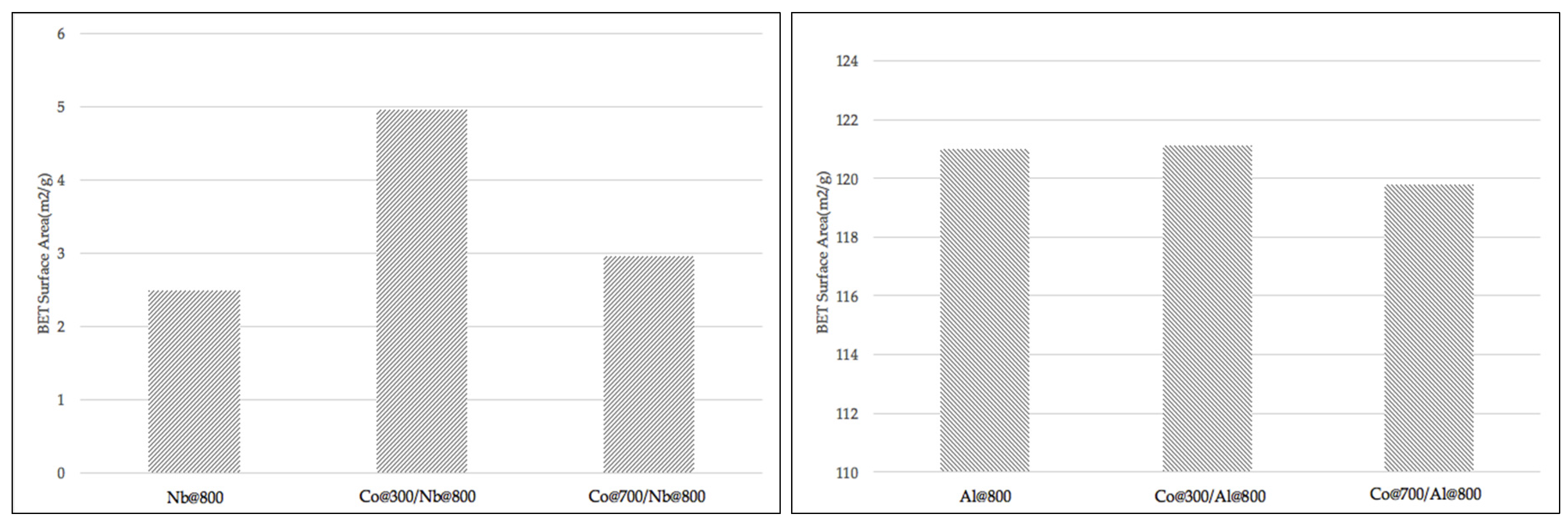
BET Tests for Catalysts and Carriers
3. Materials and Methods
3.1. Carrier Preparation
3.2. Catalyst Preparation
3.2.1. Co-Containing Catalyst Preparation
3.2.2. Pt-Containing Catalysts Preparation
3.3. Reactor Test
3.3.1. Reactor Preparation
3.3.2. Reactor Gas Flow Rate
Reactor Gas Flow without Steam for CO Oxidation
Reactor Gas Flow without Steam for Propane Oxidation
Reactor Gas Flow with Steam for Propane Oxidation
3.4. Data Management
3.4.1. Data Acquisition
3.4.2. Data Processing
3.5. Aging Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leung, E.; Shimizu, A.; Barmak, K.; Farrauto, R. Copper oxide catalyst supported on niobium oxide for CO oxidation at low temperatures. Catal. Commun. 2017. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control: Commercial Technology, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Duprez, D.; Cavani, F. Handbook of Advanced Methods and Processes in Oxidation Catalysis “from Laboratory to Industry”; Imperial College Press: London, UK, 2014; Chapters 1–8. [Google Scholar]
- Busca, G.; Daturi, M.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Willey, R.J. Transition metal mixed oxides as combustion catalysts: Preparation, characterization and activity mechanisms. Catal. Today 1997, 33, 239–249. [Google Scholar] [CrossRef]
- Thormahlen, P.; Thormählen, P.; Skoglundh, M.; Fridell, E.; Andersson, B. Low-Temperature CO Oxidation over Platinum and Cobalt Oxide Catalysts. J. Catal. 1999, 188, 300–310. [Google Scholar] [CrossRef]
- Solsona, B.; Vázquez, I.; Garcia, T.; Davies, T.E.; Taylor, S.H. Complete oxidation of short chain alkanes using a nanocrystalline cobalt oxide catalyst. Catal. Lett. 2007, 116, 116–121. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.; Canu, P. Largely Cu-doped LaCo1−xCuxO3 perovskites for TWC: Toward new PGM-free catalysts. Appl. Catal. B Environ. 2016, 180, 94–105. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.; Horsley, J.A. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Nakamura, H.; Kunimori, K.; Asano, H.; Uchijima, T. Ethane hydrogenolysis and hydrogen chemisorption over niobia-promoted rhodium catalysts: A new phase by a strong rhodium-niobia interaction. J. Catal. 1988, 112, 478–488. [Google Scholar] [CrossRef]
- Leung, E.; Lin, Q.; Farrauto, R.J.; Barmak, K. Oxygen storage and redox properties of Nb-doped ZrO2-CeO2-Y2O3 solid solutions for three-way automobile exhaust catalytic converters. Catal. Today 2016, 277, 227–233. [Google Scholar] [CrossRef]
- Sasaki, K.; Zhang, L.; Adzic, R.R. Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction. Phys. Chem. Chem. Phys. PCCP 2008, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, P.; Yang, J.Y.; He, L.; Nie, J.C.; Liu, X.; Li, Y. Ultrathin Co3O4 nanowires with high catalytic oxidation of CO. Chem. Commun. 2011, 47, 11279–11281. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Gjikaj, M.; Brockner, W. Thermal decomposition of cobalt nitrato compounds: Preparation of anhydrous cobalt (II) nitrate and its characterisation by Infrared and Raman spectra. Thermochim. Acta 2005, 432, 36–40. [Google Scholar] [CrossRef]
- Yan, L.; Rui, X.; Chen, G.; Xu, W.; Zou, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef] [PubMed]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Laboratories: East Saint Louis, IL, USA, 1987. [Google Scholar]


| Catalyst | T20 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|
| 6% Co@300/Nb@500 | 190 | 200 | 220 |
| 6% Co@300/Nb@700 | 150 | 155 | 160 |
| 6% Co@300/Nb@800 | 130 | 145 | 160 |
| 6% Co@300/Nb@900 | 130 | 155 | 185 |
| Catalyst | T20 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|
| 6% Co@300/Al@500 | 205 | 225 | 245 |
| 6% Co@300/Al@700 | 200 | 215 | - |
| 6% Co@300/Al@800 | 185 | 205 | 240 |
| 6% Co@300/Al@900 | 215 | 240 | 270 |
| Catalyst | TOF@150 °C (h−1 × 105) | TOF@170 °C (h−1 × 105) | TOF@190 °C (h−1 × 105) | TOF@210 °C (h−1 × 105) |
|---|---|---|---|---|
| 6% Co@300/Nb@800 | 897.8 | 1508 | 1508 | 1508 |
| 6% Co@300/Al@800 | 53.48 | 129.9 | 496.6 | 1241 |
| Catalyst | T20 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|
| Fresh 6% Co@300/Nb@800 | 250 | 280 | 305 |
| Aged 6% Co@300/Nb@800 | 290 | 310 | 340 |
| Fresh 3% Co@300/Nb@800 | 260 | 270 | 310 |
| Aged 3% Co@300/Nb@800 | 285 | 320 | 360 |
| Fresh 1% Co@300/Nb@800 | 290 | 315 | 345 |
| Aged 1% Co@300/Nb@800 | 315 | 355 | 390 |
| Catalysts | T20 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|
| 6% Co@300/Nb@800 | 250 | 280 | 305 |
| 1% Co@300/Nb@800 | 290 | 315 | 345 |
| 1% Pt@500/Nb@800 | 275 | 325 | 370 |
| 1% Pt@500/Al@800 | 275 | 340 | 370 |
| Catalysts | T20 (°C) | T50 (°C) | T90 (°C) |
|---|---|---|---|
| 6% Co@300/Nb@800 | 290 | 310 | 340 |
| 1% Co@300/Nb@800 | 315 | 355 | 390 |
| 1% Pt@500/Nb@800 | 290 | 335 | 375 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, W.; Han, X.; Astorsdotter, J.; Farrauto, R. Catalysts Promoted with Niobium Oxide for Air Pollution Abatement. Catalysts 2017, 7, 144. https://doi.org/10.3390/catal7050144
Xiang W, Han X, Astorsdotter J, Farrauto R. Catalysts Promoted with Niobium Oxide for Air Pollution Abatement. Catalysts. 2017; 7(5):144. https://doi.org/10.3390/catal7050144
Chicago/Turabian StyleXiang, Wendi, Xiaochen Han, Jennifer Astorsdotter, and Robert Farrauto. 2017. "Catalysts Promoted with Niobium Oxide for Air Pollution Abatement" Catalysts 7, no. 5: 144. https://doi.org/10.3390/catal7050144






