The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions
Abstract
:1. Introduction
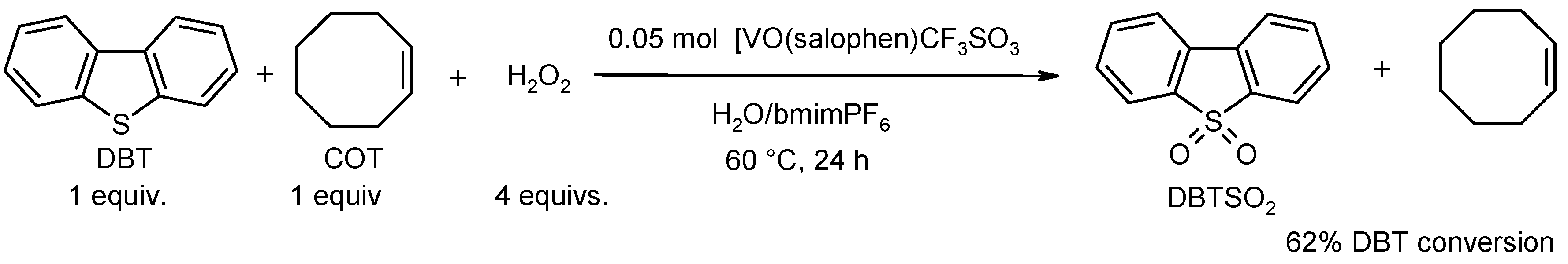
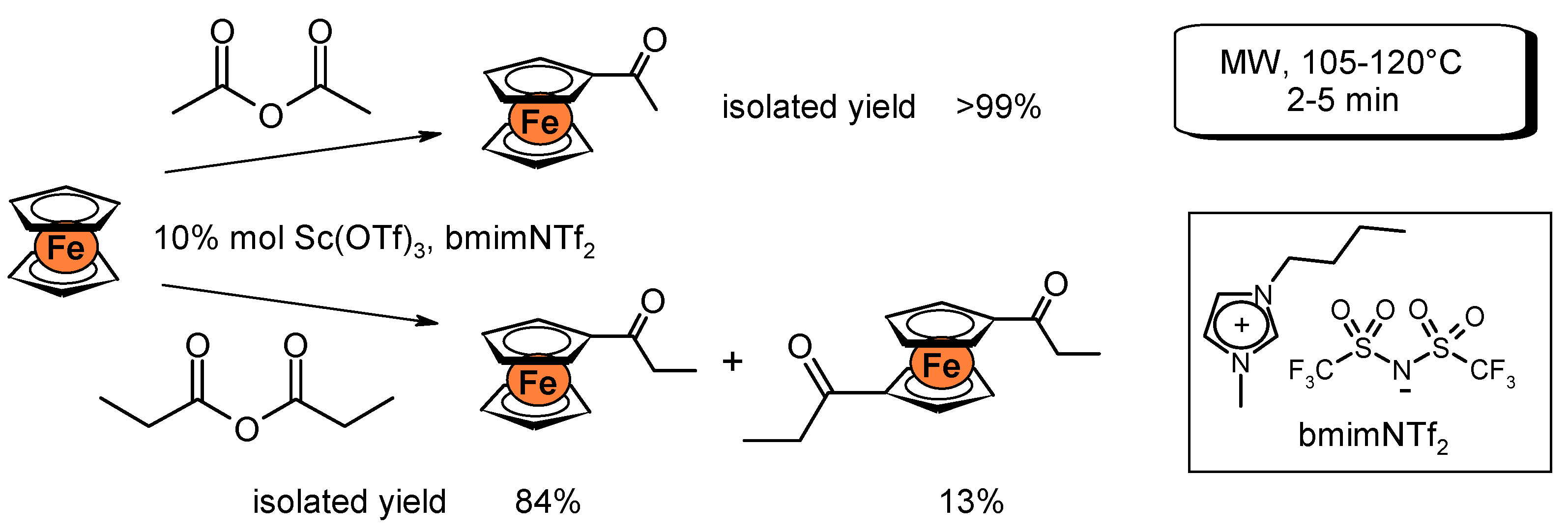
2. Oxidation Reactions
3. Carbon-Carbon Bond Formation
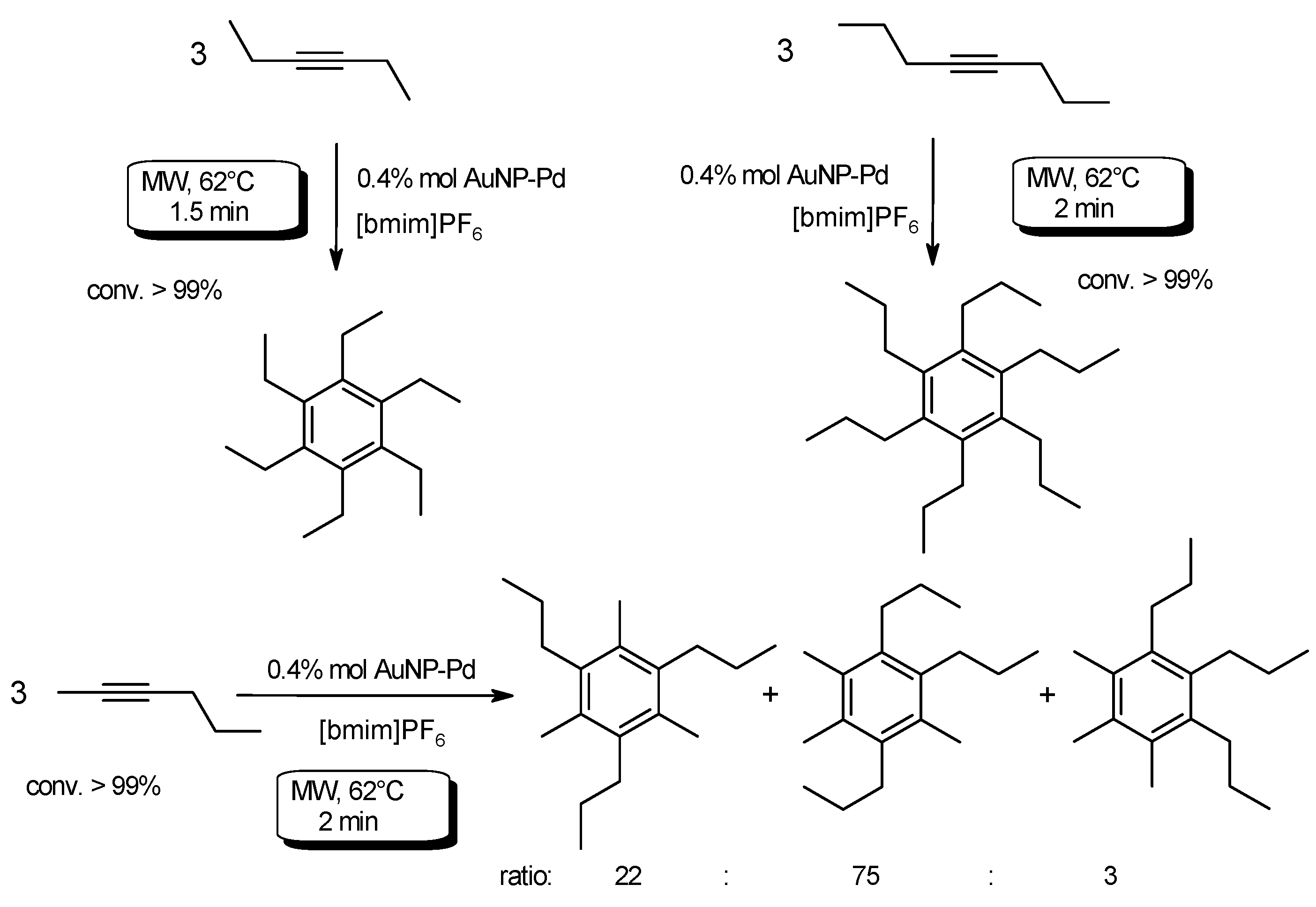
3.1. Transition Metal-Catalyzed C–CCoupling Reactions
3.2. Friedel-Crafts Aromatic Substitutions
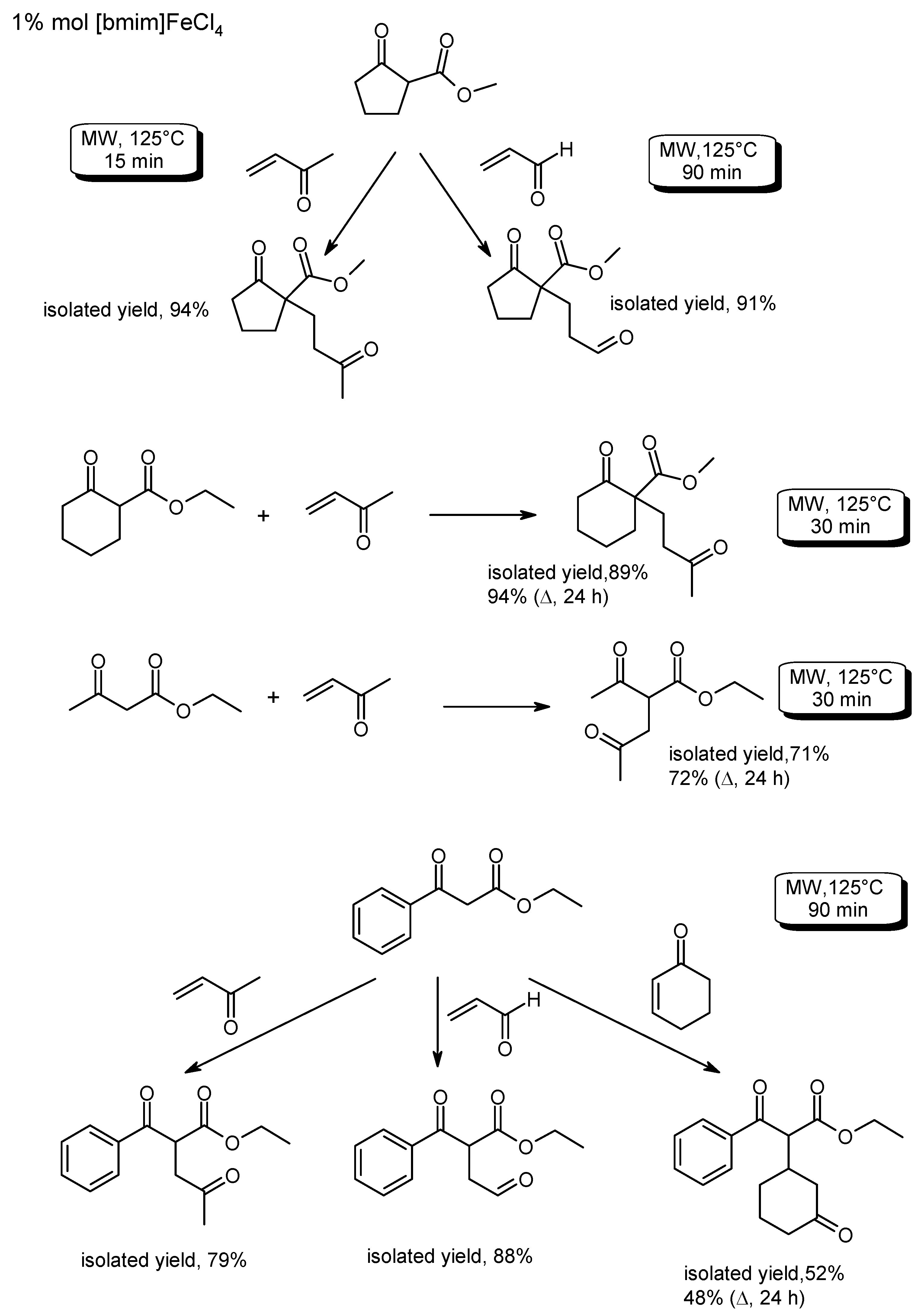
3.3. C–CBond Formation with C-Nucleophilic Reagents
3.4. Cyclization Reactions to Carbocycles with C–CBond Formation
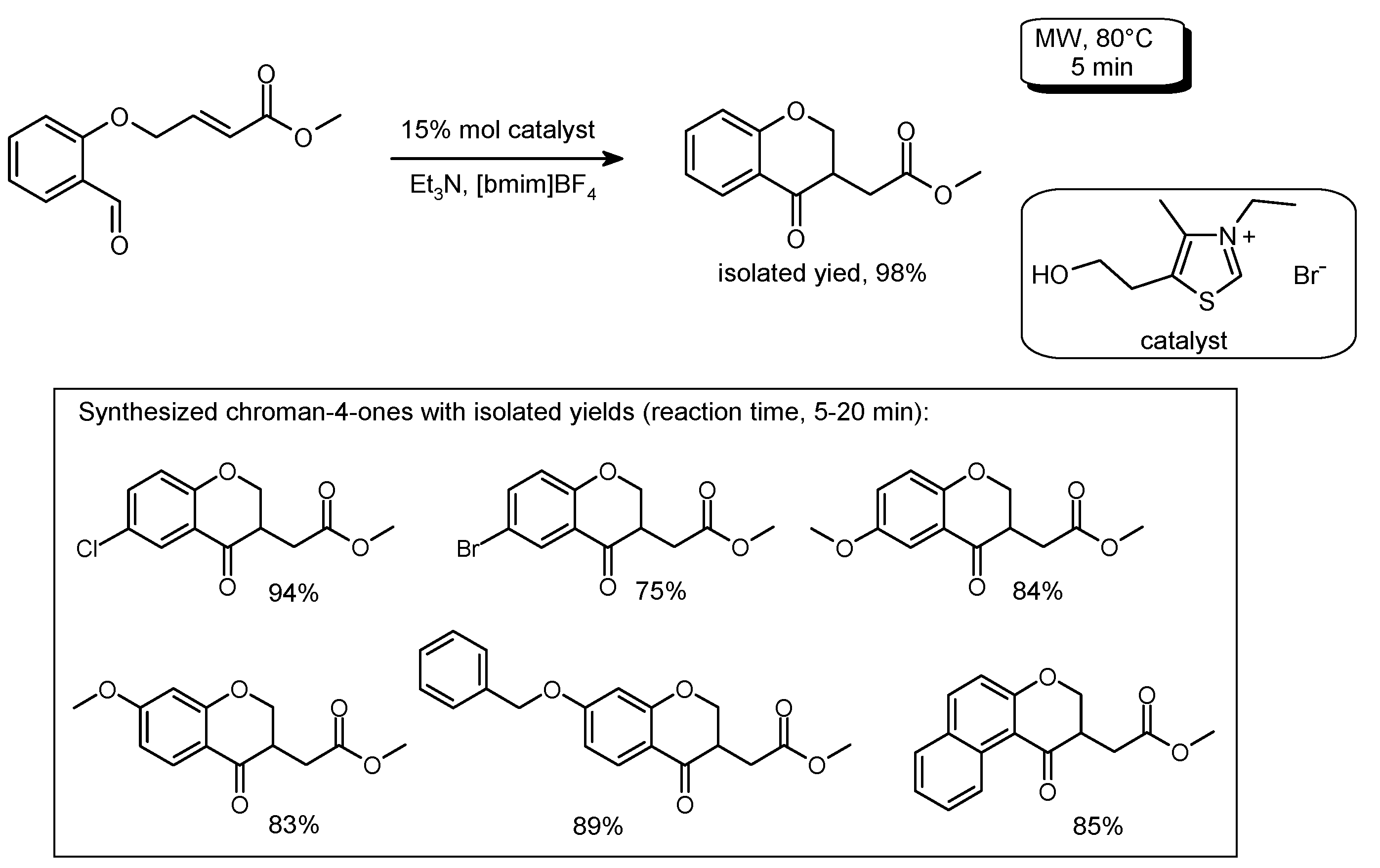
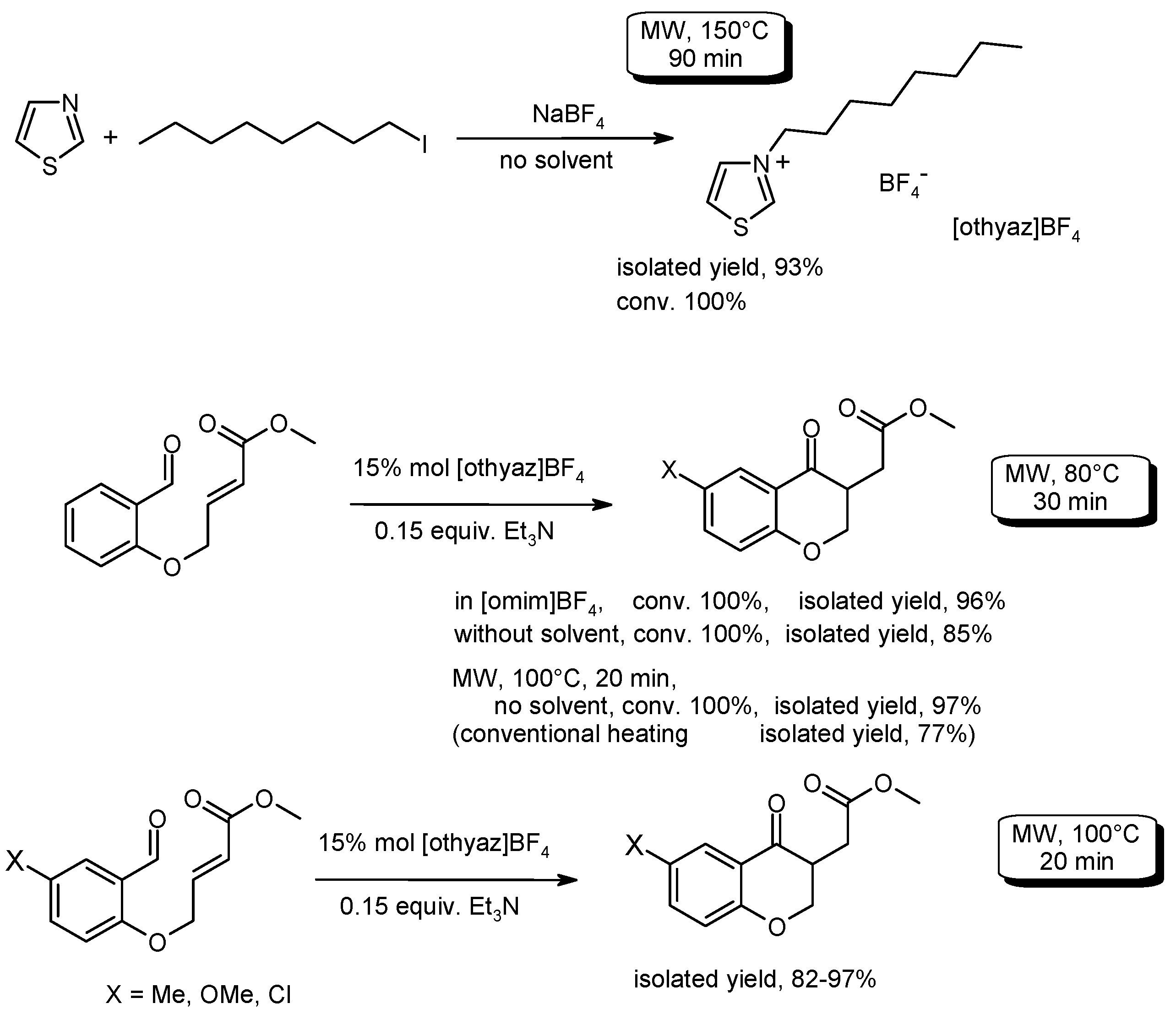
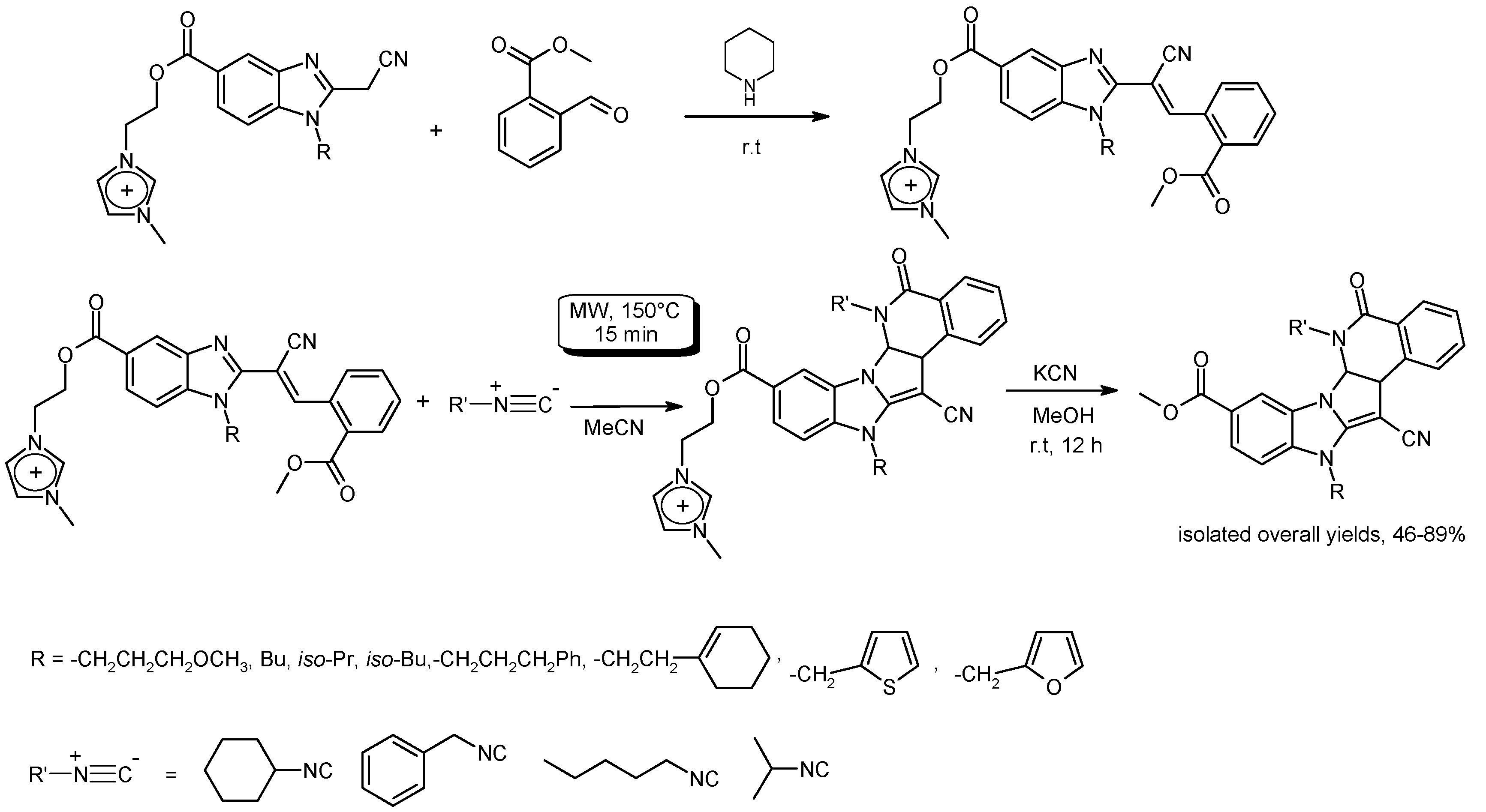
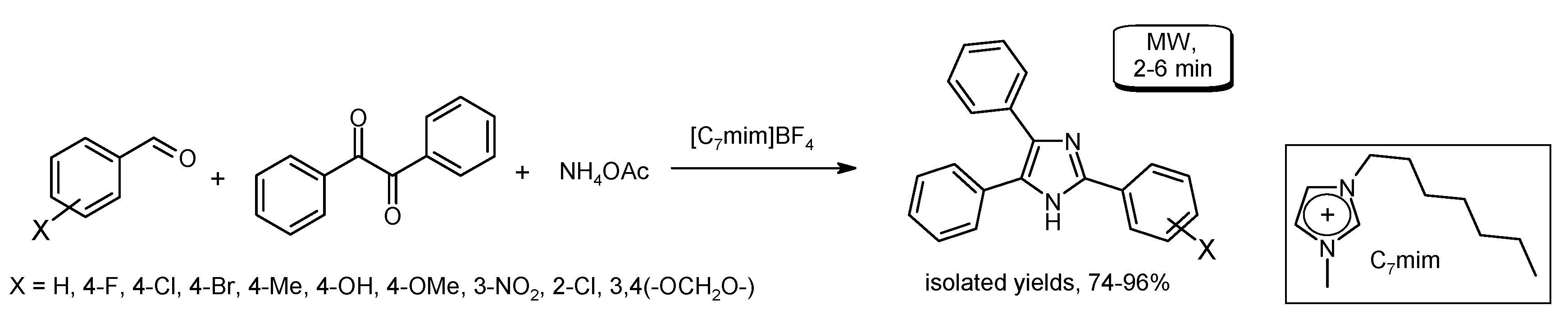
3.5. Cyclization Reactions to Heterocycles with C–C Bond Formation
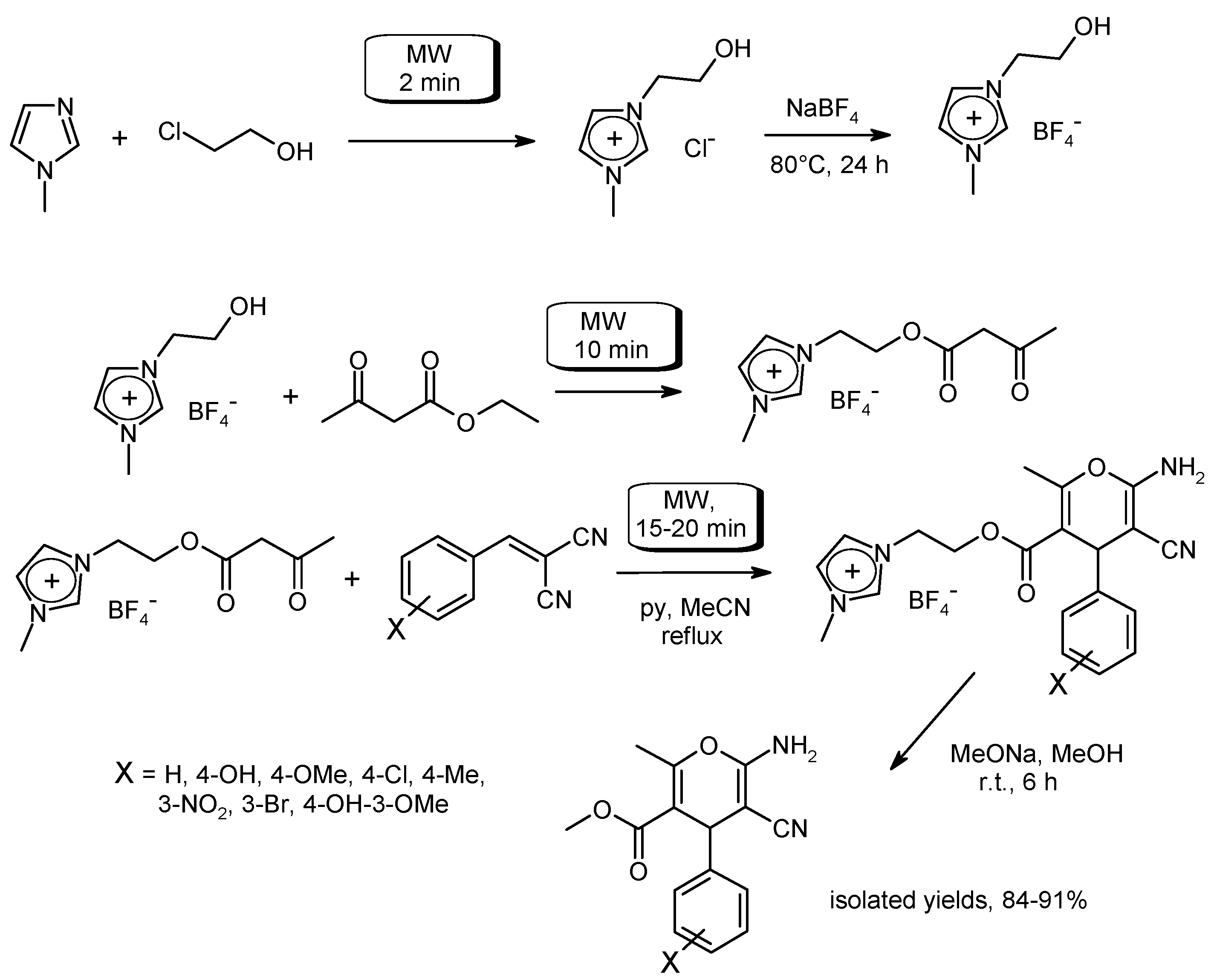
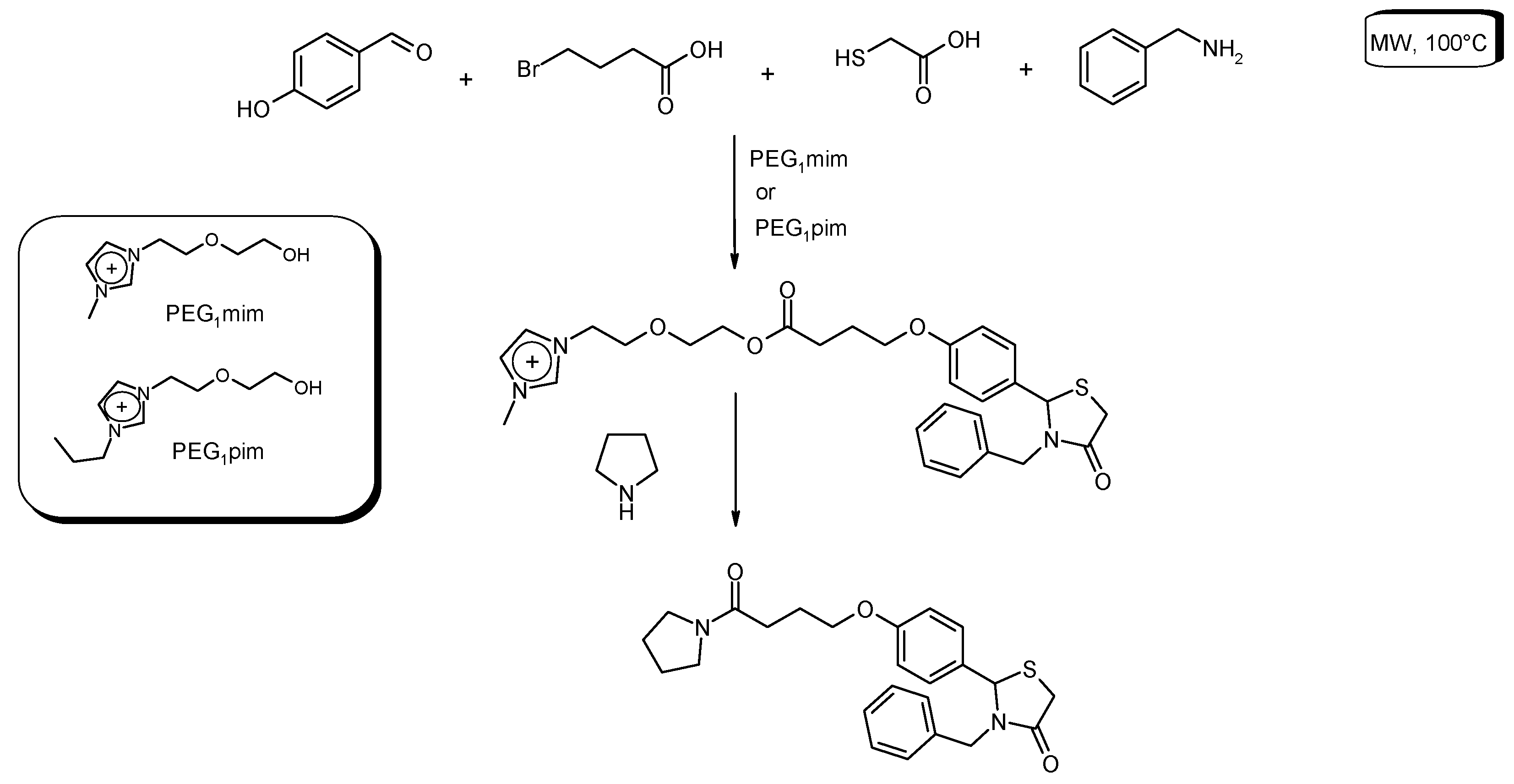
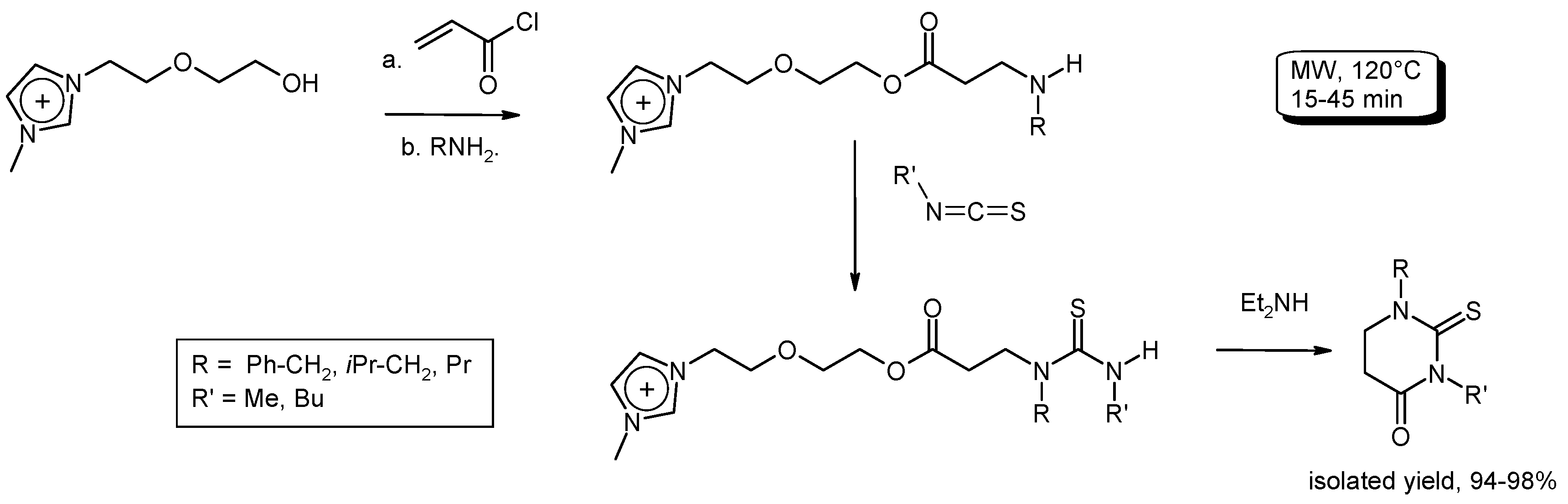
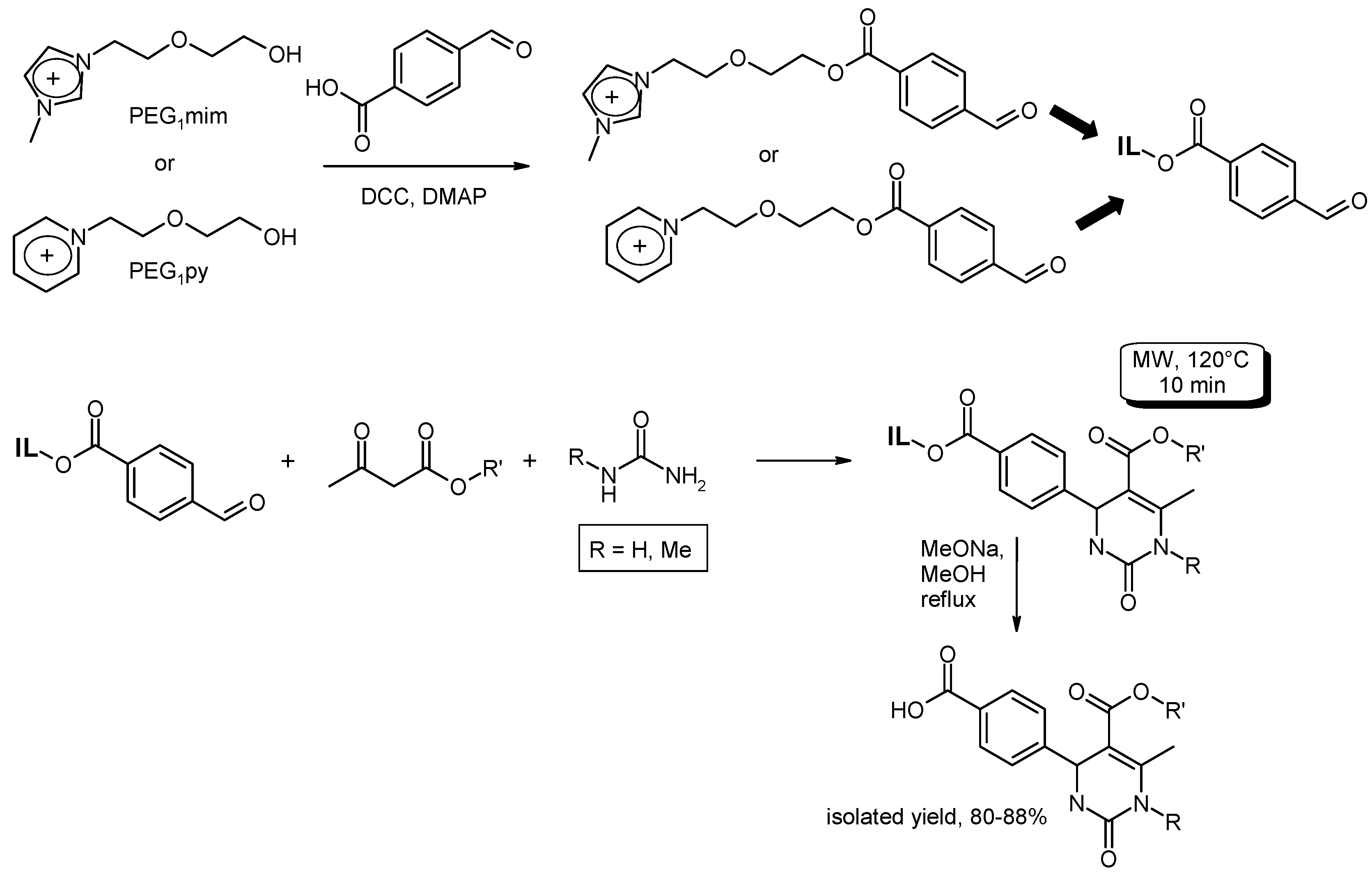
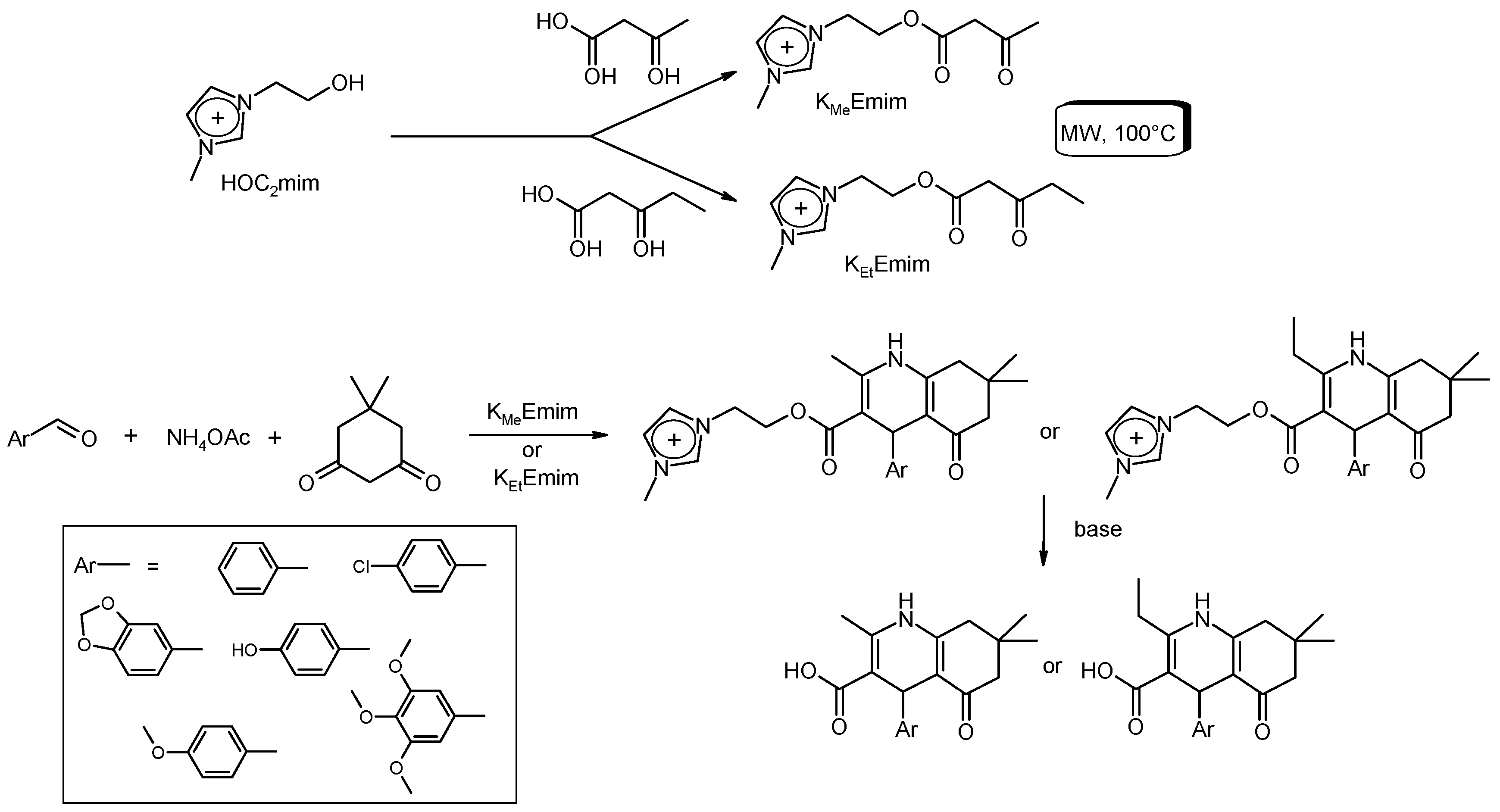
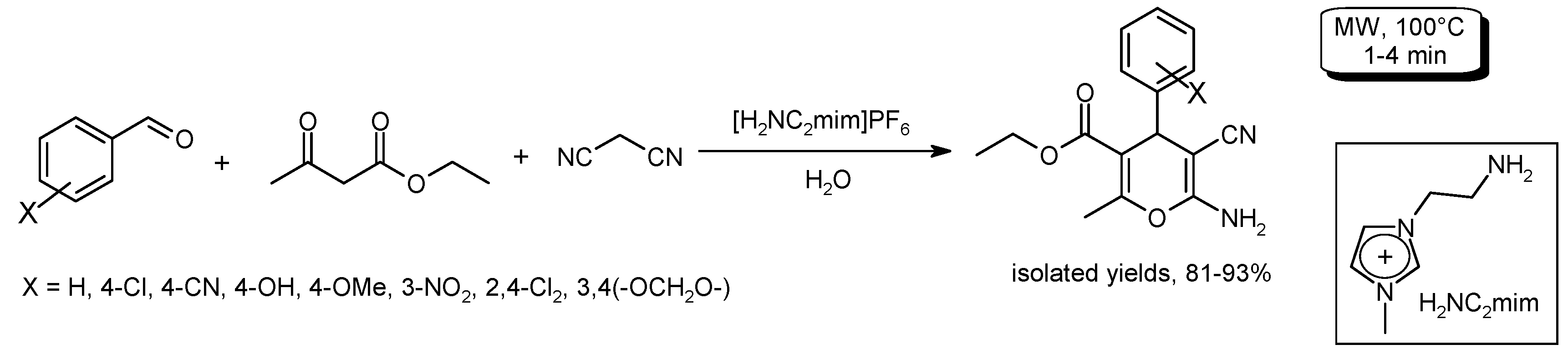
3.6. Multi-Component Reactions (MCRs) with C–CBond Formation
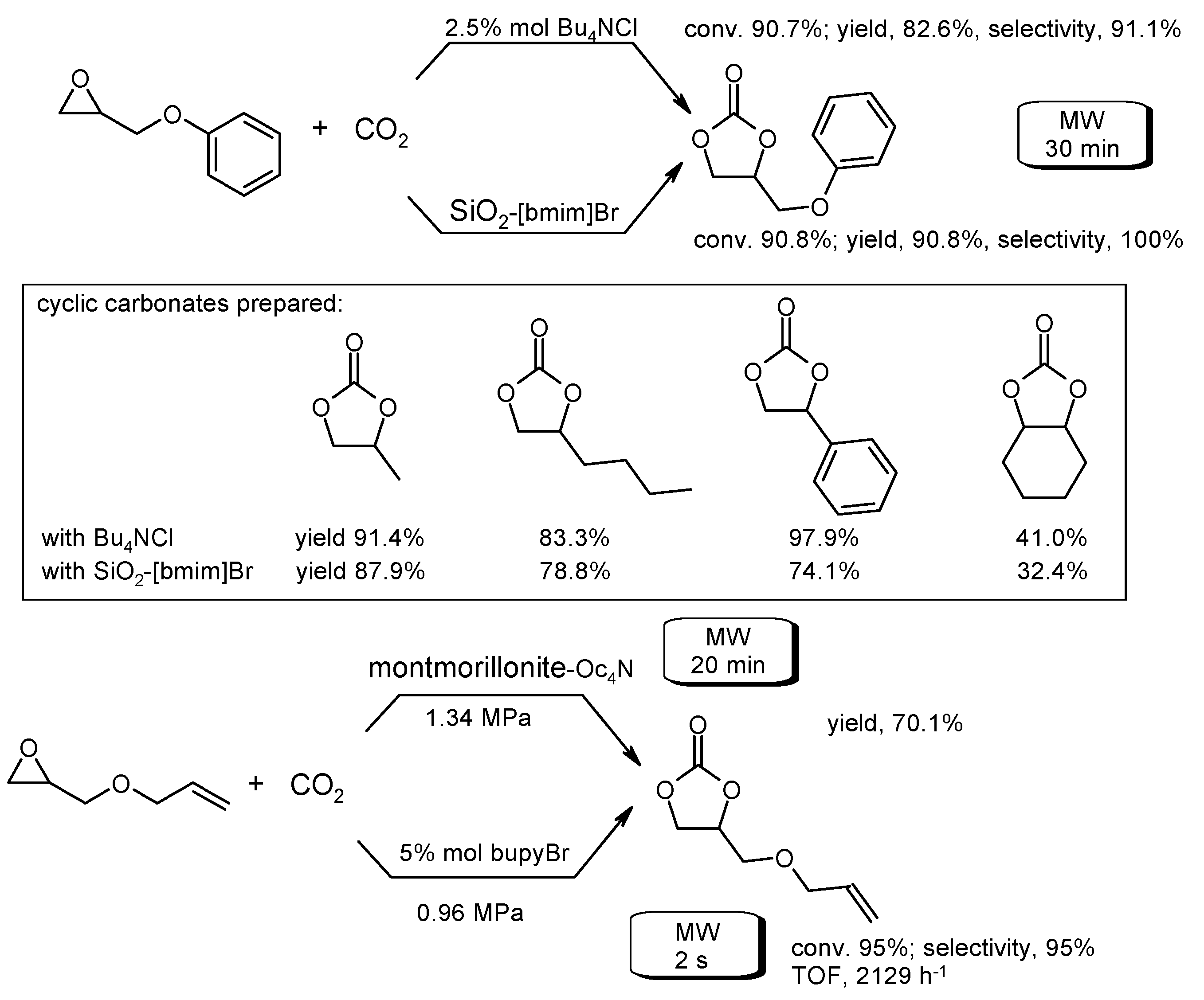
4. Carbon Dioxide Transformation
5. Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | acetyl, CH3CO |
| acac | anion of 2,4-pentanedione(acetylacetone) |
| Cp | cyclopentadienyl anion |
| dba | dibenzylideneacetone |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DMSO | dimethylsulfoxide |
| ECODS | extractive catalytic oxidative desulfurization |
| GO | graphene oxide |
| IL | ionic liquid |
| MCR | multi-component reaction |
| NP | nanoparticle |
| NTf2 | bis(trifluorometanesulfonyl) amide |
| PEG | poly(ethyleneglycol) |
| ODS | oxidative desulfurization |
| py | pyridine |
| SILLP | supported ionic liquid-like phases |
| TBHP | tert-butylhydroperoxide |
| TfO− | triflate CF3SO3- (IUPAC trifluoromethanesulfonate) |
| TOF | turnover frequency |
| tpm | hydrotris-(pyrazol-1-yl)methane |
| Ts | tosylate (p-toluenesulfonate) |
| VOC | volatile organic compound |
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Varma, R.S. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Loupy, A. (Ed.) Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Strauss, C.R. A strategic, green approach to organic chemistry with microwave assistance and predictive yield optimization as core, enabling technologies. Aust. J. Chem. 2009, 62, 3–15. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, J. Toward green catalytic synthesis—Transition metal-catalyzed reactions in non-conventional media. J. Mol. Catal. A Chem. 2007, 270, 1–43. [Google Scholar] [CrossRef]
- Keglevich, G.; Grun, A.; Balint, E.; Kiss, N.Z.; Kovacs, R.; Molnar, I.G.; Blastik, Z.; Toth, R.V.; Fehervari, A.; Csontos, I. Green chemical tools in organophosphorus chemistry—Organophosphorus tools in green chemistry. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 613–620. [Google Scholar] [CrossRef]
- Varma, R.S. “Greener” chemical syntheses using mechanochemical mixing or microwave and ultrasound irradiation. Green Chem. Lett. Rev. 2007, 1, 37–45. [Google Scholar] [CrossRef]
- Kumar, V.; Nigam, K.D.P. Process intensification in green synthesis. Green Proc. Synth. 2012, 1, 79–107. [Google Scholar] [CrossRef]
- Toma, Š.; Šebesta, R.; Mečiarova, M. Organocatalytic reactions under unusual conditions. Curr. Org. Chem. 2011, 15, 2257–2281. [Google Scholar] [CrossRef]
- Martínez-Palou, R. Microwave-assisted synthesis using ionic liquids. Mol. Divers. 2010, 14, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, N.E.; Torenius, H.M.; Tye, H. Microwave-promoted organic synthesis using ionic liquids: A mini review. Comb. Chem. High Throughput Screen. 2004, 7, 511–528. [Google Scholar] [CrossRef]
- Ogawa, C.; Kobayashi, S. Catalytic asymmetric synthesis in nonconventional media/conditions. In Catalytic Asymmetric Synthesis, 3rd ed.; Ojima, I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–35. [Google Scholar]
- Bortolini, O.; Campestrini, S.; Conte, V.; Fantin, G.; Fogagnolo, M.; Maietti, S. Sustainable epoxidation of electron-poor olefins with hydrogen peroxide in ionic liquids and recovery of the products with supercritical CO2. Eur. J. Org. Chem. 2003, 2003, 4804–4809. [Google Scholar] [CrossRef]
- Conte, V.; Fabbianesi, F.; Floris, B.; Galloni, P.; Sordi, D.; Arends, I.W.C.E.; Bonchio, M.; Rehder, D.; Bogdal, D. Vanadium-catalyzed, microwave-assisted oxidations with H2O2 in ionic liquids. Pure Appl. Chem. 2009, 81, 1265–1277. [Google Scholar] [CrossRef]
- Berardi, S.; Conte, V.; Fiorani, G.; Floris, B.; Galloni, P. Improvement of ferrocene acylation. Conventional vs. microwave heating for scandium-catalyzed reaction in alkylmethylimidazolium-based ionic liquids. J. Organomet. Chem. 2008, 693, 3015–3020. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Fu, Z.; Liu, Y.; Yi, X.; Zheng, A.; Kirk, S.R.; Yin, D. Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 2017, 24, 95–106. [Google Scholar] [CrossRef]
- Goswami, S.R.; Dumont, M.-J.; Raghavan, V. Starch to value added biochemicals. Starch 2016, 68, 274–286. [Google Scholar] [CrossRef]
- Xu, J.-K.; Chen, J.-H.; Sun, R.-C. Hydrothermal microwave valorization of eucalyptus using acidic ionic liquid as catalyst toward a green biorefinery scenario. Bioresour. Technol. 2015, 193, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Yuan, Y.; Zhang, Q.; Fang, X.; Zang, S. Hydrolysis of cellulose catalyzed by quaternary ammonium perrhenates in 1-allyl-3-methylimidazolium chloride. Bioresour. Technol. 2015, 197, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhan, Y.; Fang, X.; Yu, D. Enhanced catalytic activity and thermal stability of 2,4-dichlorophenol hydroxylase by using microwave irradiation and imidazolium ionic liquid for 2,4-dichlorophenol removal. RSC Adv. 2014, 4, 62631–62638. [Google Scholar] [CrossRef]
- Qian, E.W.; Tominaga, H.; Chen, T.L.; Isoe, R. Synthesis of functional ionic liquids and their application for the direct saccharification of cellulose. J. Chem. Eng. Jpn. 2016, 49, 466–474. [Google Scholar] [CrossRef]
- Truong, T.-K.-T.; Vo-Thanh, G. Synthesis of functionalized chiral ammonium, imidazolium, and pyridinium-based ionic liquids derived from (−)-ephedrine using solvent-free microwave activation. Applications for the asymmetric Michael addition. Tetrahedron 2010, 66, 5277–5282. [Google Scholar] [CrossRef]
- Jadhav, A.H.; Lim, A.C.; Thorat, G.M.; Jadhav, H.S.; Seo, J.G. Green solvents ionic liquids: Structural directing pioneers for microwave assisted synthesis of controlled MgO nanostructures. RSC Adv. 2016, 6, 31675–31684. [Google Scholar] [CrossRef]
- Vollmer, C.; Redel, E.; Abu-Shandi, K.; Thomann, R.; Manyar, H.; Hardacre, C.; Janiak, C. Microwave irradiation for the facile synthesis of transition-metal nanoparticles (NPs) in ionic liquids (ILs) from metal–carbonyl precursors and Ru-, Rh-, and Ir-NP/IL dispersions as biphasic liquid-liquid hydrogenation nanocatalysts for cyclohexene. Chem. Eur. J. 2010, 16, 3849–3858. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Nadagouda, M.N.; Varma, R.S. Microwave-assisted chemistry. A rapid and sustainable route to synthesis of organics and nanomaterials. Aust. J. Chem. 2009, 62, 16–26. [Google Scholar] [CrossRef]
- Lang, X.; Shi, M.; Chu, Y.; Liu, W.; Chen, Z.; Ma, C. Microwave-assisted synthesis of Pt-WC/TiO2 in ionic liquid and its application for methanol oxidation. J. Solid State Electrochem. 2013, 17, 2401–2408. [Google Scholar] [CrossRef]
- Safavi, A.; Momeni, S.; Tohidi, M. Silver-Palladium nanoalloys modified carbon ionic liquid electrode with enhanced electrocatalytic activity towards formaldehyde oxidation. Electroanalysis 2012, 24, 1981–1988. [Google Scholar] [CrossRef]
- Hu, H.; Yang, H.; Huang, P.; Cui, D.; Peng, Y.; Zhang, J.; Lu, F.; Liand, J.; Shi, D. Unique role of ionic liquid in microwave-assisted synthesis of monodisperse magnetite nanoparticles. Chem. Commun. 2010, 46, 3866–3868. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Cravotto, G.; Garella, D.; Tagliapietra, S.; Bonrath, W. Esterification of terpene alcohols catalyzed by acidic Brønsted ionic liquids. Org. Prep. Proced. Int. 2012, 44, 175–179. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Kuang, Y.; Tan, Z.; Song, L.; Han, X. Catalytic activity and process variables optimization during microwave synthesis of methyl caprylate over various multi-SO3H functionalized ionic liquids. J. Taiwan Inst. Chem. Eng. 2015, 49, 51–57. [Google Scholar] [CrossRef]
- Xiong, X.; Yi, C.; Han, Q.; Shi, L.; Li, S. I2/ionic liquid as a highly efficient catalyst for per-O-acetylation of sugar under microwave irradiation. Chin. J. Catal. 2015, 36, 237–243. [Google Scholar] [CrossRef]
- Arfan, A.; Bazureau, J.P. Efficient combination of recyclable task specific ionic liquid and microwave dielectric heating for the synthesis of lipophilic esters. Org. Process Res. Dev. 2005, 9, 745–748. [Google Scholar] [CrossRef]
- Altimari, J.M.; Delaney, J.P.; Servinis, L.; Squire, J.S.; Thornton, M.T.; Khosa, S.K.; Long, B.M.; Johnstone, M.D.; Fleming, C.L.; Pfeffer, F.M.; et al. Rapid formation of diphenylmethyl ethers and thioethers using microwave irradiation and protic ionic liquids. Tetrahedron Lett. 2012, 53, 2035–2039. [Google Scholar] [CrossRef]
- Zare, A.; Hasaninejad, A.; Khalafi-Nezhad, A.; Moosavi Zare, A.R.; Parhami, A. Organic reactions in ionic liquids: MgO as efficient and reusable catalyst for the Michael addition of sulfonamides to α,β-unsaturated esters under microwave irradiation. Arch. Org. Chem. 2007, 2007, 105–115. [Google Scholar]
- Zare, A.; Hasaninejad, A.; Moosavi Zare, A.R.; Parhami, A.; Sharghi, H.; Khalafi-Nezhad, A. Zinc oxide as a new, highly efficient, green, and reusable catalyst for microwave-assisted Michael addition of sulfonamides to α,β-unsaturated esters in ionic liquids. Can. J. Chem. 2007, 85, 438–444. [Google Scholar] [CrossRef]
- Hernández, J.G.; Juaristi, E. Recent efforts directed to the development of more sustainable asymmetric organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saima; Shard, A.; Andhare, N.H.; Richa; Sinha, A.K. Thiol–Ene “Click” Reaction Triggered by Neutral Ionic Liquid: The “Ambiphilic” Character of [hmim]Br in the Regioselective Nucleophilic Hydrothiolation. Angew. Chem. Int. Ed. 2015, 54, 828–832. [Google Scholar] [CrossRef] [PubMed]
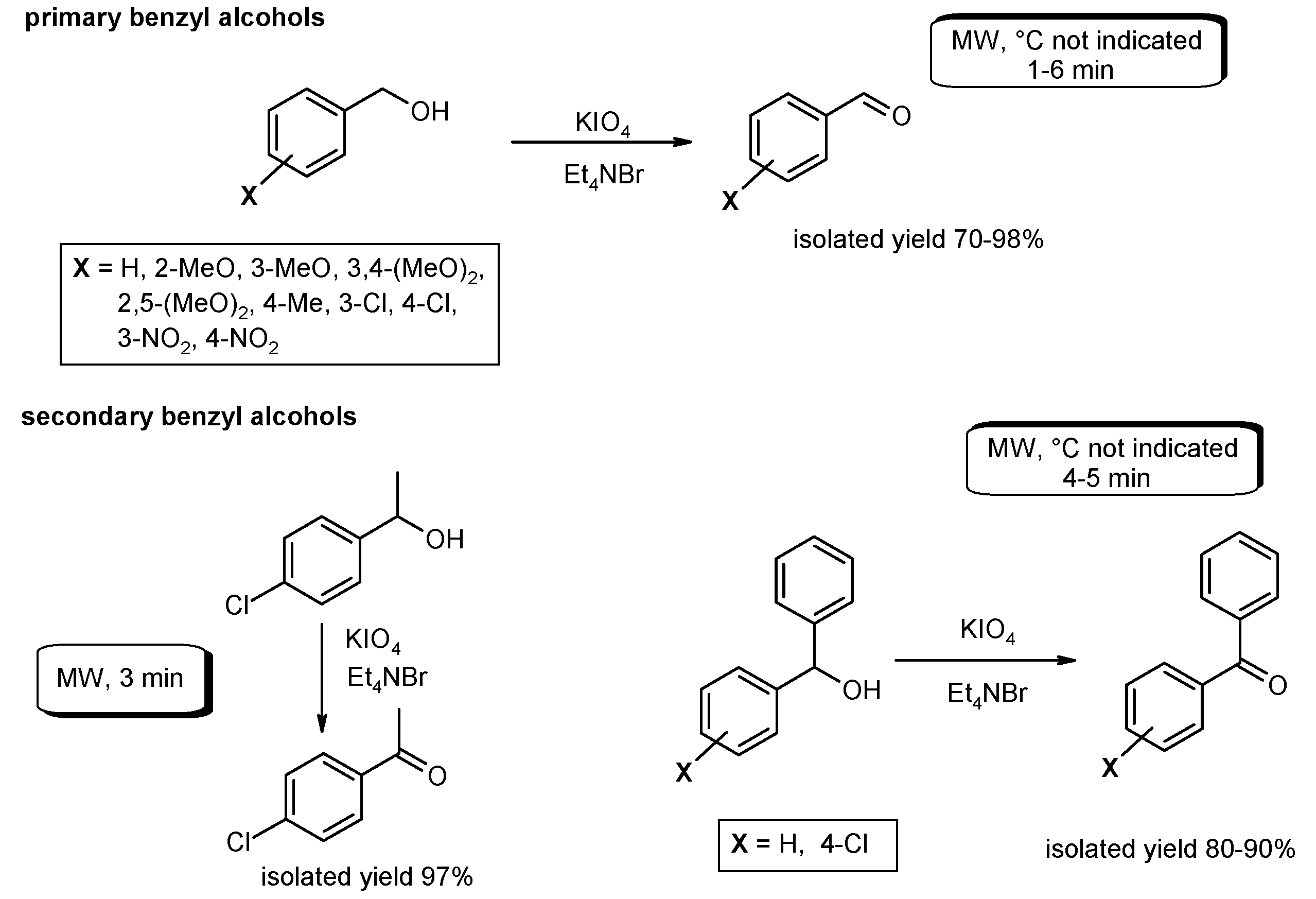
- Hajipour, A.R.; Rafiee, F.; Ruoho, A.E. Oxidation of benzylic alcohols to their corresponding carbonyl compounds using KIO4 in ionic liquid by microwave irradiation. Synth. Commun. 2006, 36, 2563–2568. [Google Scholar] [CrossRef]
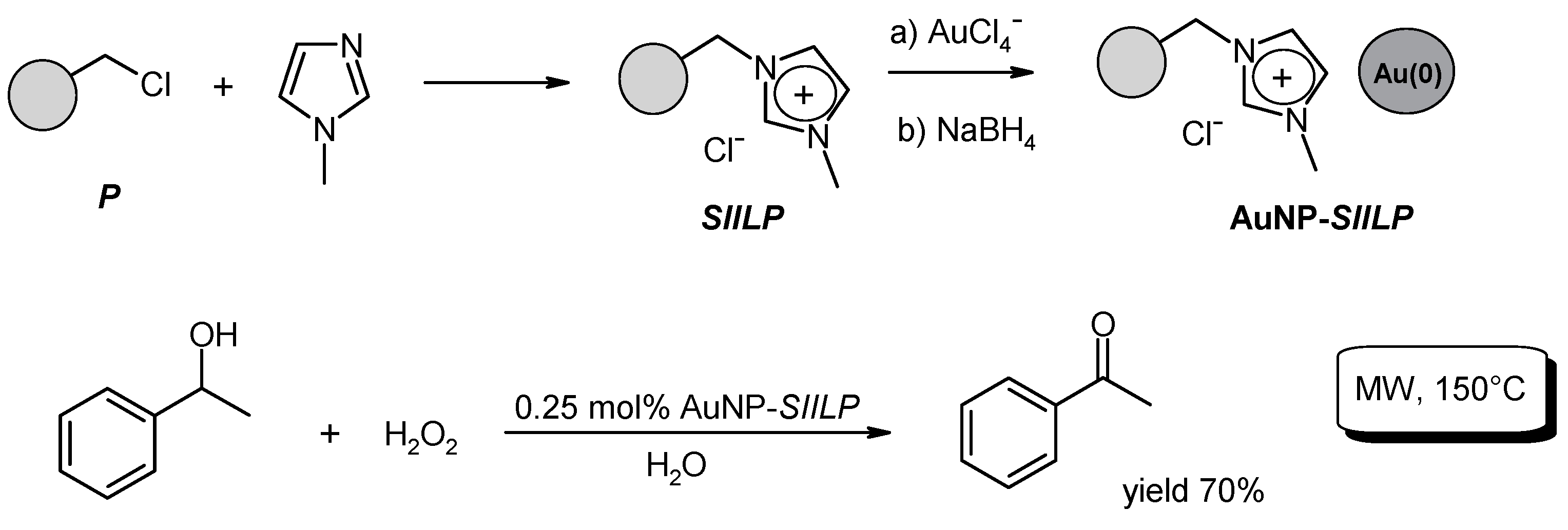
- Restrepo, J.; Lozano, P.; Burguete, M.I.; Garcia-Verdugo, E.; Luis, S.V. Gold nanoparticles immobilized onto supported ionic liquid-like phases for microwave phenylethanol oxidation in water. Catal. Today 2015, 255, 97–101. [Google Scholar] [CrossRef]
- Haider, P.; Kimmerle, B.; Krumeich, F.; Kleist, W.; Grunwaldt, J.-D.; Baiker, A. Gold-catalyzed aerobic oxidation of benzyl alcohol: Effect of gold particle size on activity and selectivity in different solvents. Catal. Lett. 2008, 125, 169–176. [Google Scholar] [CrossRef]
- Díaz-Urrutia, C.; Hurisso, B.B.; Gauthier, P.M.P.; Sedai, B.; Singer, R.D.; Baker, R. Catalytic aerobic oxidation of lignin-derived bio-oils using oxovanadium and copper complex catalysts and ionic liquids. J. Mol. Catal. A Chem. 2016, 423, 414–422. [Google Scholar] [CrossRef]
- Chatel, G.; Monnier, C.; Kardos, N.; Voiron, C.; Andrioletti, B.; Draye, M. Green, selective and swift oxidation of cyclic alcohols to corresponding ketones. Appl. Catal. A Gen. 2014, 478, 157–164. [Google Scholar] [CrossRef]
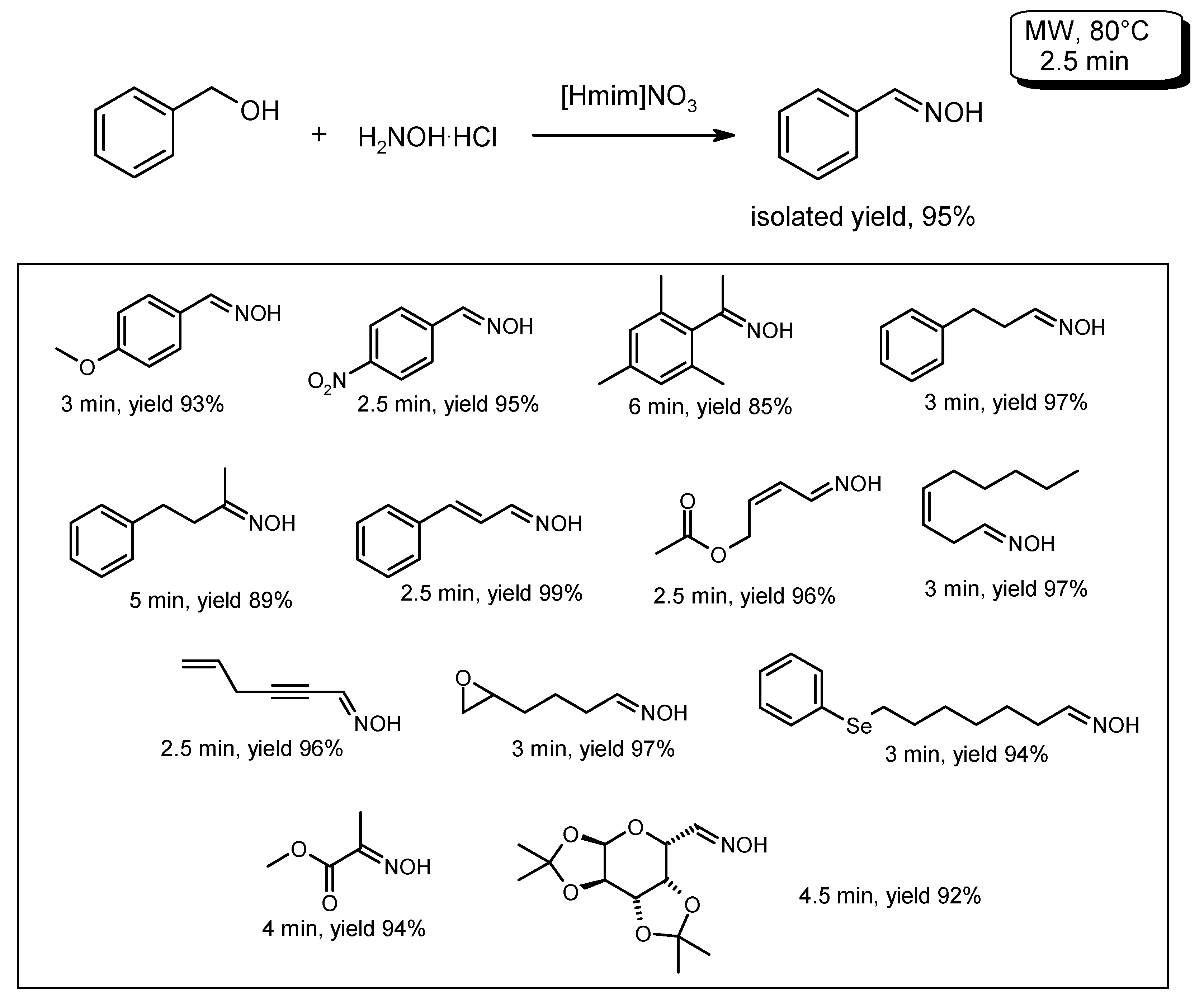
- Mirjafari, A.; Mobarrez, N.; O’Brien, R.A.; Davis, J.H., Jr.; Noei, J. Microwave-promoted one-pot conversion of alcohols to oximes using 1-methylimidazolium nitrate, [Hmim][NO3], as a green promoter and medium. Comptes Rendus Chim. 2011, 14, 1065–1070. [Google Scholar] [CrossRef]
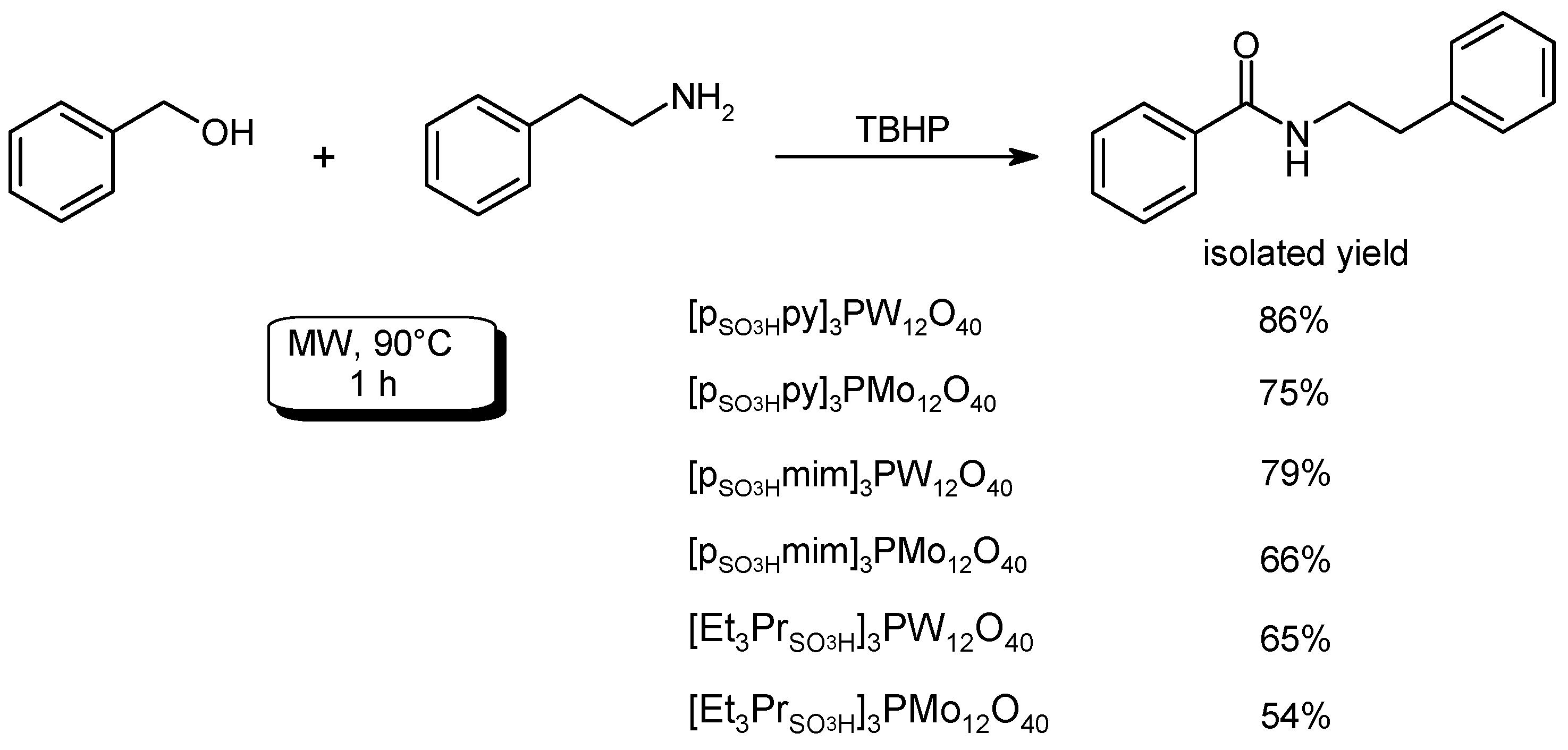
- Fu, R.; Yang, Y.; Feng, W.; Ge, Q.; Feng, Y.; Zeng, X.; Chai, W.; Yi, J.; Yuan, R. An efficient, eco-friendly and sustainable tandem oxidative amidation of alcohols with amines catalyzed by heteropolyanion-based ionic liquids via a bifunctional catalysis process. Tetrahedron 2016, 72, 8319–8326. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Jin, W.; Gu, H.; Zeng, X.; Chai, W.; Ma, Y.; Wang, Q.; Yi, J.; Yuan, R. Microwave-assisted heteropolyanion-based ionic liquid promoted sustainable protocol to N-heteroaryl amides via N-directing dual catalyzed oxidative amidation of aldehydes. RSC Adv. 2016, 6, 107699–107707. [Google Scholar] [CrossRef]
- Abrantes, M.; Neves, P.; Antunes, M.M.; Gago, S.; Almeida Paz, F.A.; Rodrigues, A.E.; Pillinger, M.; Goncalves, I.S.; Silva, C.M.; Valente, A.A. Microwave-assisted molybdenum-catalyzed epoxidation of olefins. J. Mol. Catal. A Chem. 2010, 320, 19–26. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bruno, S.M.; Gago, S.; Lopes, R.P.; Machado, D.A.; Carminatti, A.P.; Valente, A.A.; Pillinger, M.; Goncalves, I.S. Epoxidation of cyclooctene using soluble or MCM-41-supported molybdenum tetracarbonyl-pyridylimine complexes as catalyst precursors. J. Organomet. Chem. 2011, 696, 3543–3550. [Google Scholar] [CrossRef]
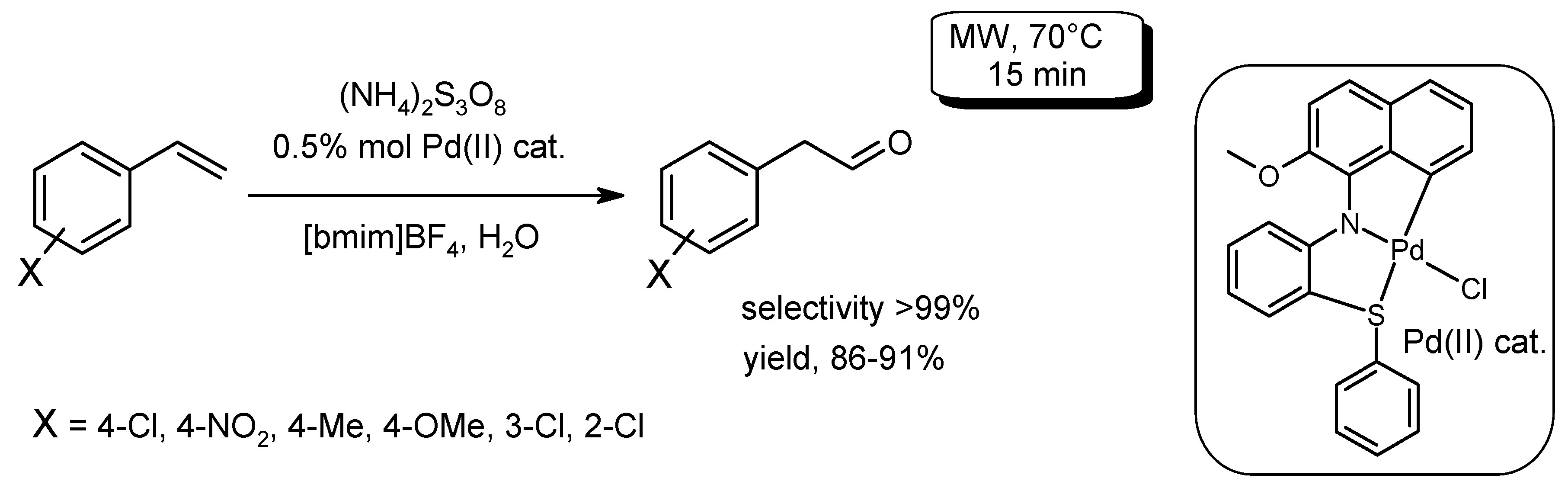
- Sarma, K.; Devi, N.; Sutradhar, D.; Sarma, B.; Chandra, A.K.; Barman, P. Synthesis of a novel six membered CNS palladacycle; TD-DFT study and catalytic activity towards microwave-assisted selective oxidation of terminal olefin to aldehyde. J. Organomet. Chem. 2016, 822, 20–28. [Google Scholar] [CrossRef]
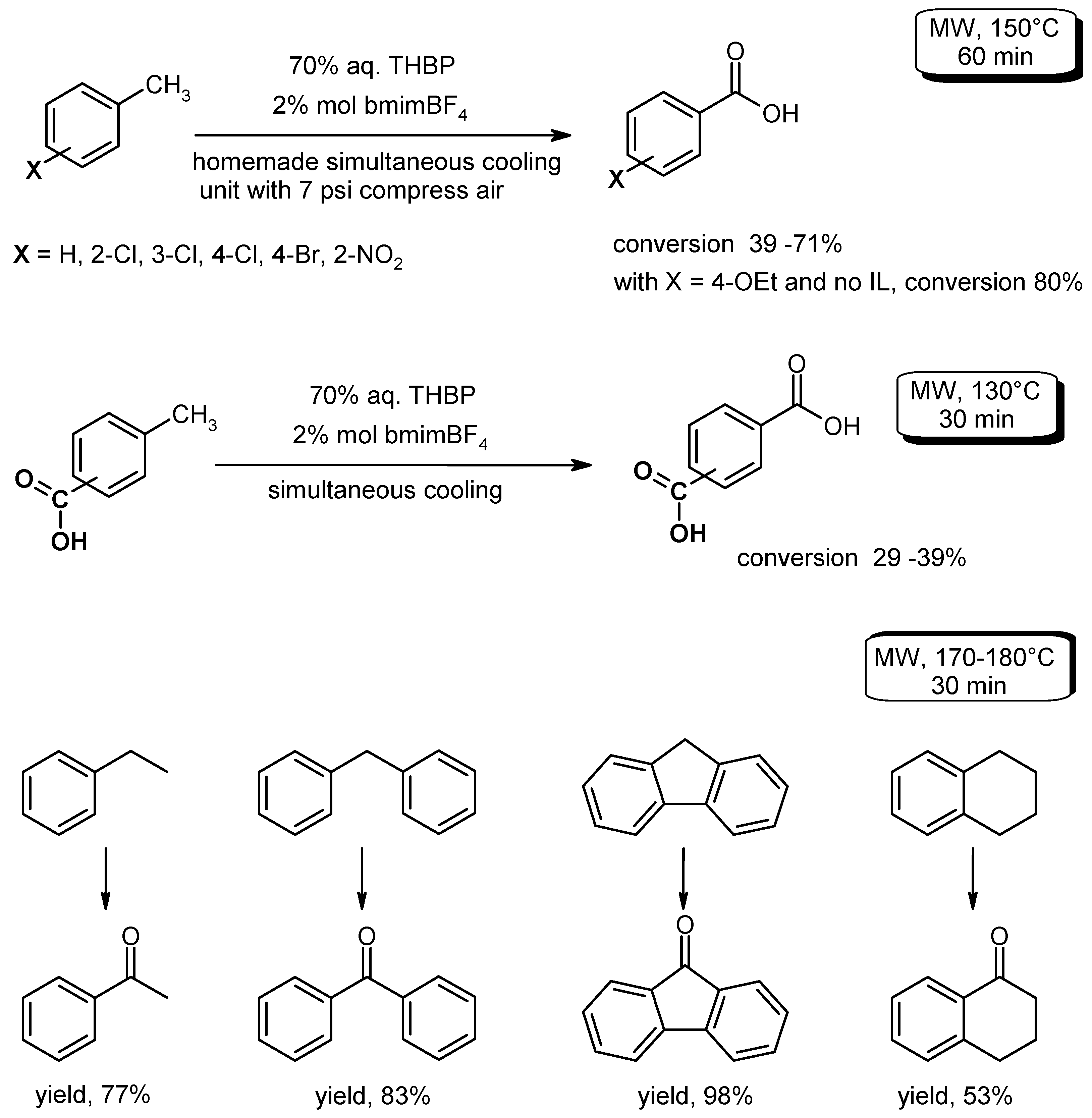
- He, H.; Pei, B.-J.; Lee, A.W.M. Metal free oxidation of alkyl substituted aromatics with aqueous tert-butyl hydroperoxide under microwave irradiation. Green Chem. 2009, 11, 1857–1861. [Google Scholar] [CrossRef]
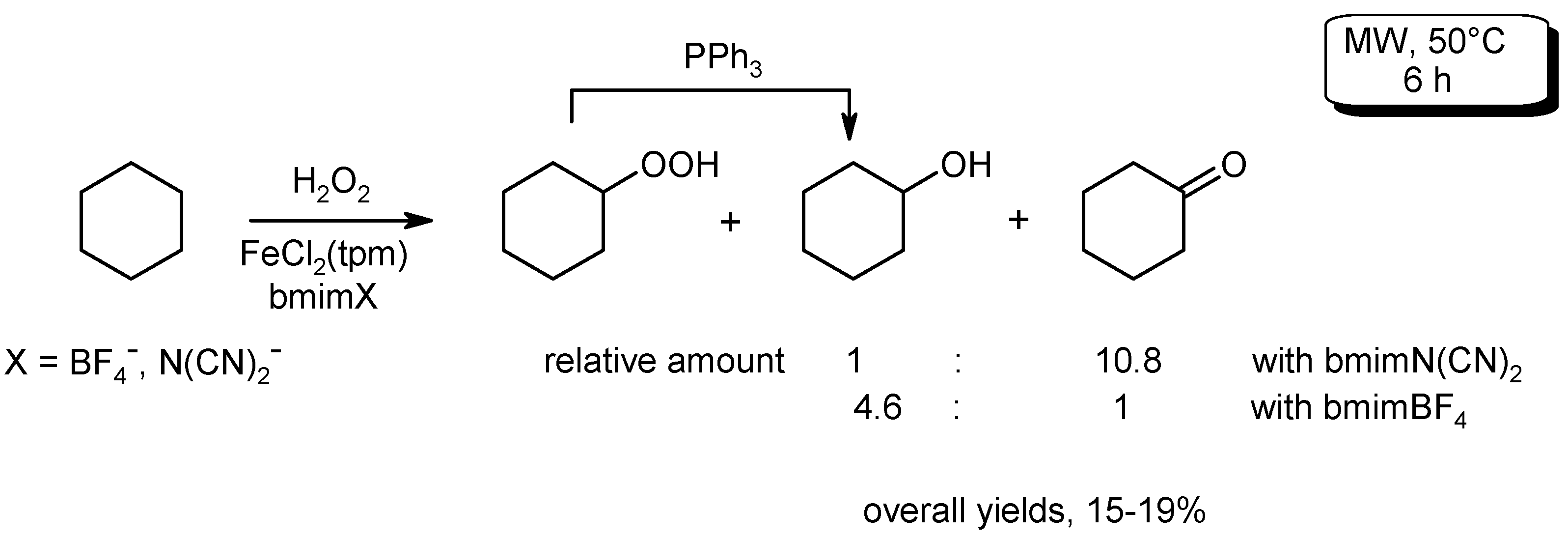
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning cyclohexane oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Coletti, A. From Catalysis to Electron Transfer Processes: Sustainability as Guideline. Ph.D. Thesis, Università di Roma Tor Vergata, Rome, Italy, 2012. [Google Scholar]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable vanadium-catalyzed oxidation of organic substrates with H2O2. Catal. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
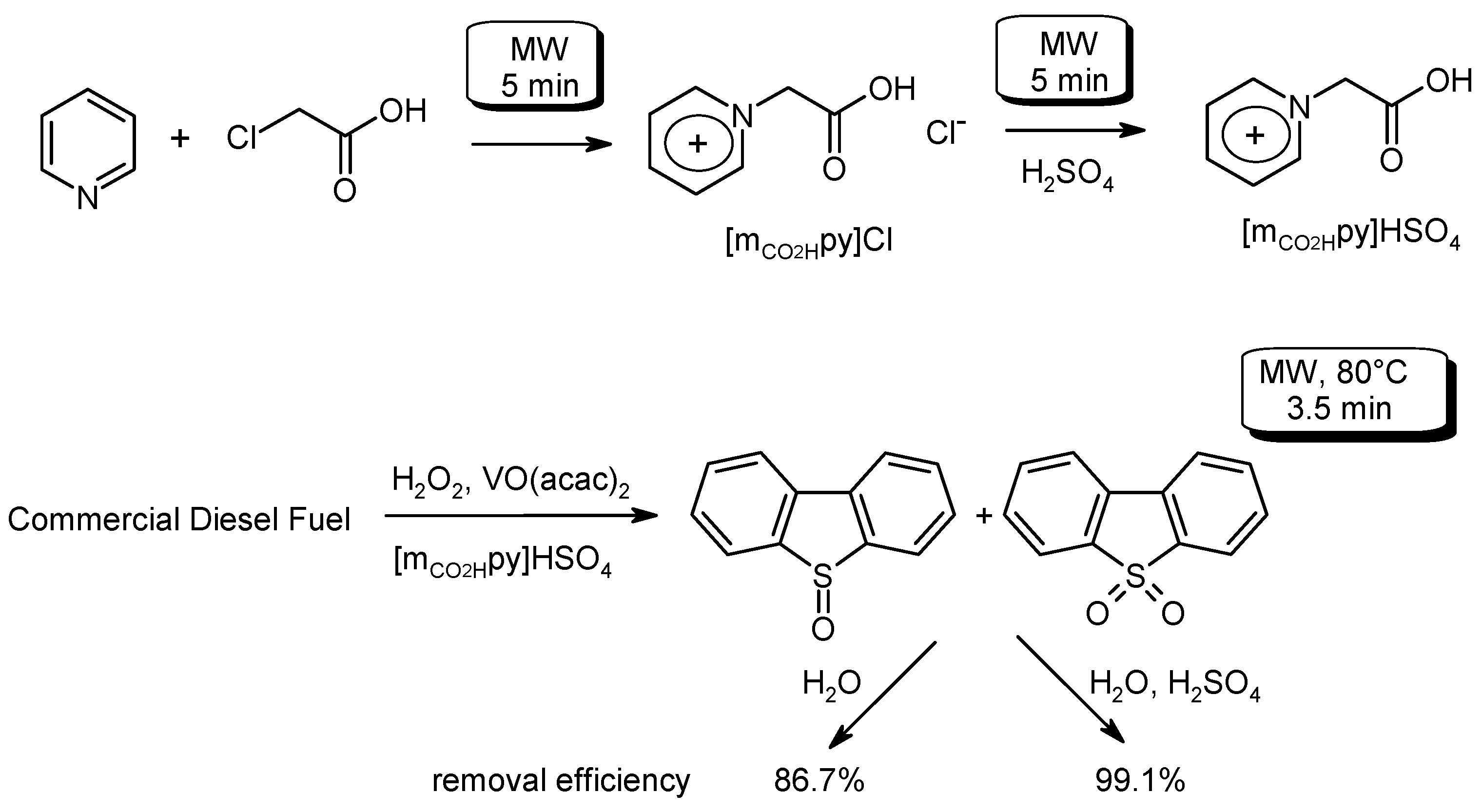
- Conte, V.; Galloni, P.; Centazzo, M.; Coletti, A. Process for Desulphurisation of Fuels. Patent PCT WO2015 162162 A1, 29 October 2015. [Google Scholar]
- Mesdour, S.; Lekbir, C.; Doumandji, L.; Hamada, B. Microwave-assisted extractive catalytic-oxidative desulfurization of diesel fuel via a VO(acac)2/ionic liquid system with H2O2 and H2SO4 as oxidizing agents. J. Sulfur Chem. 2017. [Google Scholar] [CrossRef]
- Mehta, V.P.; Van der Eycken, E.V. Microwave-assisted C–C bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2011, 40, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Kalck, P.; Urrutigoïty, M. Recent improvements in the alkoxycarbonylation reaction catalyzed by transition metal complexes. Inorg. Chim. Acta 2015, 431, 110–121. [Google Scholar] [CrossRef]
- Doháňošová, J.; Lásiková, A.; Toffano, M.; Gracza, T.; Vo-Thanh, G. Kinetic resolution of pent-4-ene-1,3-diol by Pd(II)-catalysed oxycarbonylation in ionic liquids. New J. Chem. 2012, 36, 1744–1750. [Google Scholar] [CrossRef]
- Bai, L.; Wang, J.-X. Environmentally friendly Suzuki aryl-aryl cross-coupling reaction. Curr. Org. Chem. 2005, 9, 535–553. [Google Scholar] [CrossRef]
- Dawood, K.M.; El-Deftar, M.M. Microwave-assisted C–C cross-coupling reactions of aryl and heteroaryl halides in water. Arch. Org. Chem. 2010, 2010, 319–330. [Google Scholar]
- Shaaban, M.R.; Darweesh, A.F.; Dawood, K.M. Mizoroki-Heck cross-couplings of 2-acetyl-5-bromobenzofuran and aryl halides under microwave irradiation. Arch. Org. Chem. 2010, 2010, 208–225. [Google Scholar]
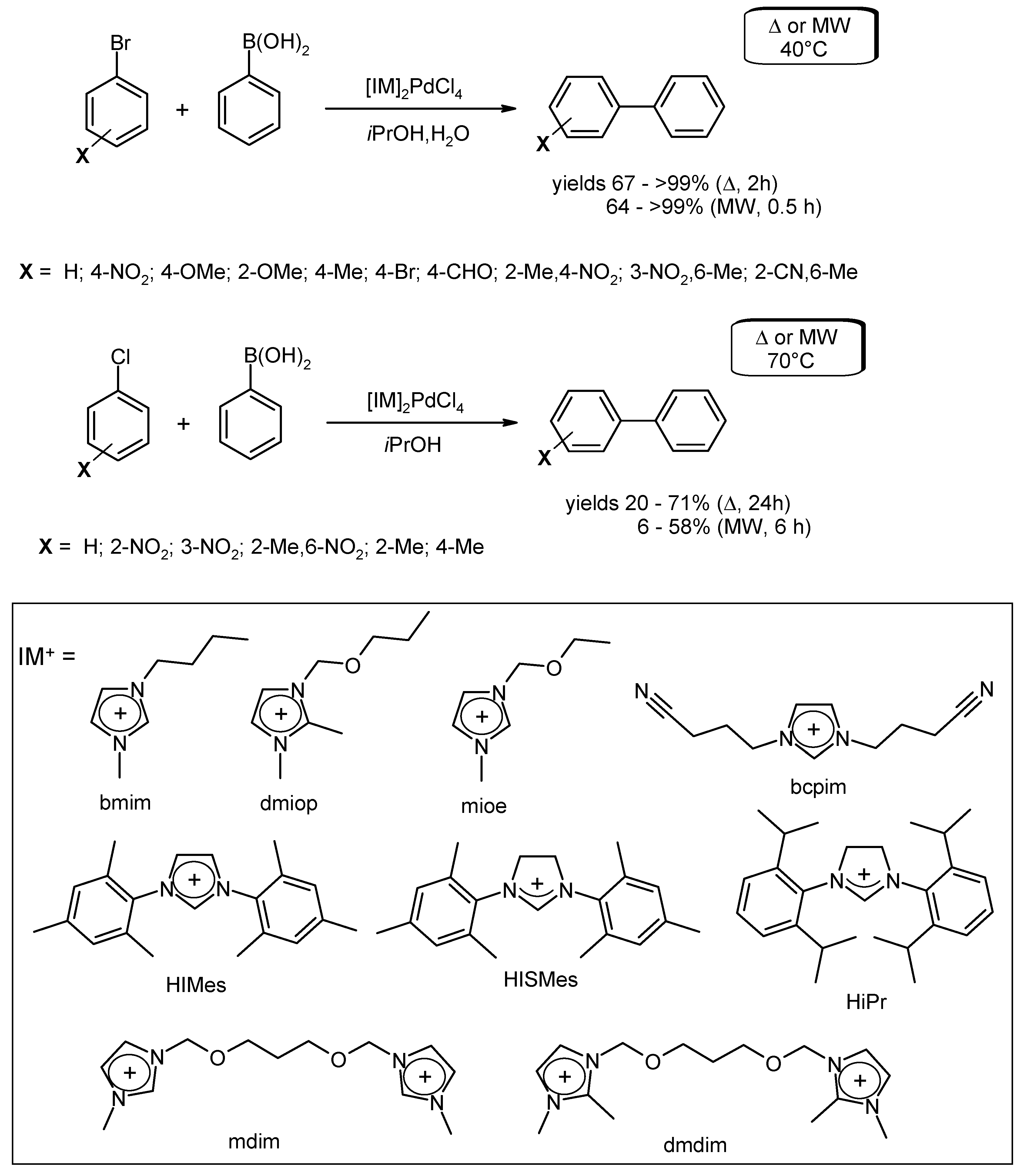
- Silarska, E.; Trzeciak, A.M.; Pernak, J.; Skrzypczak, A. [IL]2[PdCl4] complexes (IL = imidazolium cation) as efficient catalysts for Suzuki-Miyaura cross-coupling of aryl bromides and aryl chlorides. Appl. Catal. A Gen. 2013, 466, 216–223. [Google Scholar] [CrossRef]
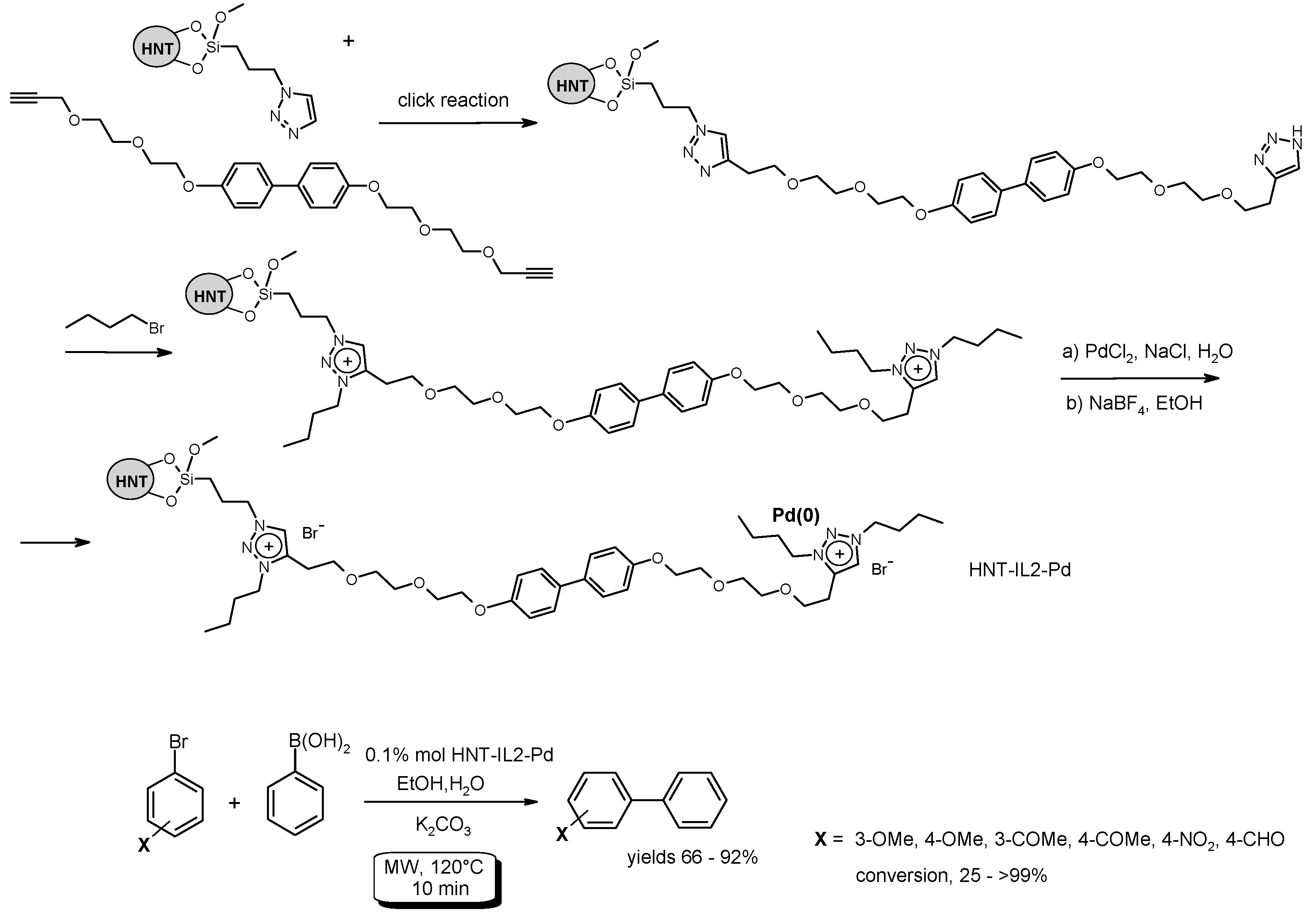
- Massaro, M.; Riela, S.; Cavallaro, G.; Gruttadauria, M.; Milioto, S.; Noto, R.; Lazzara, G. Eco-friendly functionalization of natural halloysite clay nanotube with ionic liquids by microwave irradiation for Suzuki coupling reaction. J. Organomet. Chem. 2014, 749, 410–415. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Parisi, F.; Lazzara, G. Palladium supported on halloysite-triazolium salts as catalyst for ligand free Suzuki cross-coupling in water under microwave irradiation. J. Mol. Catal. A Chem. 2015, 408, 12–19. [Google Scholar] [CrossRef]
- Campisciano, V.; La Parola, V.; Liotta, L.F.; Giacalone, F.; Gruttadauria, M. Fullerene-Ionic liquid conjugates: A new class of hybrid materials with unprecedented properties. Chem. Eur. J. 2015, 21, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
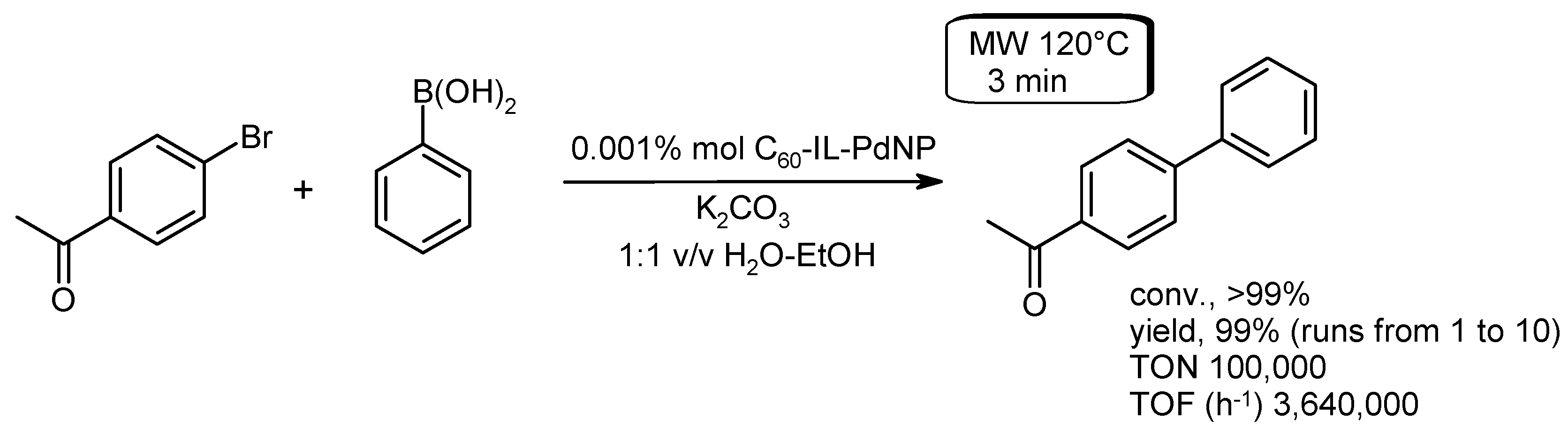
- Giacalone, F.; Campisciano, V.; Calabrese, C.; La Parola, V.; Liotta, L.F.; Aprile, C.; Gruttadauria, M. Supported C60-IL-PdNPs as extremely active nanocatalysts for C–C cross-coupling reactions. J. Mater. Chem. A 2016, 4, 17193–17206. [Google Scholar] [CrossRef]
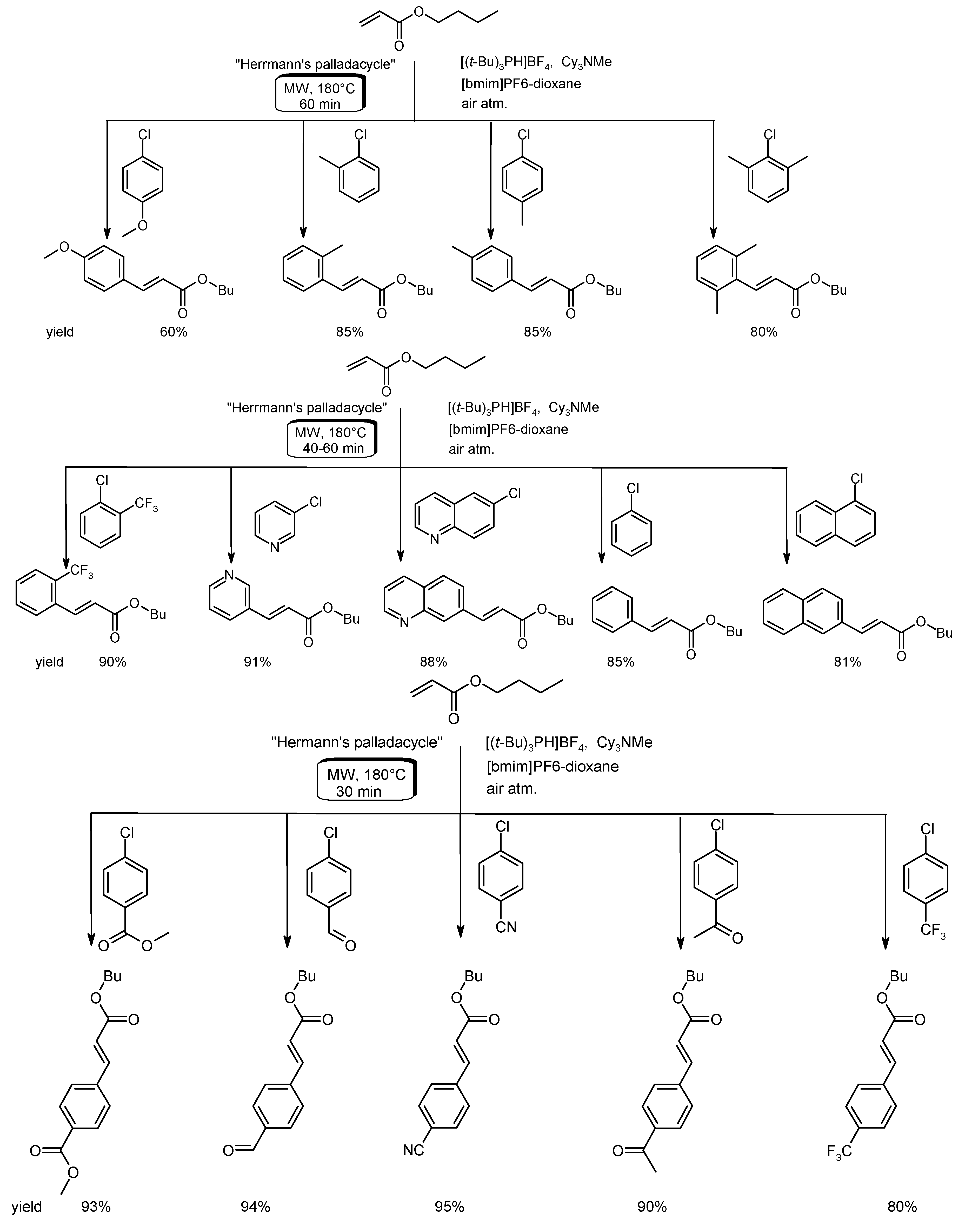
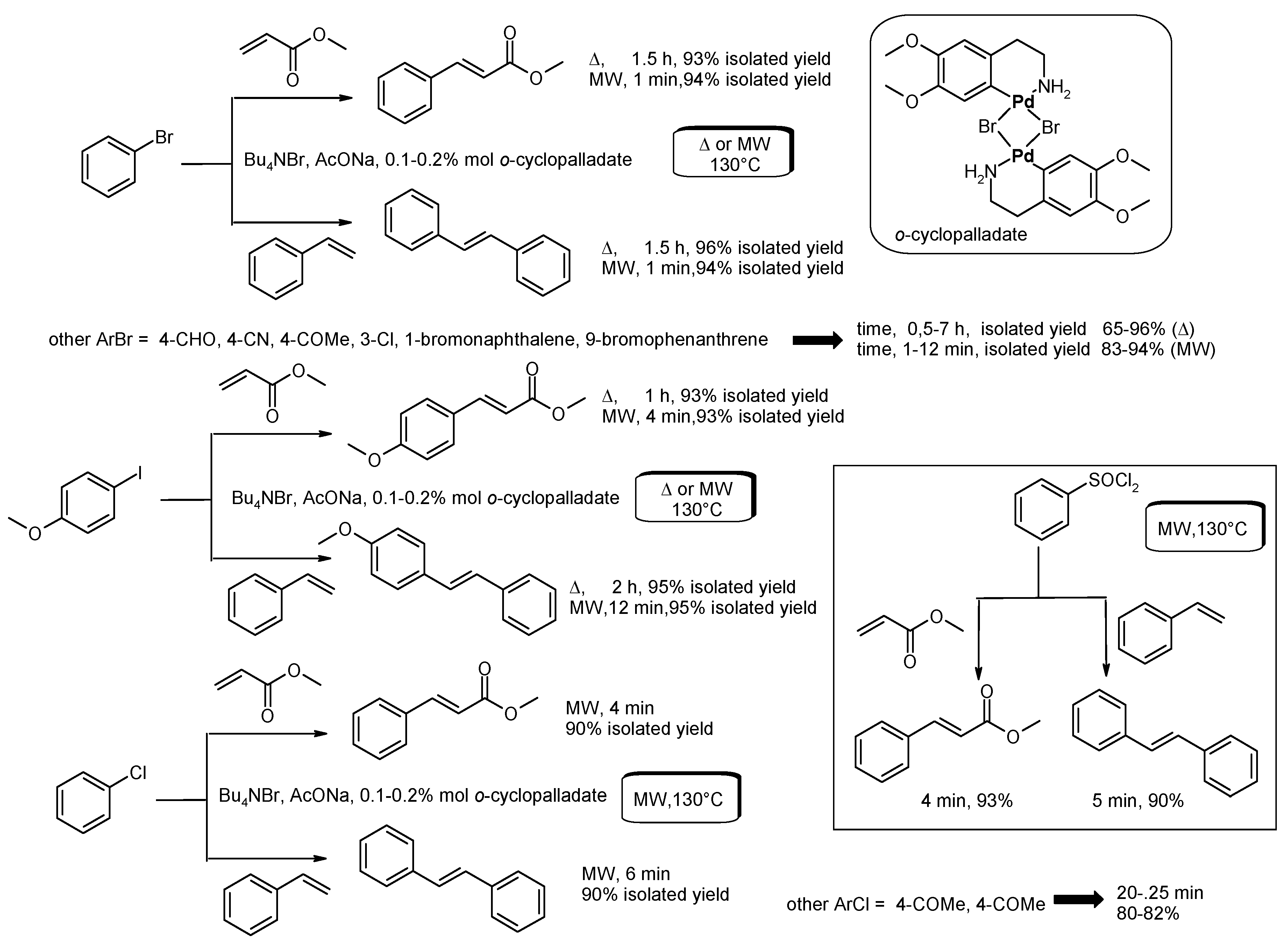
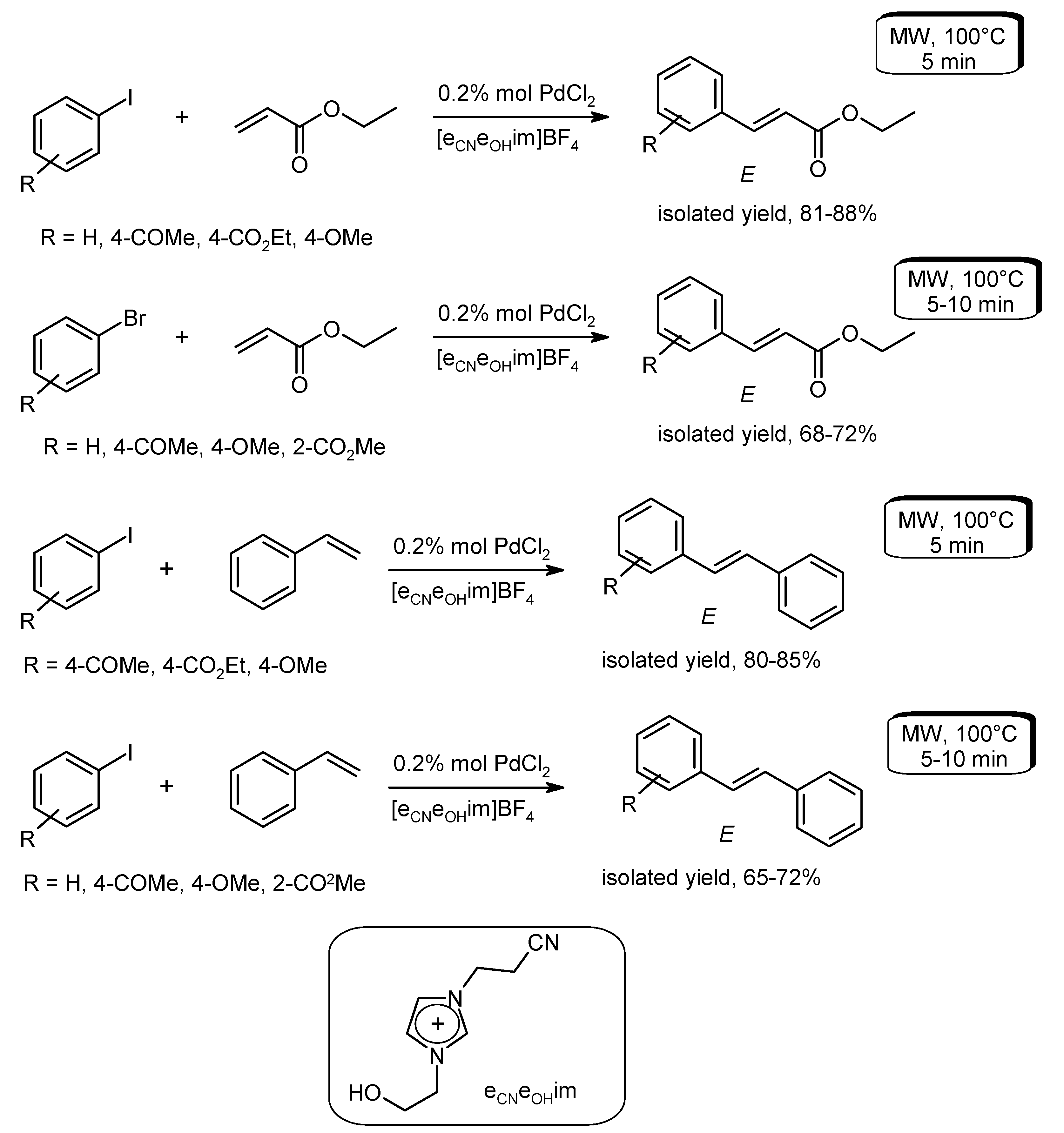
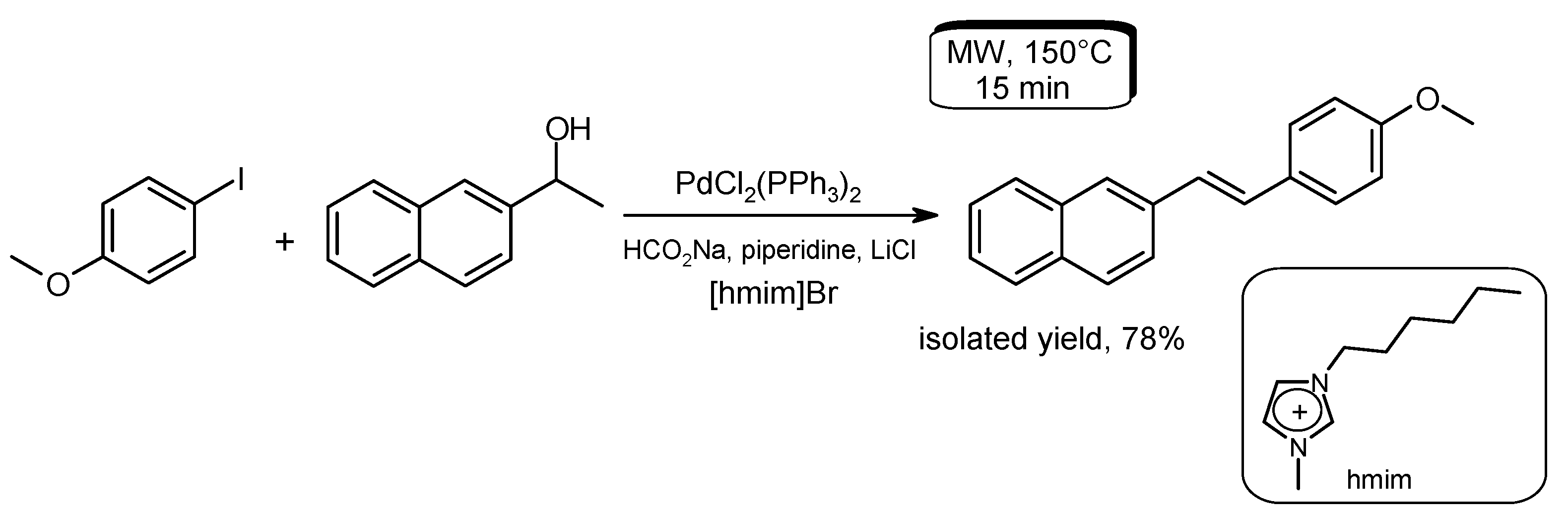
- Alonso, F.; Beletskaya, I.P.; Yus, M. Non-conventional methodologies for transition-metal catalyzed carbon-carbon coupling: A critical overview. Part 1: The Heck reaction. Tetrahedron 2005, 61, 11771–11835. [Google Scholar] [CrossRef]
- Arvela, R.K.; Pasquini, S.; Larhed, M. Highly regioselective internal Heck arylation of hydroxyalkyl vinyl ethers by aryl halides in water. J. Org. Chem. 2007, 72, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Vallin, K.S.A.; Emilsson, P.; Larhed, M.; Hallberg, A. High-speed Heck reactions in ionic liquid with controlled microwave heating. J. Org. Chem. 2002, 67, 6243–6246. [Google Scholar] [CrossRef] [PubMed]
- Datta, G.K.; Vallin, K.S.A.; Larhed, M. A rapid microwave protocol for Heck vinylation of aryl chlorides under air. Mol. Divers. 2003, 7, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, J.; Chen, B.; Han, J.; She, X.; Pan, X. Pd/C-catalyzed Heck reaction in ionic liquid accelerated by microwave heating. Tetrahedron Lett. 2004, 45, 809–811. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Accelerated Heck reaction using ortho-palladated complex in a nonaqueous ionic liquid with controlled microwave heating. Appl. Organomet. Chem. 2011, 25, 542–551. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, Y.; Du, Z.; Hu, Q.; Xue, J.; Shi, J. Highly regioselective Heck-Mizoroki reaction catalyzed by Pd/phosphine ligand in DMSO/[bmim][BF4] under microwave irradiation. Arch. Org. Chem. 2012, 2012, 164–172. [Google Scholar]
- Dighe, M.G.; Degani, M.S. Microwave-assisted ligand-free, base-free Heck reactions in a task-specific imidazolium ionic liquid. Arch. Org. Chem. 2011, 2011, 189–197. [Google Scholar]
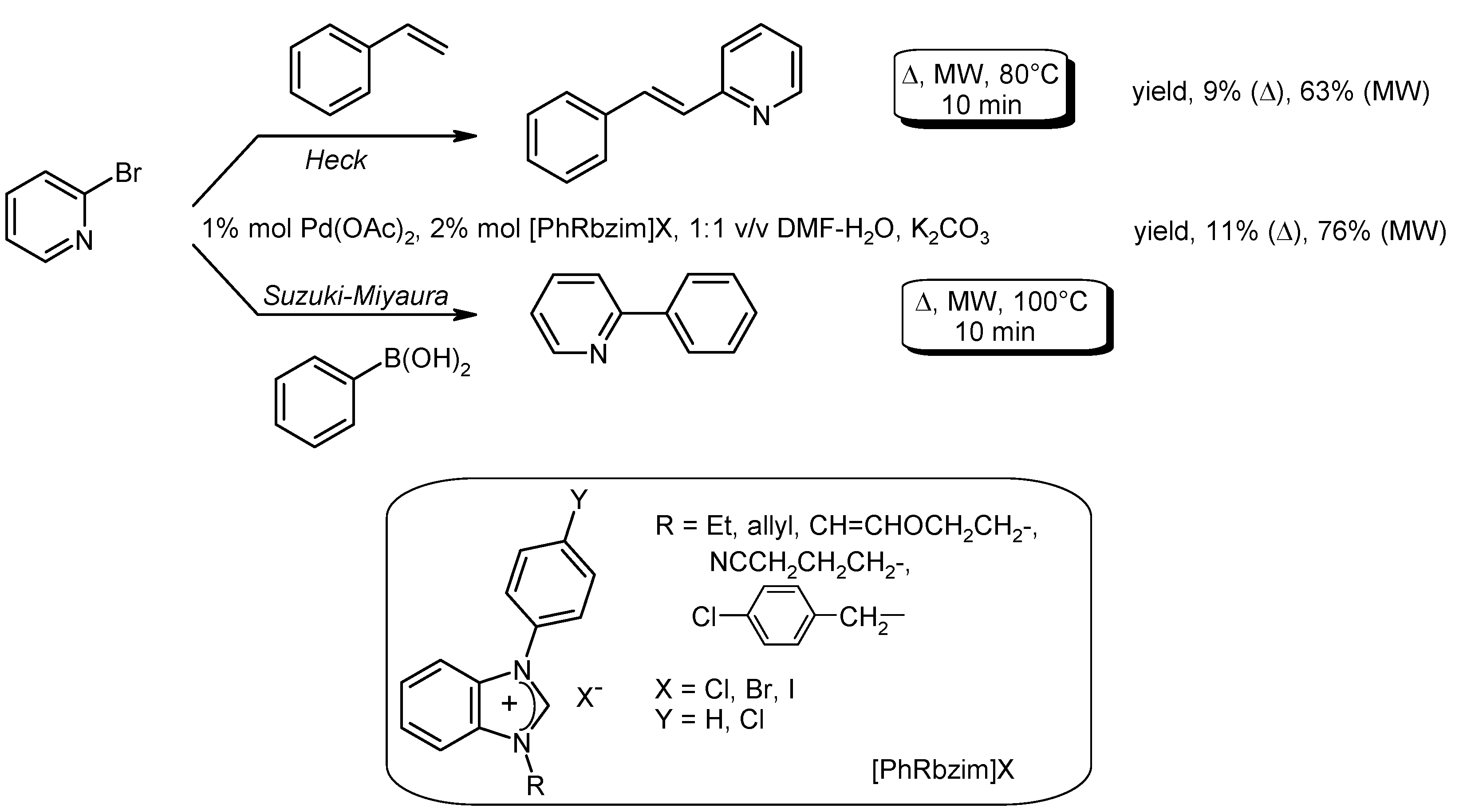
- Yilmaz, U.; Kucukbay, H.; Deniz, S.; Sireci, N. Synthesis, characterization and microwave-promoted catalytic activity of novel N-phenylbenzimidazolium salts in Heck-Mizoroki and Suzuki-Miyaura cross-coupling reactions under mild conditions. Molecules 2013, 18, 2501–2517. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shard, A.; Bharti, R.; Thopate, Y.; Kumar Sinha, A. Palladium-catalyzed dehydrative Heck olefination of secondary aryl alcohols in ionic liquids: Towards a waste-free strategy for tandem synthesis of stilbenoids. Angew. Chem. Int. Ed. 2012, 51, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, H.; Jiang, H.; Chen, K. Rapid and efficient Pd-catalyzed Sonogashira coupling of aryl chlorides. J. Org. Chem. 2008, 73, 6037–6040. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Markina, N.A.; Larock, R.C. An efficient, microwave-assisted, one-pot synthesis of indoles under Sonogashira conditions. Tetrahedron 2009, 65, 8908–8915. [Google Scholar] [CrossRef] [PubMed]
- Sajith, A.M.; Muralidharan, A. Exploration of copper and amine-free Sonogashira cross coupling reactions of 2-halo-3-alkyl imidazo[4,5-b]pyridines using tetrabutyl ammonium acetate as an activator under microwave enhanced conditions. Tetrahedron Lett. 2012, 53, 5206–5210. [Google Scholar] [CrossRef]
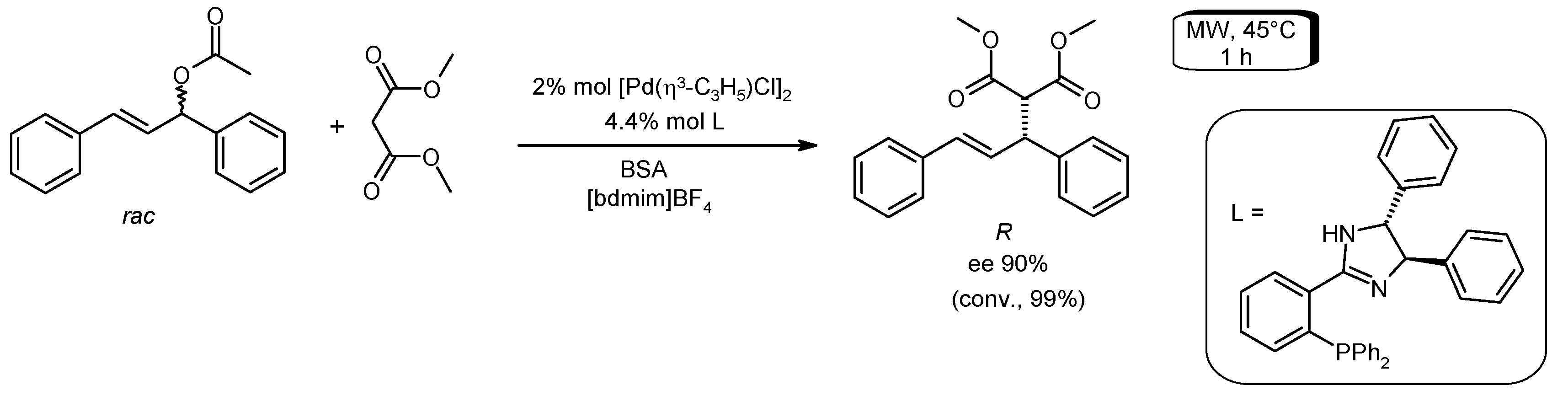
- De la Fuente, V.; Fleury-Bregeot, N.; Castillon, S.; Claver, C. Recycling of allylic alkylation Pd catalysts containing phosphine-imidazoline ligands in ionic liquids. Green Chem. 2012, 14, 2715–2718. [Google Scholar] [CrossRef]
- Li, L.-H.; Pan, Z.-L.; Duan, X.-H.; Liang, Y.-M. An environmentally benign procedure for the synthesis of aryl and arylvinyl nitriles assisted by microwave in ionic liquid. Synlett 2006, 13, 2094–2098. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, G.; Han, H.; Song, J.; Liu, Y.; Chu, W.; Sun, Z. An effective and environment-friendly system for Cu NPs@RGO-catalyzed C–C homocoupling of aryl halides or arylboronic acids in ionic liquids under microwave irradiation. RSC Adv. 2016, 6, 41108–41113. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H. Rare-Earth metal triflates in organic synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef] [PubMed]
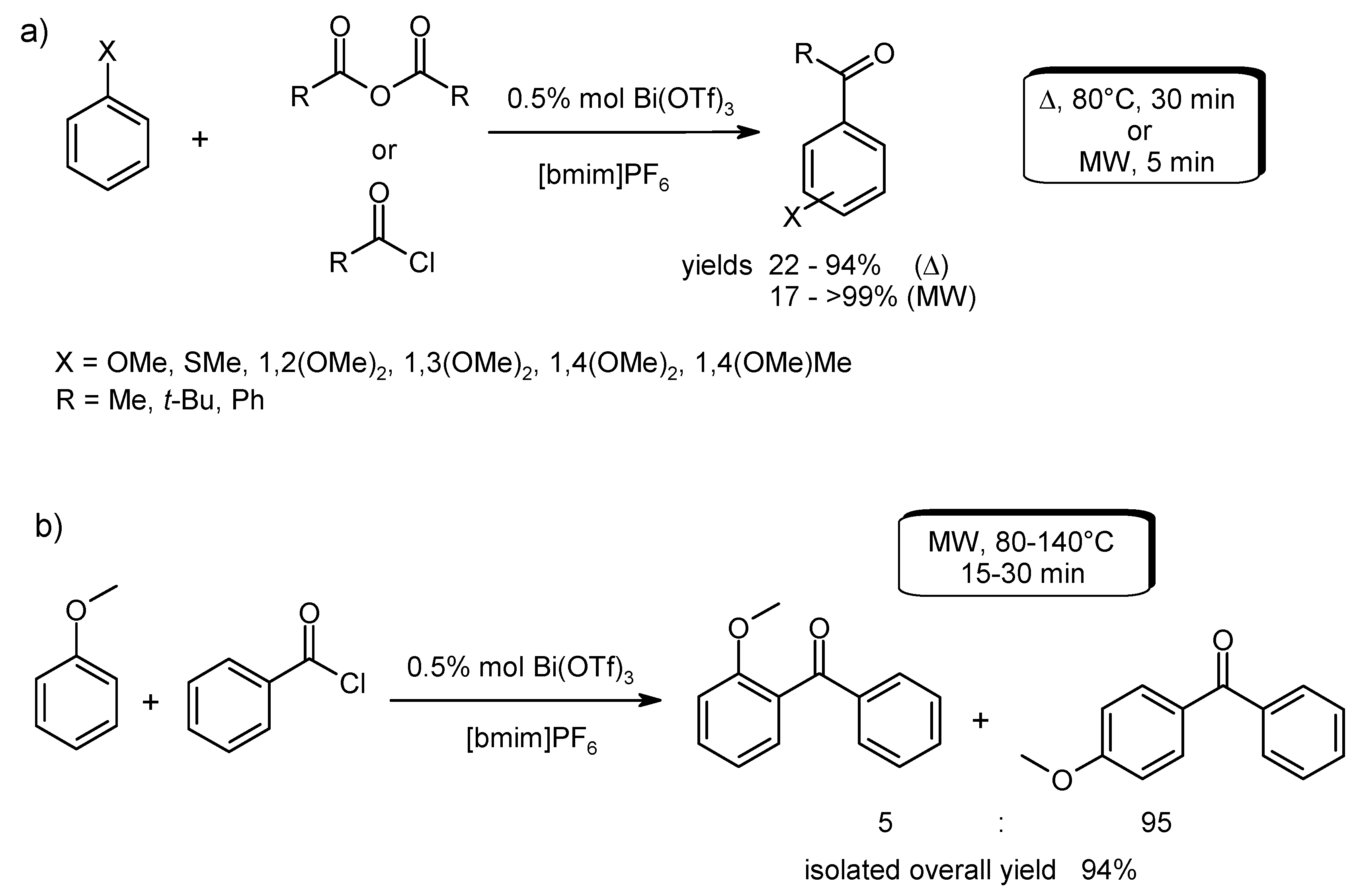
- Tran, P.H.; Duus, F.; Le, T.N. Friedel-Crafts acylation using bismuth triflate in [BMI][PF6]. Tetrahedron Lett. 2012, 53, 222–224. [Google Scholar] [CrossRef]
- Tran, P.H.; BichLe Do, N.; NgocLe, T. Improvement of the Friedel-Crafts benzoylation by using bismuth trifluoromethanesulfonate in 1-butyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid under microwave irradiation. Tetrahedron Lett. 2014, 55, 205–208. [Google Scholar] [CrossRef]
- Tran, P.H.; Hansen, P.E.; Hoang, H.M.; Nguyen Chau, D.-K.N.; Le, T.N. Indium triflate in 1-isobutyl-3-methylimidazolium dihydrogen phosphate: An efficient and green catalytic system for Friedel-Crafts acylation. Tetrahedron Lett. 2015, 56, 2187–2192. [Google Scholar] [CrossRef]
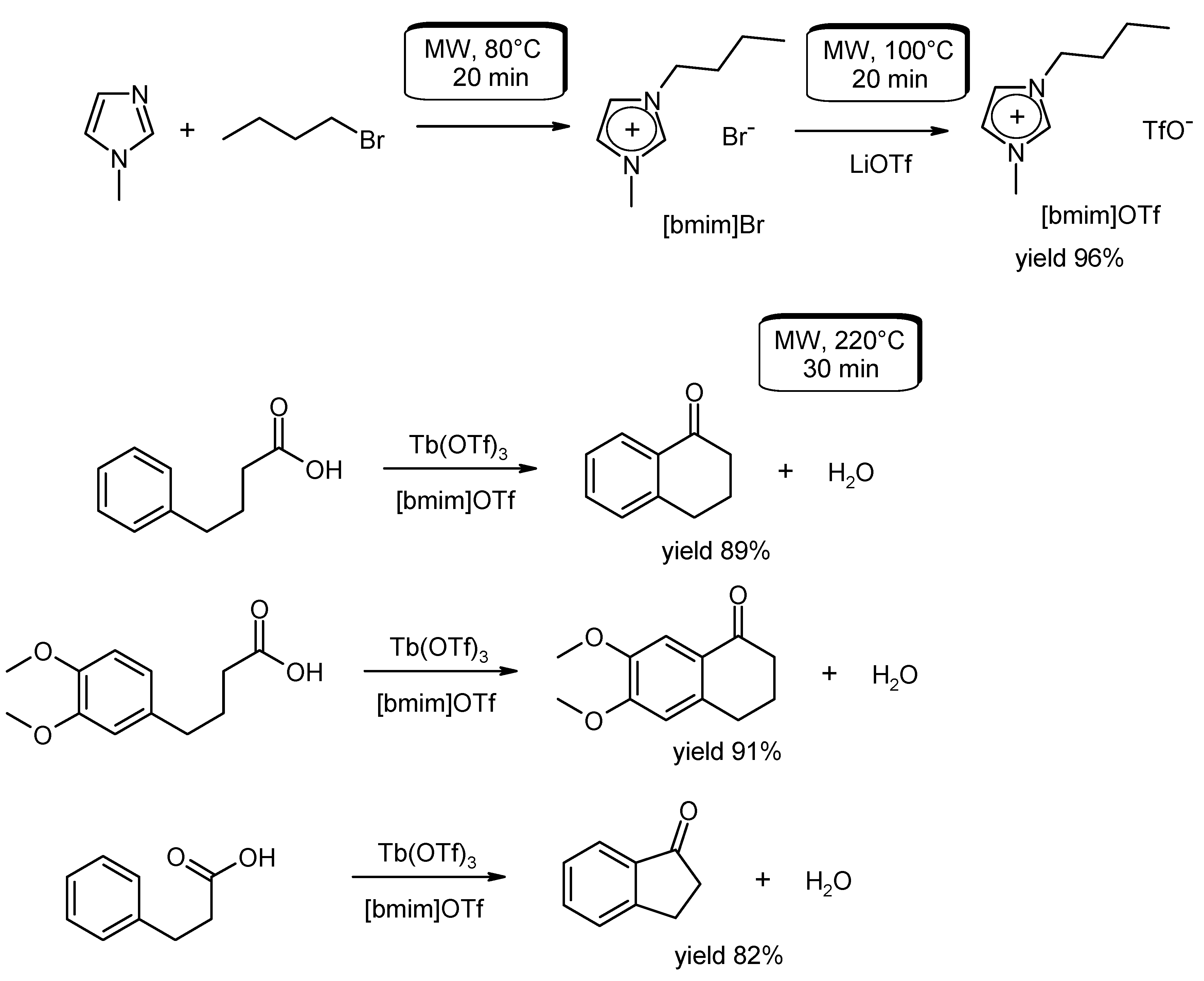
- Tran, P.H.; Huynh, V.H.; Hansen, P.E.; Nguyen Chau, D.-K.N.; Le, T.N. An efficient and green synthesis of 1-indanone and 1-tetralone via intramolecular Friedel-Craft acylation reaction. Asian J. Org. Chem. 2015, 4, 482–486. [Google Scholar] [CrossRef]
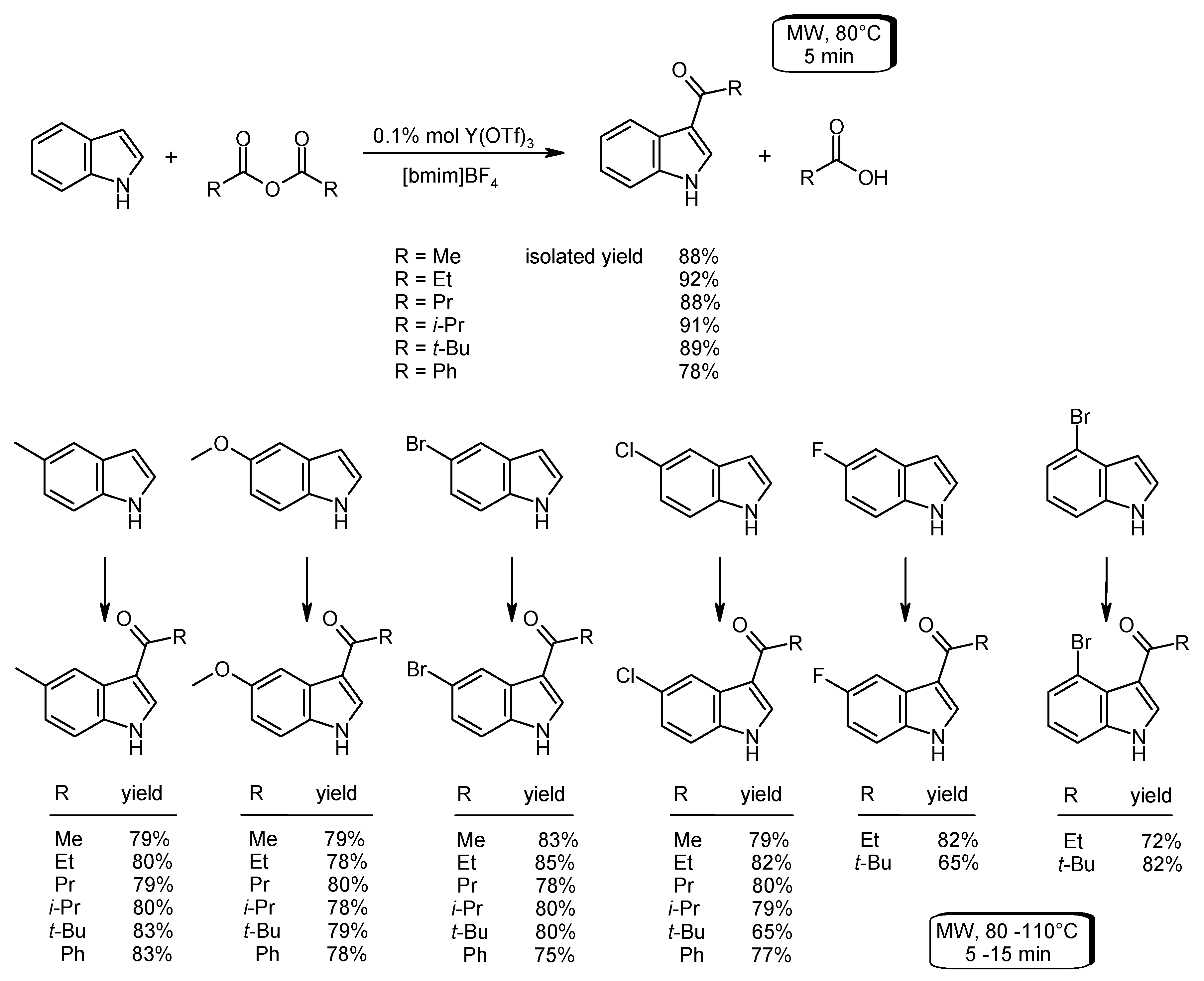
- Tran, P.H.; Tran, H.N.; Hansen, P.E.; Do, M.H.N.; Le, T.N. A simple, effective, green method for the regioselective 3-acylation of unprotected indoles. Molecules 2015, 20, 19605–19619. [Google Scholar] [CrossRef] [PubMed]
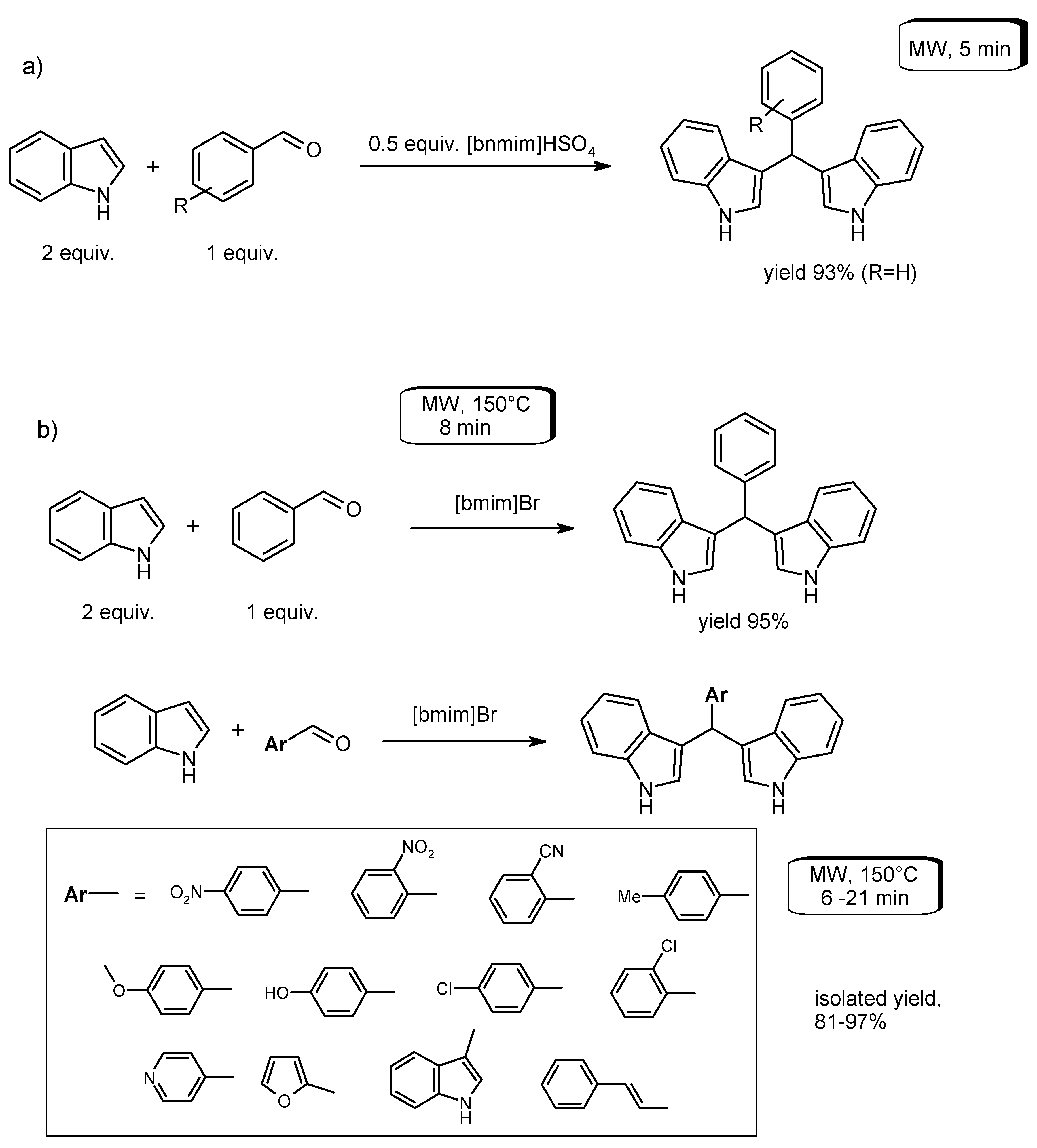
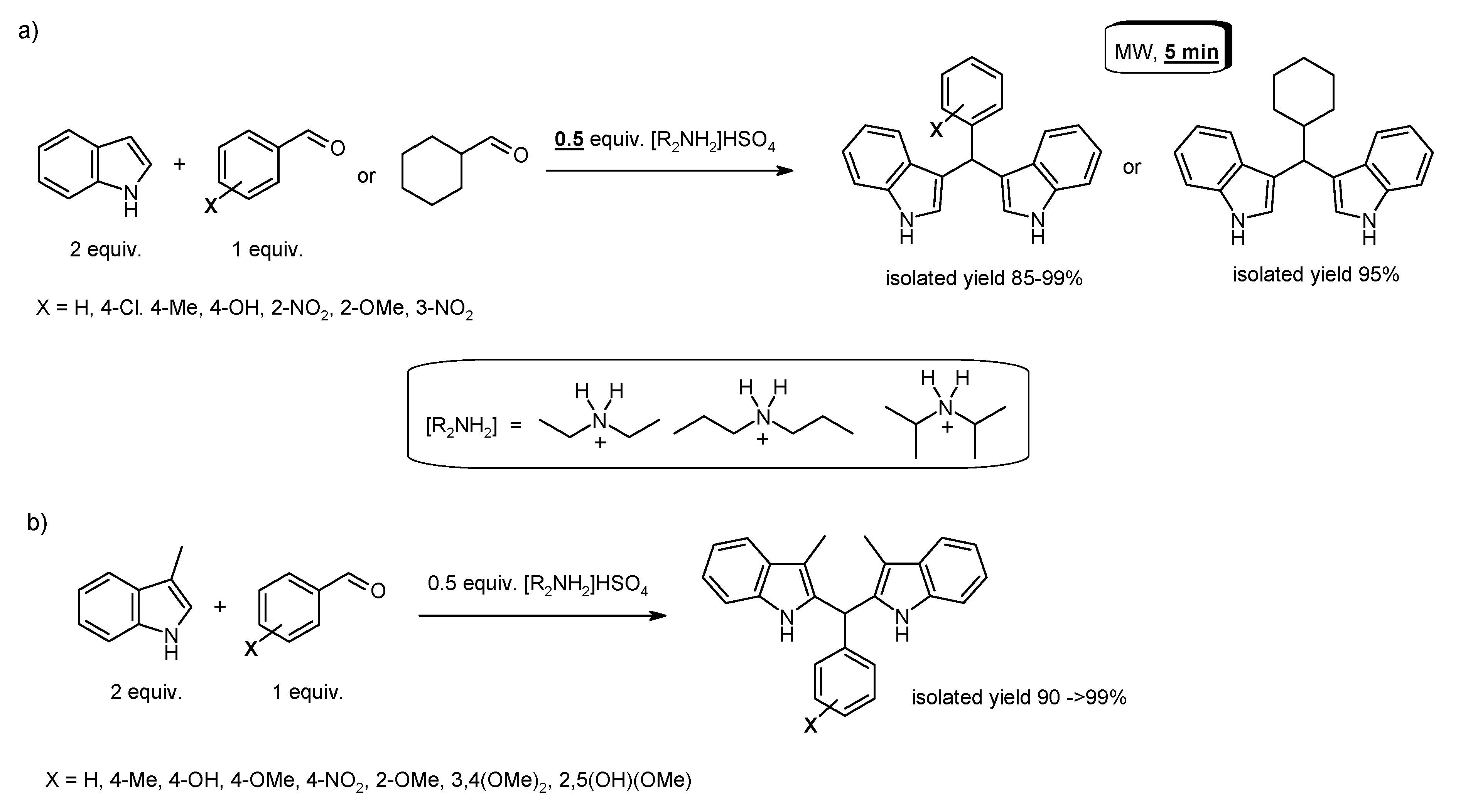
- Sadaphal, S.A.; Shelke, K.F.; Sonar, S.S.; Shingare, M.S. Ionic liquid promoted synthesis of bis(indolyl)methanes. Cent. Eur. J. Chem. 2008, 6, 622–626. [Google Scholar] [CrossRef]
- Zare, A.; Parhami, A.; Moosavi Zare, A.R.; Hasaninejad, A.; Khalafi-Nezhad, A.; Beyzavi, M.H. A catalyst-free protocol for the green and efficient condensation of indoles with aldehydes in ionic liquids. Can. J. Chem. 2009, 87, 416–421. [Google Scholar] [CrossRef]
- Das, P.J.; Das, J. Synthesis of aryl/alkyl(2,2′-bis-3-methylindolyl)methanes and aryl(3,3′-bisindolyl)methanes promoted by secondary amine based ionic liquids and microwave irradiation. Tetrahedron Lett. 2012, 53, 4718–4720. [Google Scholar] [CrossRef]
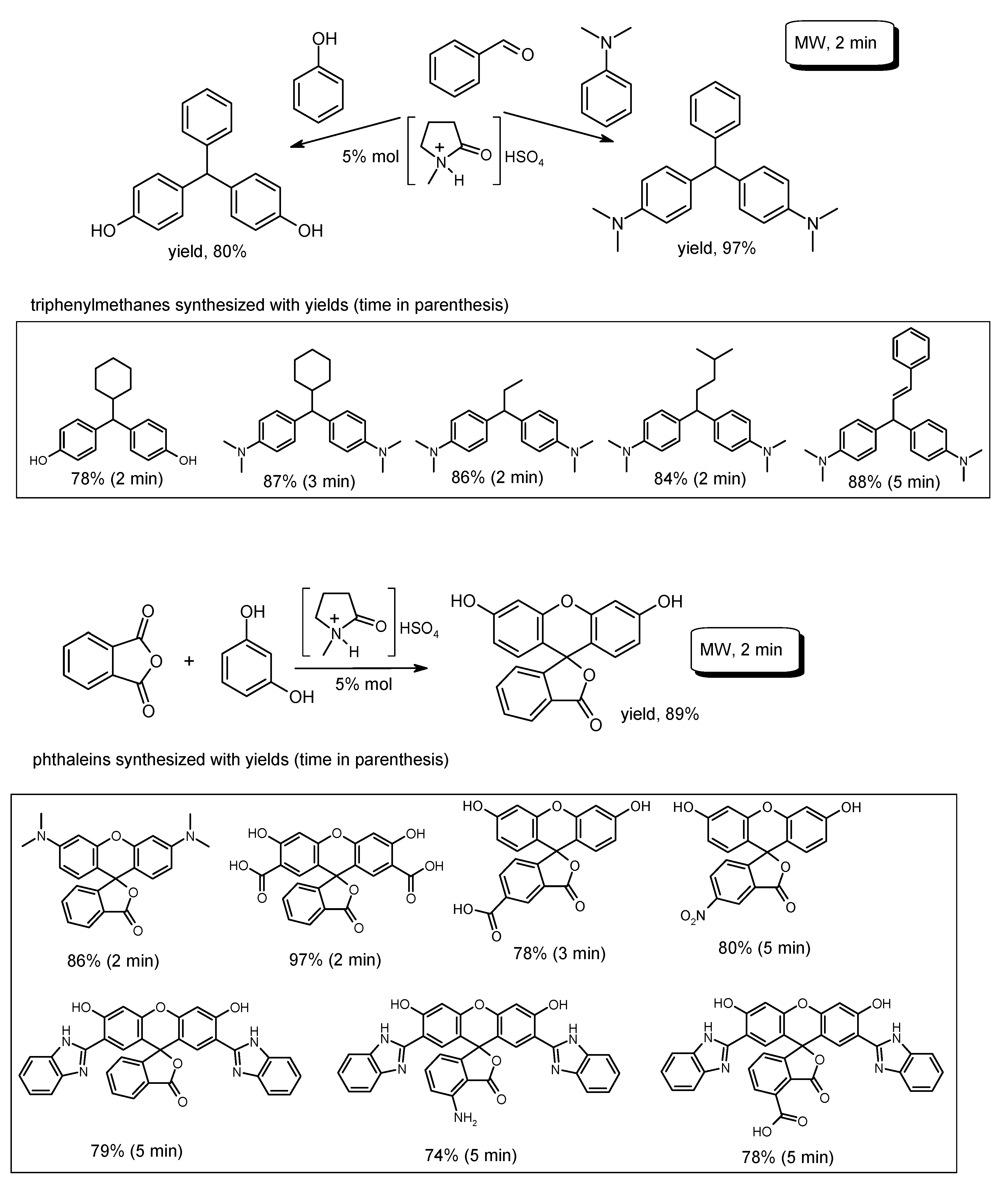
- Chaudhari, A.S.; Parab, Y.S.; Patil, V.; Sekar, N.; Shukla, S.R. Intrinsic catalytic activity of Bronsted acid ionic liquids for the synthesis of triphenylmethane and phthalein under microwave irradiation. RSC Adv. 2012, 2, 12112–12117. [Google Scholar] [CrossRef]
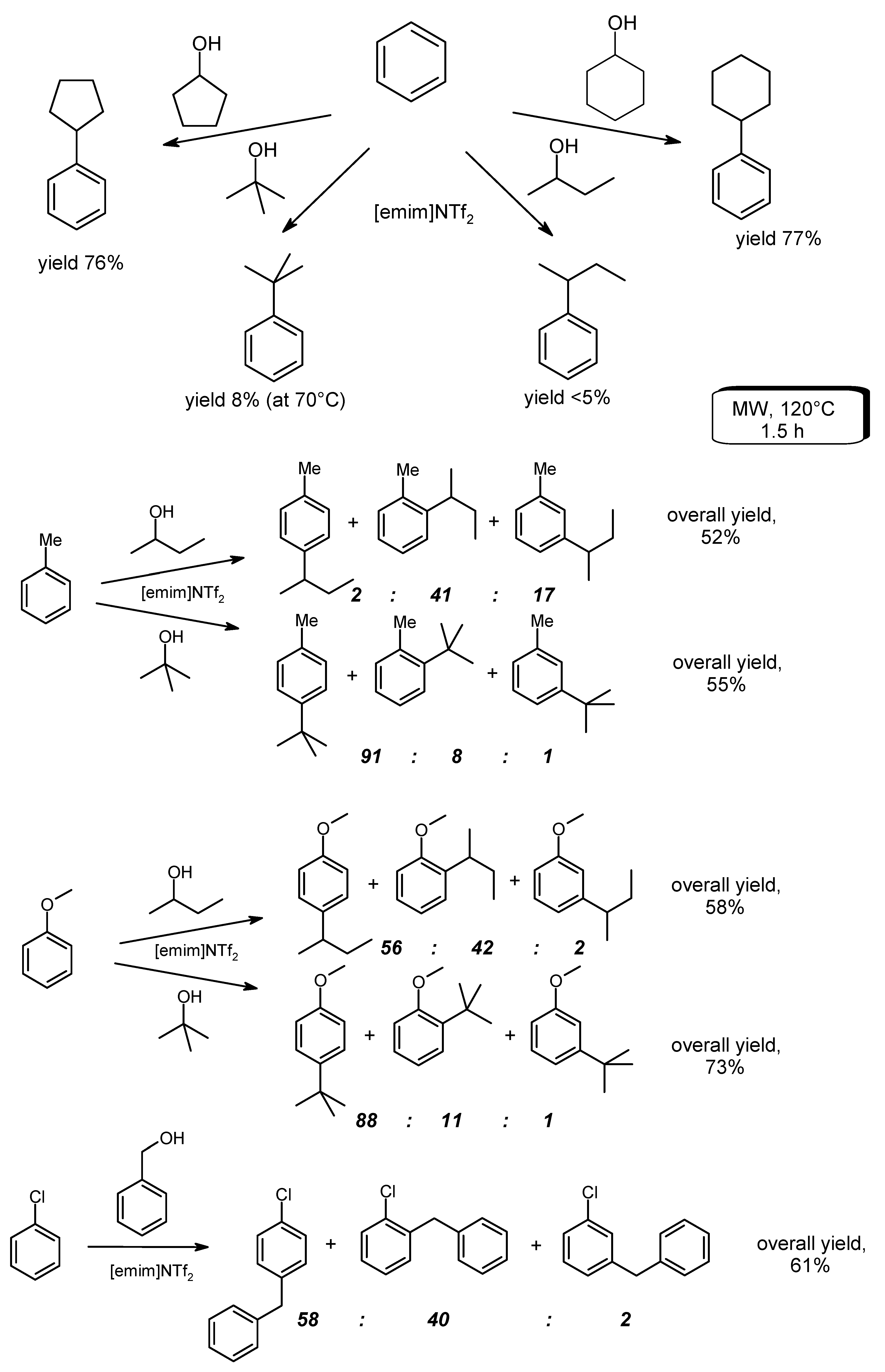
- Mack, R.; Askins, G.; Lowry, J.; Hurley, N.; Reeves, P.C. Indium(III) triflate—A catalyst for greener aromatic alkylation reactions. Can. J. Chem. 2013, 91, 1262–1265. [Google Scholar] [CrossRef]
- Mestres, R. A green look at the aldol reaction. Green Chem. 2004, 6, 583–603. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Wang, D.; Han, B.; Liu, L.; Huang, J.; Zhang, J.; Sun, D. Direct aldol reactions catalyzed by 1,1,3,3-tetramethylguanidine lactate without solvent. Green Chem. 2005, 7, 514–517. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Han, B.; Huang, J.; Zhang, J.; Ma, X. Study on guanidine-based task-specific ionic liquids as catalysts for direct aldol reactions without solvent. New J. Chem. 2006, 30, 736–740. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, T.; Zhang, Z.; Zhu, A.; Han, B.; Song, J.; Xie, Y.; Li, W. Functional ionic liquid from biorenewable materials: Synthesis and application as a catalyst in direct aldol reactions. Tetrahedron Lett. 2007, 48, 5613–5617. [Google Scholar] [CrossRef]
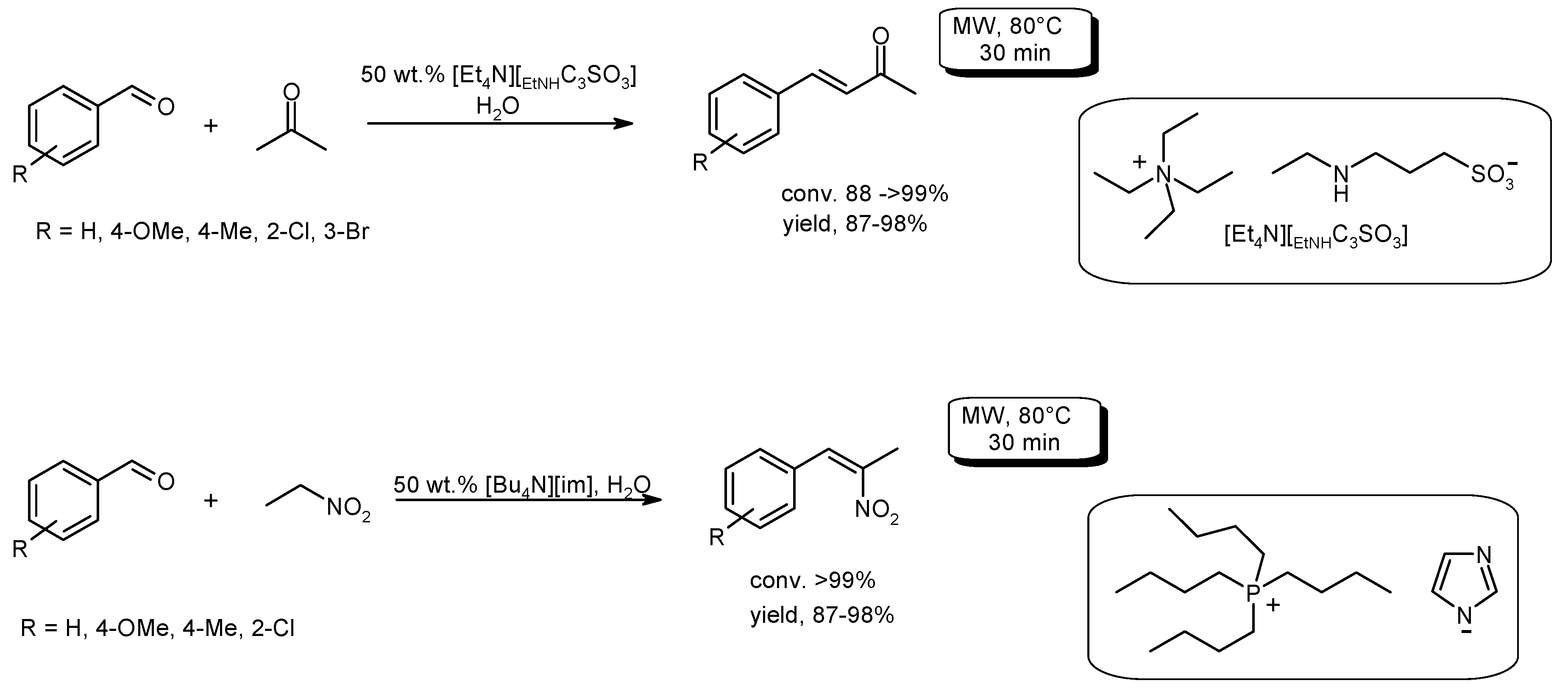
- Limnios, D.; Kokotos, C.G. Microwave-assisted organocatalytic cross-aldol condensation of aldehydes. RSC Adv. 2013, 3, 4496–4499. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Leng, W.; Gao, Y. Rapid and efficient functionalized ionic liquid-catalyzed aldol condensation reactions associated with microwave irradiation. Int. J. Mol. Sci. 2014, 15, 1284–1299. [Google Scholar] [CrossRef] [PubMed]
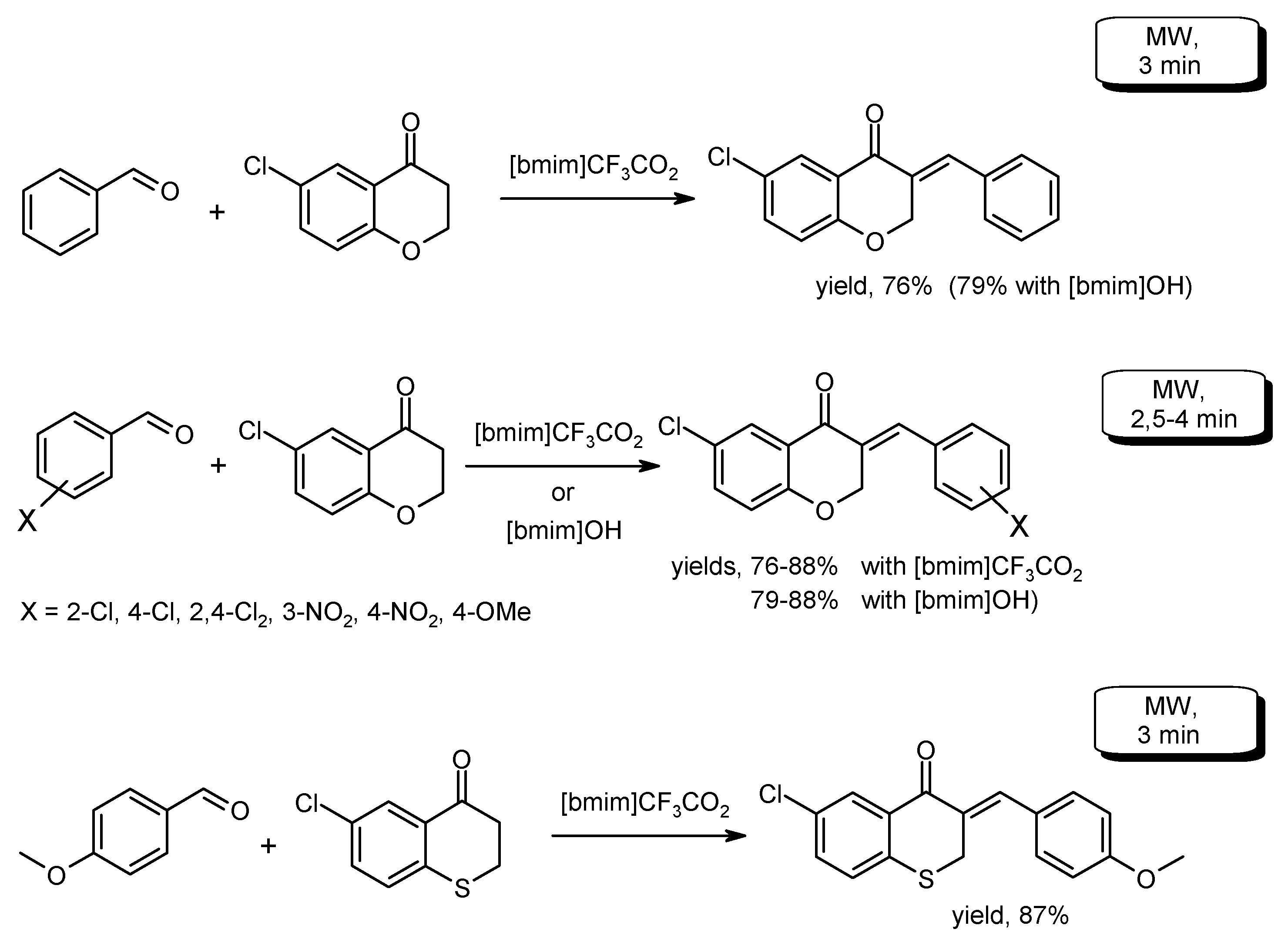
- Li, H.-Y.; Li, S.-N.; Wang, Q.; Wang, S.-X.; Zhu, B.-C. Imidazolium ionic liquids as catalyst for synthesis of (E)-3-arylidene(thio)chroman-4-ones under microwave irradiation. J. Chem. Res. 2012, 36, 635–637. [Google Scholar] [CrossRef]
- Shelke, K.F.; Sapkal, S.B.; Shitole, N.V.; Shingate, B.B.; Shingare, M.S. Microwave-assisted synthesis of 3-styrylchromones in alkaline ionic liquid. Bull. Korean Chem. Soc. 2009, 30, 2883–2886. [Google Scholar] [CrossRef]
- Li, B.L.; Liang, H.D.; Zhong, A.G.; Xu, D.Q. The novel SO3H-functionalized ionic liquids promoted synthesis of xanthenedione derivatives in Aqueous media under microwave irradiation. Adv. Mater. Res. 2012, 391–392, 1354–1357. [Google Scholar] [CrossRef]
- Estager, J.; Leveque, J.-M.; Turgis, R.; Draye, M. Solventless and swift benzoin condensation catalyzed by 1-alkyl-3-methylimidazolium ionic liquids under microwave irradiation. J. Mol. Catal. A Chem. 2006, 256, 261–264. [Google Scholar] [CrossRef]
- Estager, J.; Leveque, J.-M.; Turgis, R.; Draye, M. Neat benzoin condensation in recyclable room-temperature ionic liquids under ultrasonic activation. Tetrahedron Lett. 2007, 48, 755–759. [Google Scholar] [CrossRef]
- Aupoix, A.; Pegot, B.; Vo-Thanh, G. Synthesis of imidazolium and pyridinium-based ionic liquids and application of 1-alkyl-3-methylimidazolium salts as pre-catalysts for the benzoin condensation using solvent-free and microwave activation. Tetrahedron 2010, 66, 1352–1356. [Google Scholar] [CrossRef]
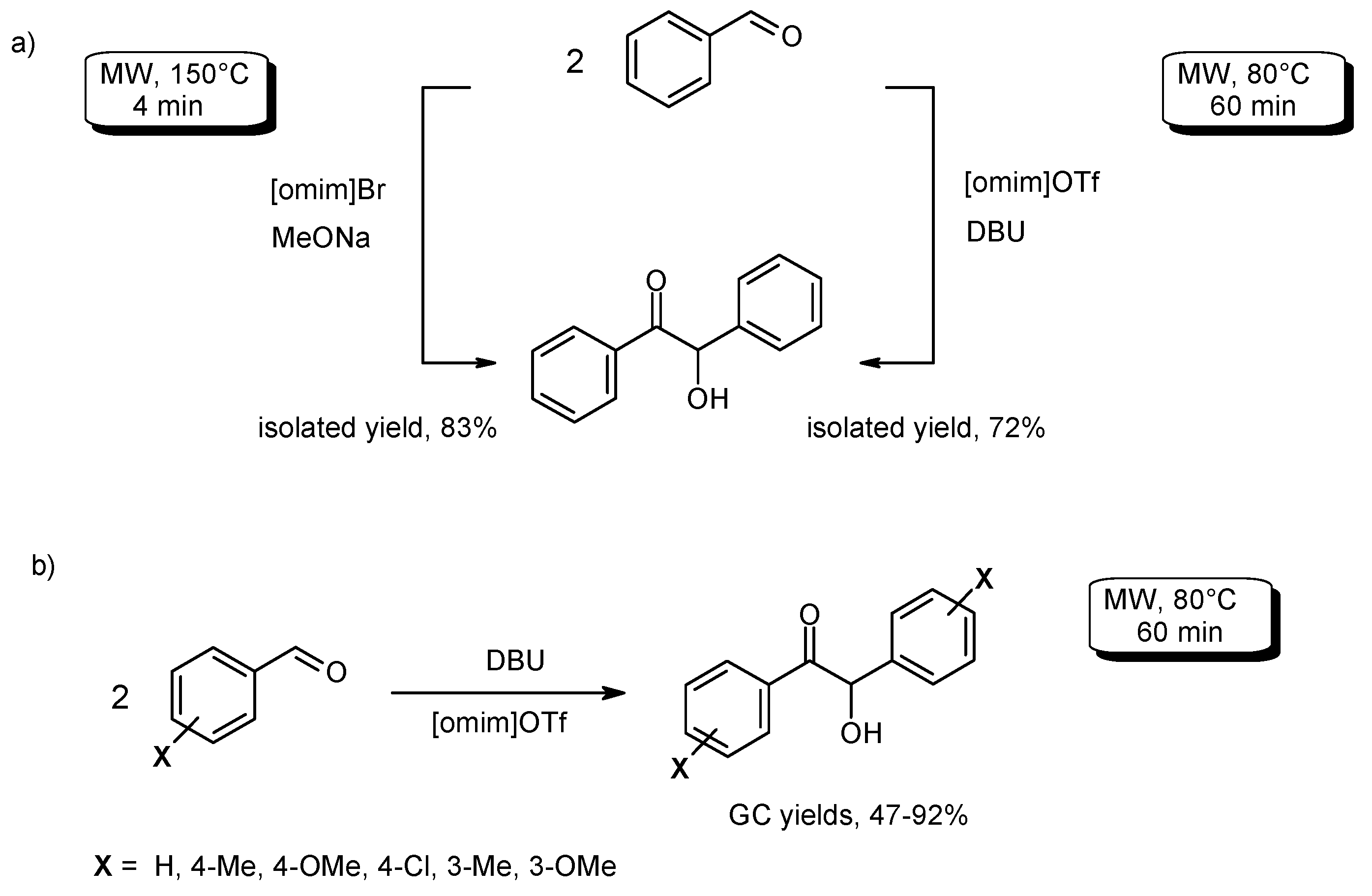
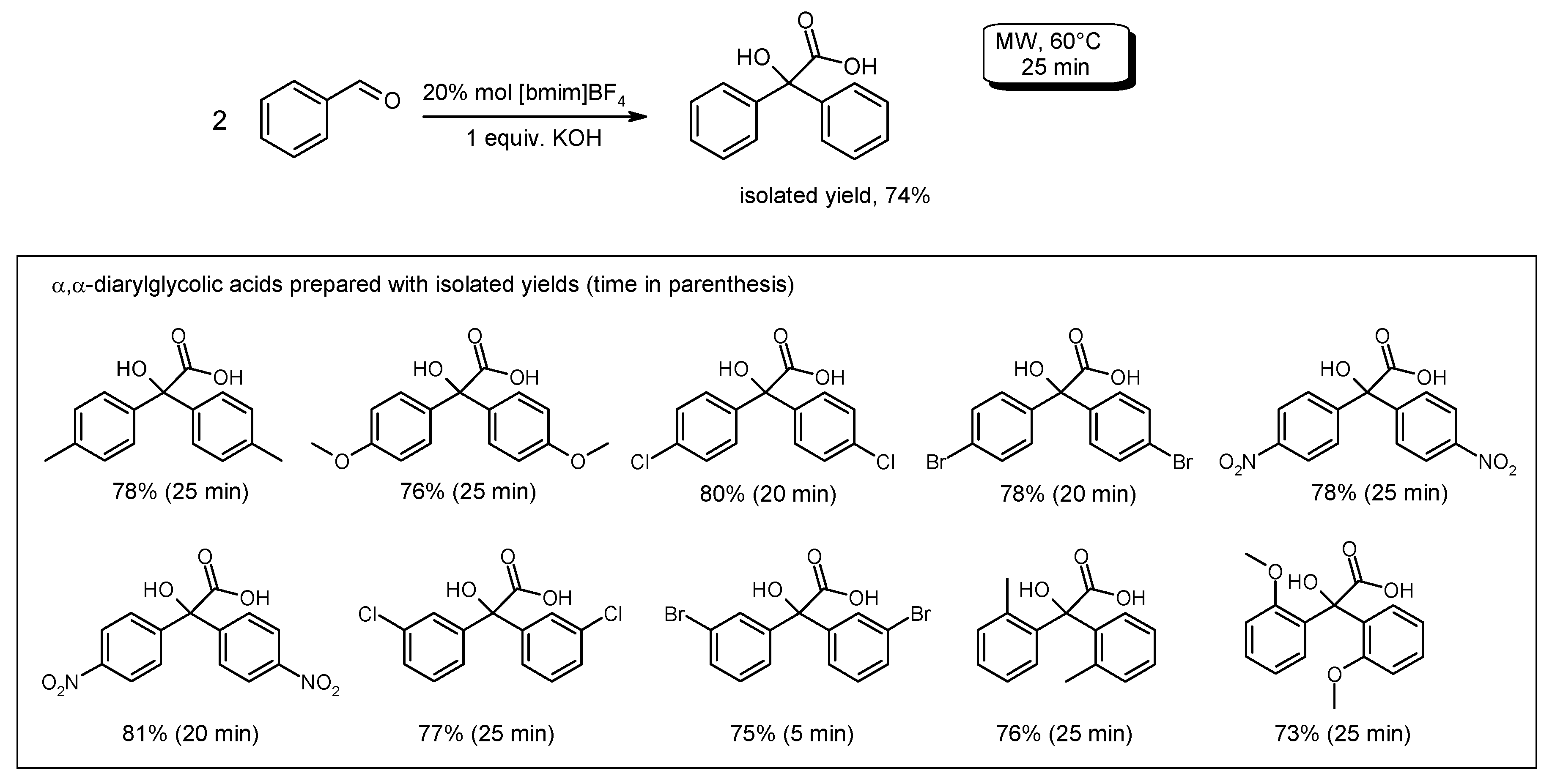
- Singh, N.; Singh, S.K.; Khanna, R.S.; Singh, K.N. Ionic liquid/potassium hydroxide catalyzed solvent-free, one-pot synthesis of diarylglycolic acids from aromatic aldehydes under microwave. Tetrahedron Lett. 2011, 52, 2419–2422. [Google Scholar] [CrossRef]
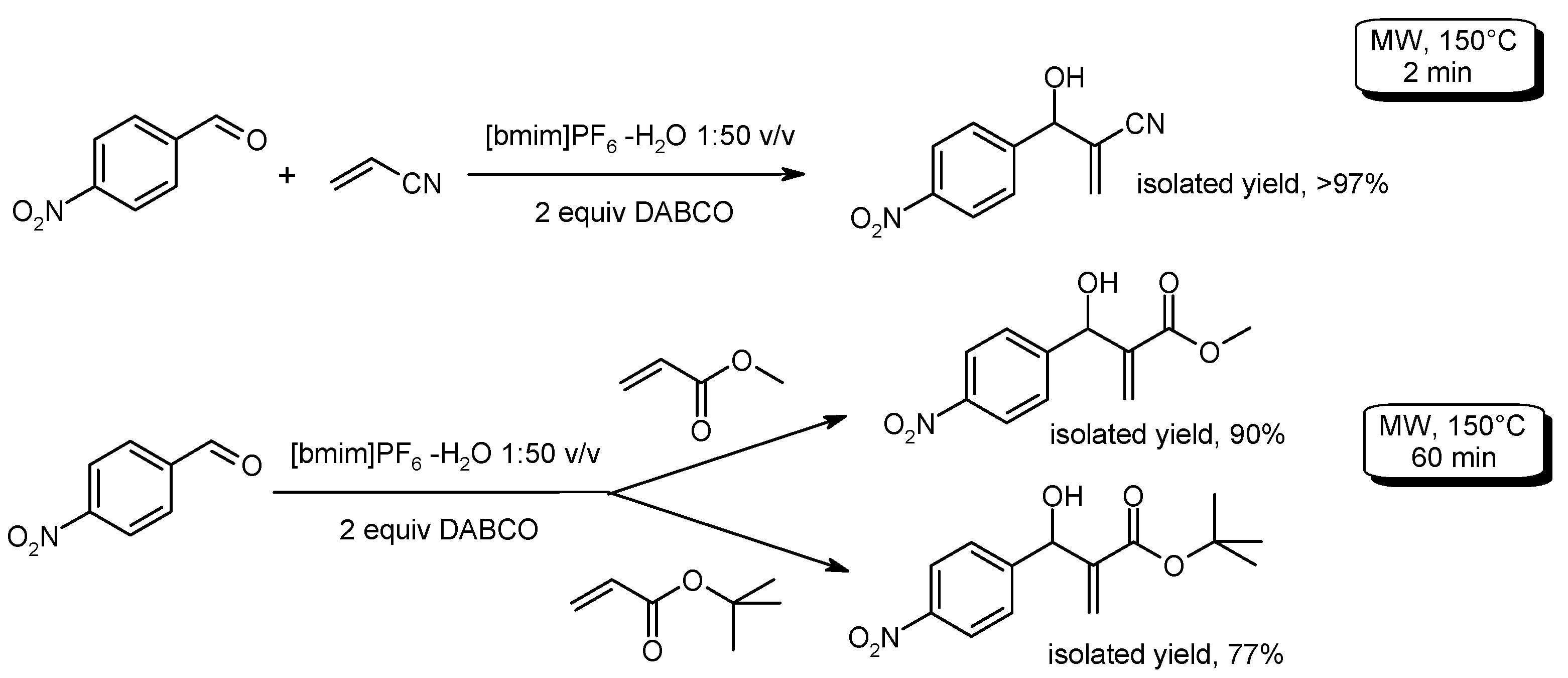
- De Souza, R.O.M.A.; de Souza, A.L.F.; Fernandez, T.L.; Silva, A.C.; Pereira, V.L.P.; Esteves, P.M.; Vasconcellos, M.L.A.A.; Antunes, O.A.C. Morita-Baylis-Hillman reaction in water/ionic liquids under microwave irradiation. Lett. Org. Chem. 2008, 5, 379–382. [Google Scholar] [CrossRef]
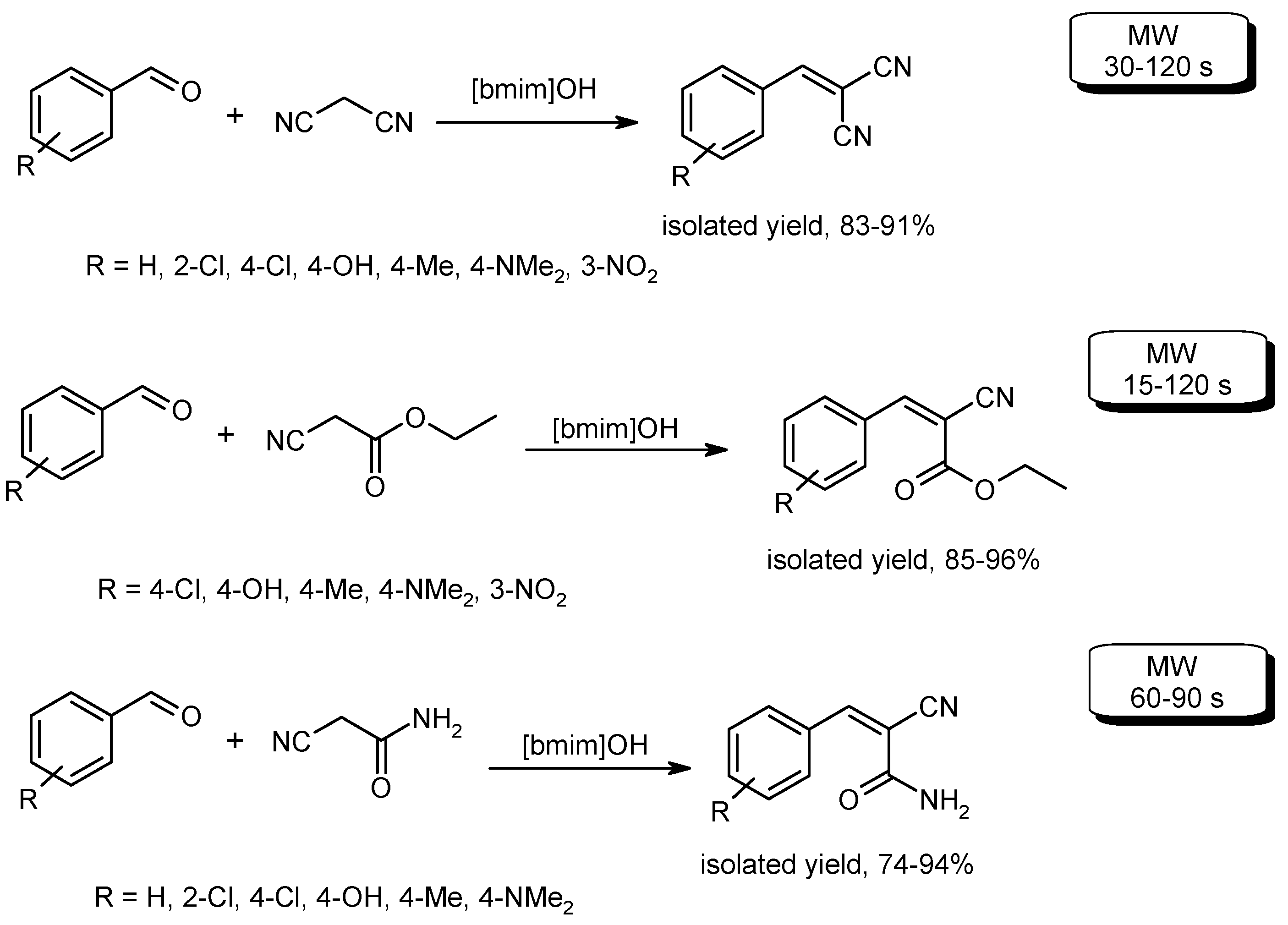
- Wang, D.-Y.; Xi, G.-H.; Ma, J.-J.; Wang, C.; Zhang, X.-C.; Wang, Q.-Q. Condensation reactions of aromatic aldehydes with active methylene compounds catalyzed by alkaline ionic liquid. Synth. Commun. 2011, 41, 3060–3065. [Google Scholar] [CrossRef]
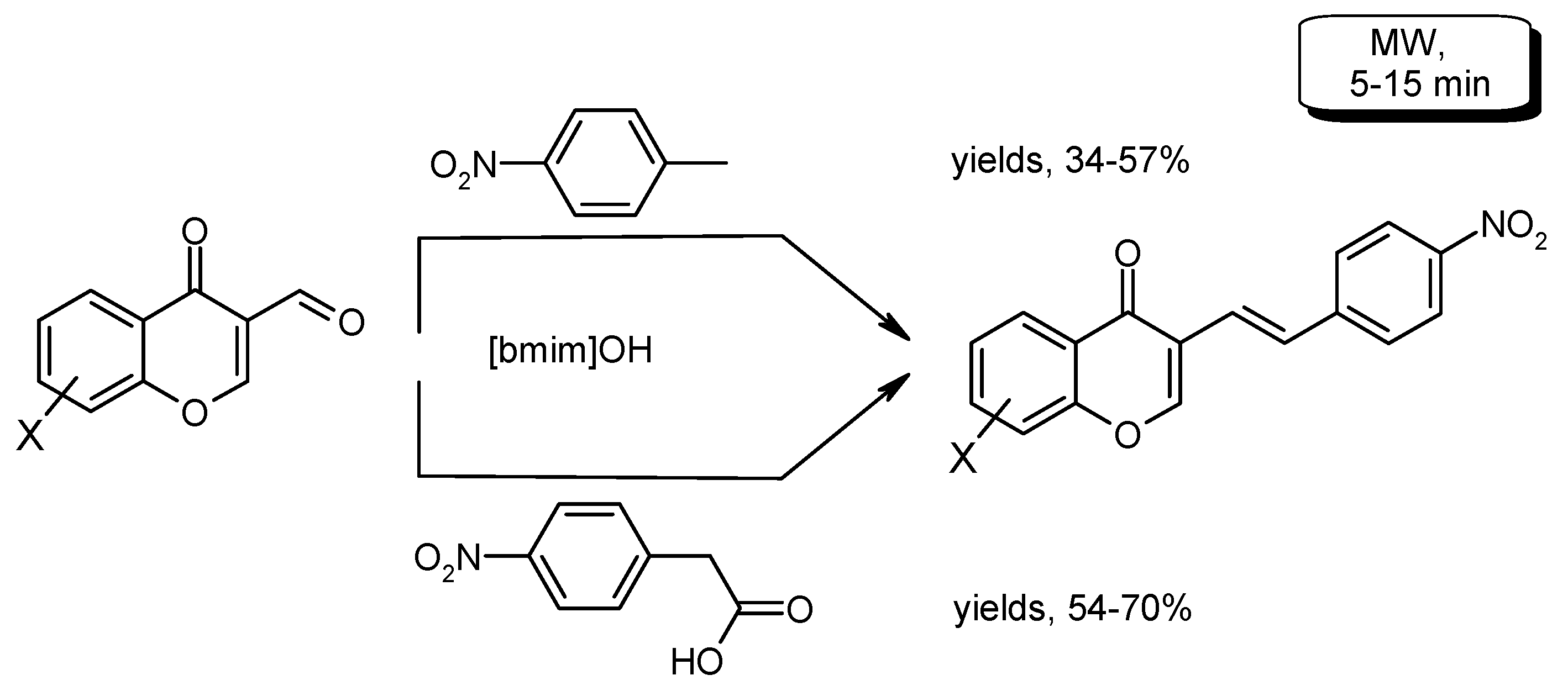
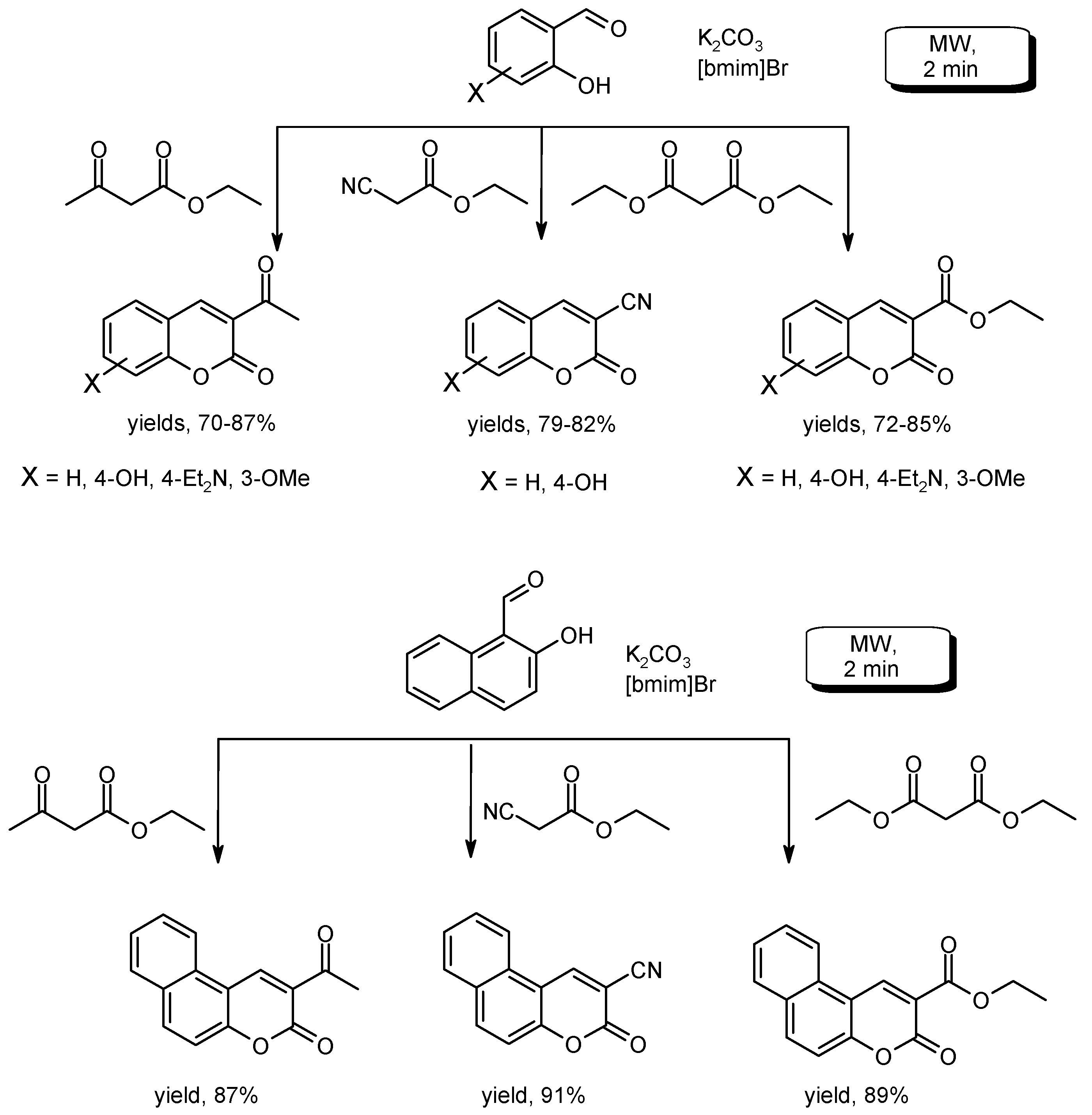
- Valizadeh, H.; Shockravi, A.; Gholipur, H. Microwave assisted synthesis of coumarins via potassium carbonate catalyzed Knoevenagel condensation in 1-n-butyl-3-methylimidazolium bromide ionic liquid. J. Heterocycl. Chem. 2007, 44, 867–870. [Google Scholar] [CrossRef]
- Vasiloiu, M.; Gaertner, P.; Bica, K. Iron-catalyzed Michael addition. Chloroferrate ionic liquids as efficient catalysts under microwave conditions. Sci. China Chem. 2012, 55, 1614–1619. [Google Scholar] [CrossRef]
- Paul, C.E.; Gotor-Fernandez, V.; Lavandera, I.; Montejo-Bernardo, J.; Garcia-Granda, S.; Gotor, V. Chemoenzymatic preparation of optically active 3-(1H-imidazol-1-yl)cyclohexanol-based ionic liquids: Application in organocatalysis and toxicity studies. RSC Adv. 2012, 2, 6455–6463. [Google Scholar] [CrossRef]
- Zhou, Z.-Z.; Ji, F.-Q.; Cao, M.; Yang, G.-F. An efficient intramolecular Stetter reaction in room temperature ionic liquids promoted by microwave irradiation. Adv. Synth. Catal. 2006, 348, 1826–1830. [Google Scholar] [CrossRef]
- Aupoix, A.; Vo-Thanh, G. Solvent-free synthesis of alkylthiazolium-based ionic liquids and their use as catalysts in the intramolecular Stetter reaction. Synlett 2009, 12, 1915–1920. [Google Scholar]
- Lin, Y.-Y.; Tsai, S.-C.; Yu, S.J. Highly efficient and recyclable Au nanoparticle-supported Palladium(II) interphase catalysts and microwave-assisted alkyne cyclotrimerization reactions in ionic liquids. J. Org. Chem. 2008, 73, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Janus, E.; Syguda, A.; Materna, K. Ionic liquids—Deanol derivatives as the Diels-Alder reaction solvents. Cent. Eur. J. Chem. 2010, 8, 1140–1146. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Torenius, H.M. A study of the ionic liquid mediated microwave heating of organic solvents. J. Org. Chem. 2002, 67, 3145–3148. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-H.; Young, J.-N.; Yu, S.J. Recyclable organotungsten Lewis acid and microwave-assisted Diels-Alder reactions in water and in ionic liquids. Tetrahedron 2004, 60, 11903–11909. [Google Scholar] [CrossRef]
- Fuentes, A.; Martinez-Palou, R.; Jimenez-Vazquez, H.A.; Delgado, F.; Reyes, A.; Tamariz, J. Diels-Alder reactions of 2-oxazolidinone dienes in polar solvents using catalysis or non-conventional energy sources. Monatsh. Chem. 2005, 136, 177–192. [Google Scholar] [CrossRef]
- López, I.; Silvero, G.; Arevalo, M.J.; Babiano, R.; Palacios, J.C.; Bravo, J.L. Enhanced Diels-Alder reactions: On the role of mineral catalysts and microwave irradiation in ionic liquids as recyclable media. Tetrahedron 2007, 63, 2901–2906. [Google Scholar] [CrossRef]
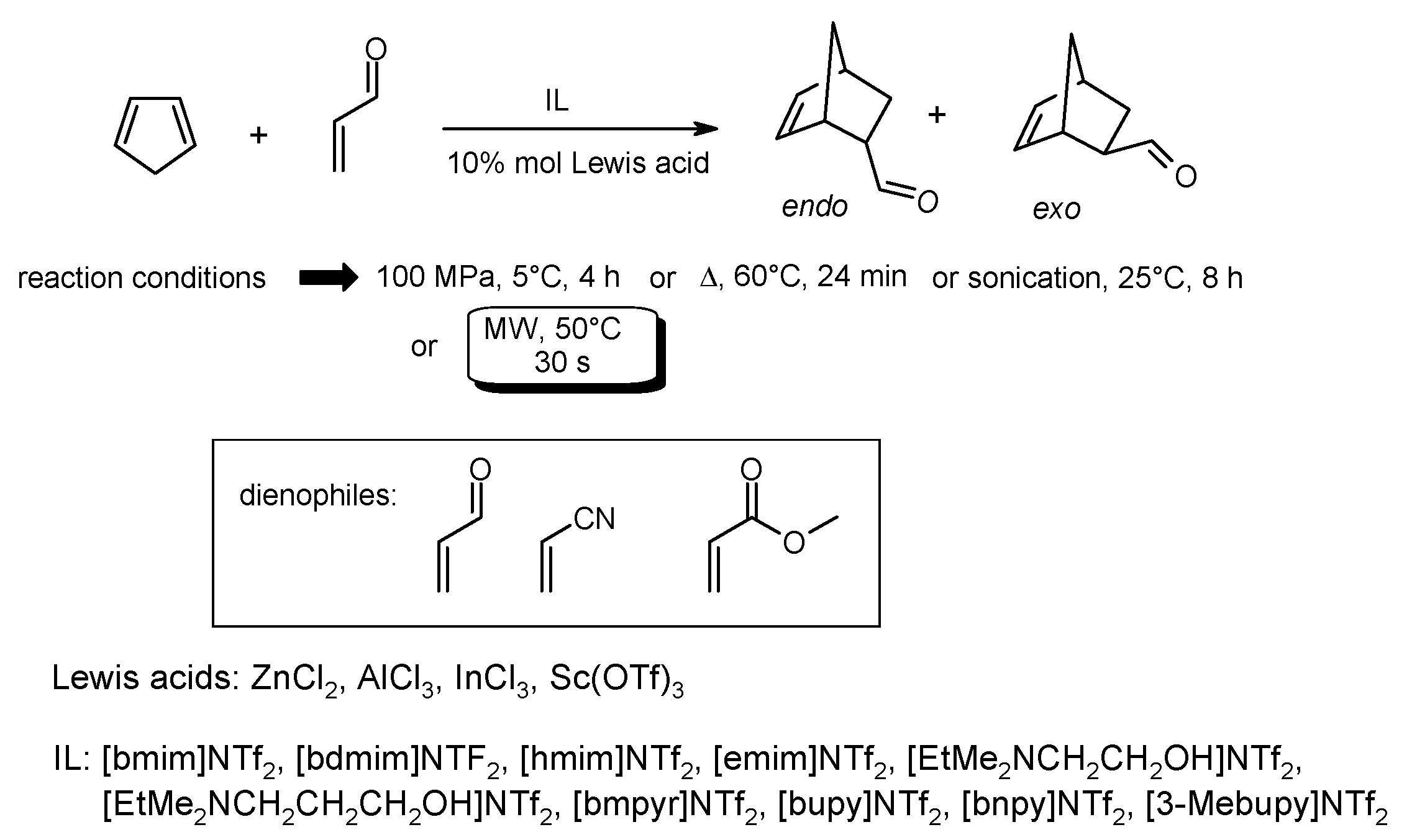
- Vidiš, A.; Kusters, E.; Sedelmeier, G.; Dyson, P.J. Effect of Lewis acids on the Diels-Alder reaction in ionic liquids with different activation modes. J. Phys. Org. Chem. 2008, 21, 264–270. [Google Scholar] [CrossRef]
- Mancini, P.M.E.; Ormachea, C.M.; Della Rosa, C.D.; Kneeteman, M.N.; Suarez, A.G.; Domingo, L.R. Ionic liquids and microwave irradiation as synergistic combination for polar Diels–Alder reactions using properly substituted heterocycles as dienophiles. A DFT study related. Tetrahedron Lett. 2012, 53, 6508–6511. [Google Scholar] [CrossRef]
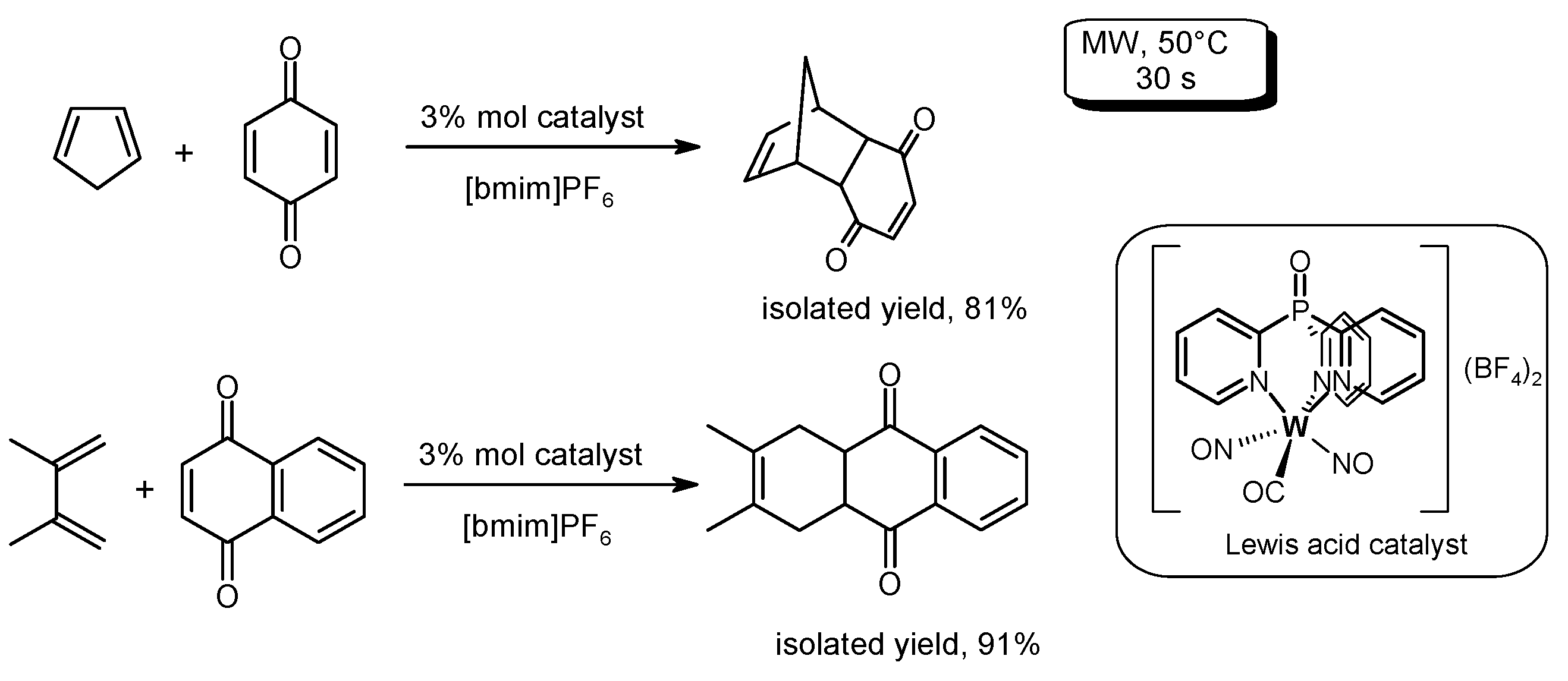
- Rajendran, A.; Kumar, G.V. Sustainable Diels-Alder syntheses in imidazolium ionic liquids. Synth. Commun. 2016, 46, 483–496. [Google Scholar]
- Rajendran, A.; Kumar, G.V. Ultra-rapid green microwave Diels-Alder reactions using ionic liquid. Curr. Microw. Chem. 2016, 3, 55–59. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Prajapati, N.P.; Vekariya, R.H.; Borad, M.A.; Patel, H.D. Recent advances in the synthesis of 2-substituted benzothiazoles: A review. RSC Adv. 2014, 4, 60176–60208. [Google Scholar] [CrossRef]
- Sapkal, S.B.; Shelke, K.F.; Shingate, B.B.; Shingare, M.S. An efficient synthesis of benzofuran derivatives under conventional/non-conventional method. Chin. Chem. Lett. 2010, 21, 1439–1442. [Google Scholar] [CrossRef]
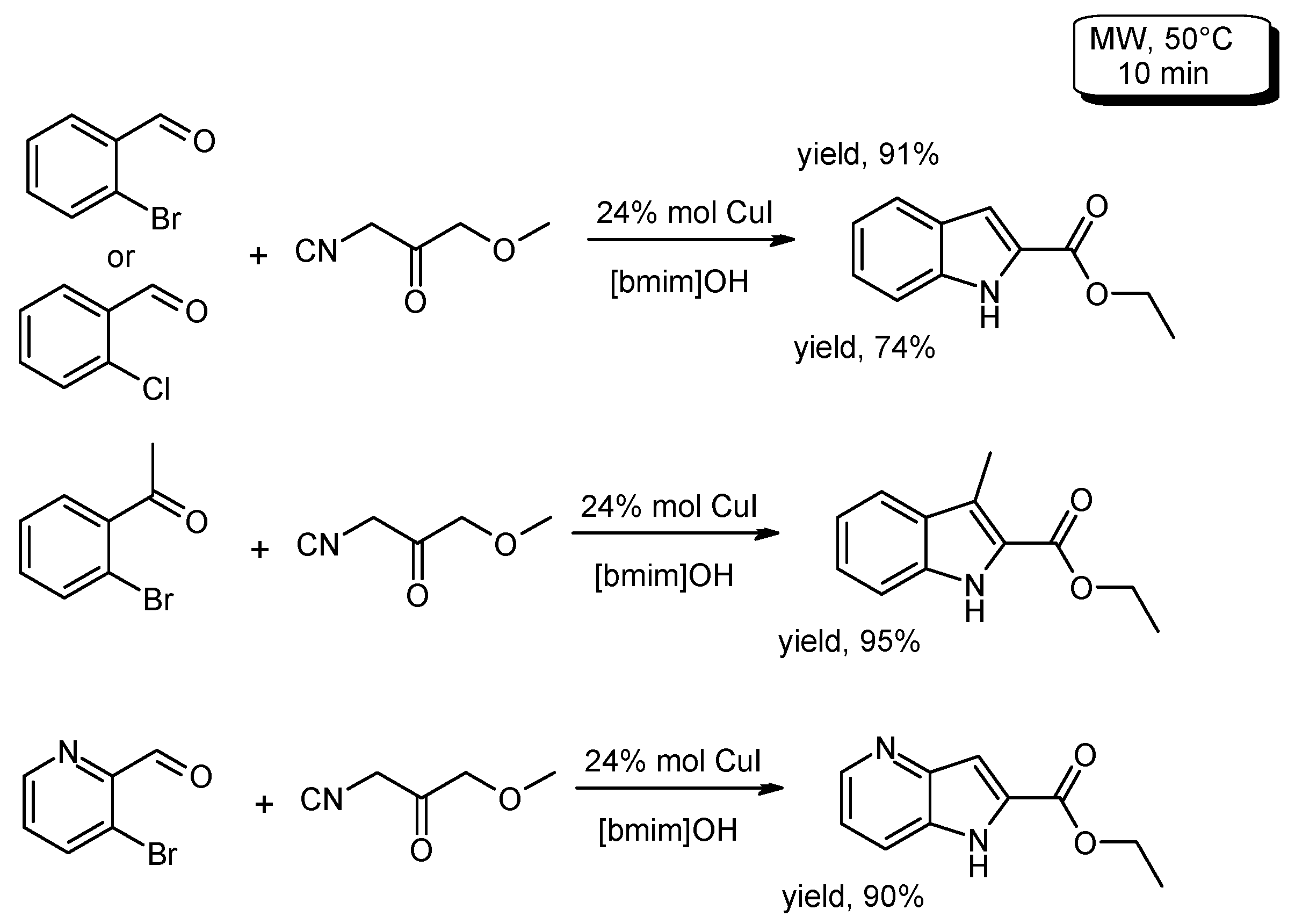
- Gu, L.; Li, X. Microwave-assisted synthesis of indole-2-carboxylic acid esters in ionic liquid. J. Braz. Chem. Soc. 2011, 22, 2036–2039. [Google Scholar] [CrossRef]
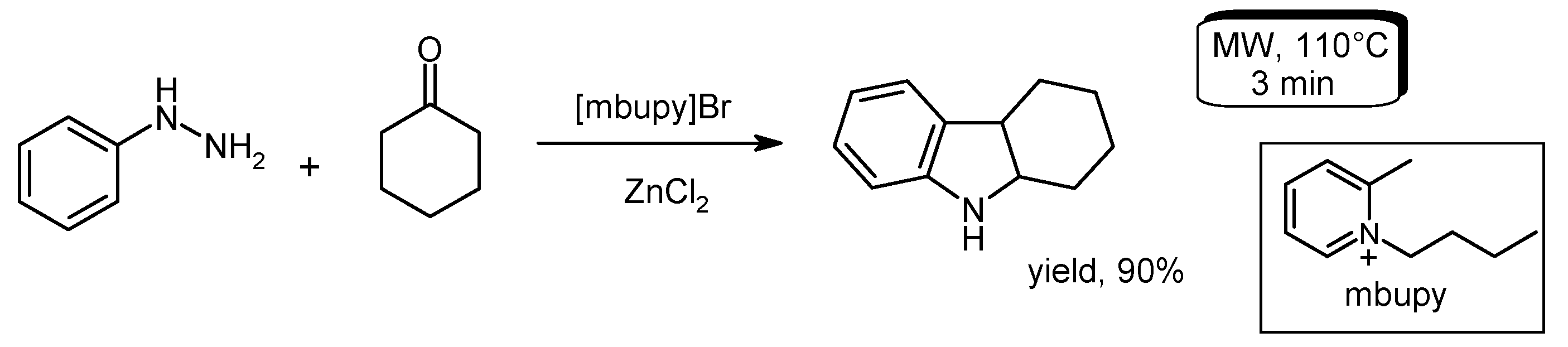
- Eduque, R.M., Jr.; Creencia, E.C. Microwave-assisted Fischer Indole synthesis of 1,2,3,4-tetrahydrocarbazole using pyridinium-based ionic liquids. Procedia Chem. 2015, 16, 413–419. [Google Scholar] [CrossRef]
- Yi, F.; Peng, Y.; Song, G. Microwave-assisted liquid-phase synthesis of methyl 6-amino-5-cyano-4-aryl-2-methyl-4H-pyran-3-carboxylate using functional ionic liquid as soluble support. Tetrahedron Lett. 2005, 46, 3931–3933. [Google Scholar] [CrossRef]
- Mamaghani, M.; Nia, R.H. Recent developments in the MCRs synthesis of pyridopyrimidines and spiro-pyridopyrimidines. J. Heterocycl. Chem. 2017, 54, 1700–1722. [Google Scholar] [CrossRef]
- Sreevalli, W.; Ramachandran, G.; Madhuri, W.; Sathiyanarayanan, K.I. Green trends in Mannich reaction. Mini-Rev. Org. Chem. 2014, 11, 97–115. [Google Scholar] [CrossRef]
- Syamala, M. A decade of advances in three-component reactions. A review. Org. Prep. Proced. Int. 2005, 37, 103–171. [Google Scholar] [CrossRef]
- Patil, V.; Padalkar, V.; Chaudhari, A. Intrinsic catalytic activity of an acidic ionic liquid as a solvent for quinazoline synthesis. Catal. Sci. Technol. 2012, 2, 1681–1684. [Google Scholar] [CrossRef]
- Mert-Balci, F.; Imrich, H.-G.; Conrad, J.; Beifuss, U. Influence of guanidinium salts and other ionic liquids on the three-component aza-Diels-Alder reaction. Helv. Chim. Acta 2013, 96, 1681–1692. [Google Scholar] [CrossRef]
- Fraga-Dubreuil, J.; Bazureau, J.P. Efficient combination of task-specific ionic liquid and microwave dielectric heating applied to one-pot three component synthesis of a small library of 4-thiazolidinones. Tetrahedron 2003, 59, 6121–6130. [Google Scholar] [CrossRef]
- Hakkou, H.; Vanden Eynde, J.J.; Hamelin, J.; Bazureau, J.P. Ionic liquid phase organic synthesis (IoLiPOS) methodology applied to the three component preparation of 2-thioxotetrahydropyrimidin-4-(1H)-ones under microwave dielectric heating. Tetrahedron 2004, 60, 3745–3753. [Google Scholar] [CrossRef]
- Legeay, J.-C.; Vanden Eynde, J.J.; Bazureau, J.P. Ionic liquid phase technology supported the three component synthesis of Hantzsch 1,4-dihydropyridines and Biginelli 3,4-dihydropyrimidin-2(1H)-ones under microwave dielectric heating. Tetrahedron 2005, 61, 12386–12397. [Google Scholar] [CrossRef]
- Legeay, J.C.; Goujon, J.Y.; Vanden Eynde, J.J.; Toupet, L.; Bazureau, J.P. Liquid-phase synthesis of polyhydroquinoline using task-specific ionic liquid technology. J. Comb. Chem. 2006, 8, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Narhe, B.D.; Tsai, M.-H.; Sun, C.-M. Rapid two-Sstep synthesis of benzimidazo[1′,2′:1,5]pyrrolo[2,3-c]isoquinolines by a three-component coupling reaction. ACS Comb. Sci. 2014, 16, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Song, G. Amino-functionalized ionic liquid as catalytically active solvent for microwave-assisted synthesis of 4H-pyrans. Catal. Commun. 2007, 8, 111–114. [Google Scholar] [CrossRef]
- Xia, M.; Lu, Y. A novel, neutral ionic liquid-catalyzed, solvent-free synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation. J. Mol. Catal. A Chem. 2007, 265, 205–208. [Google Scholar] [CrossRef]
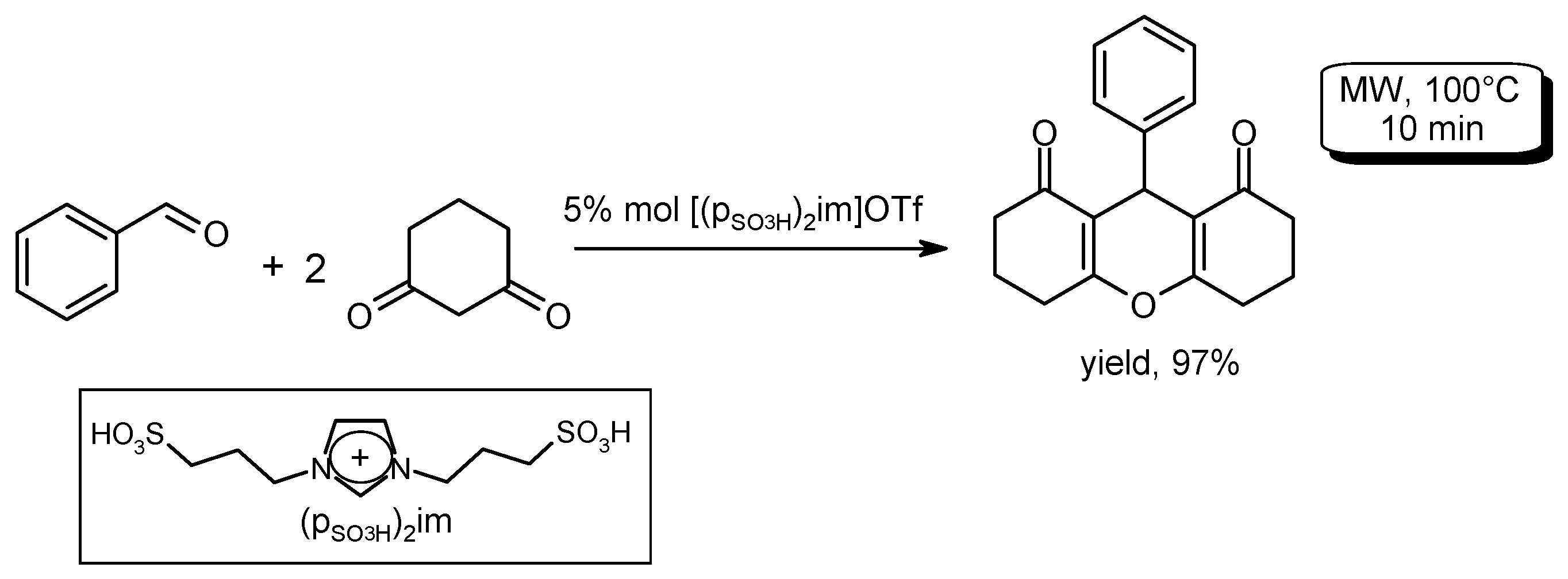
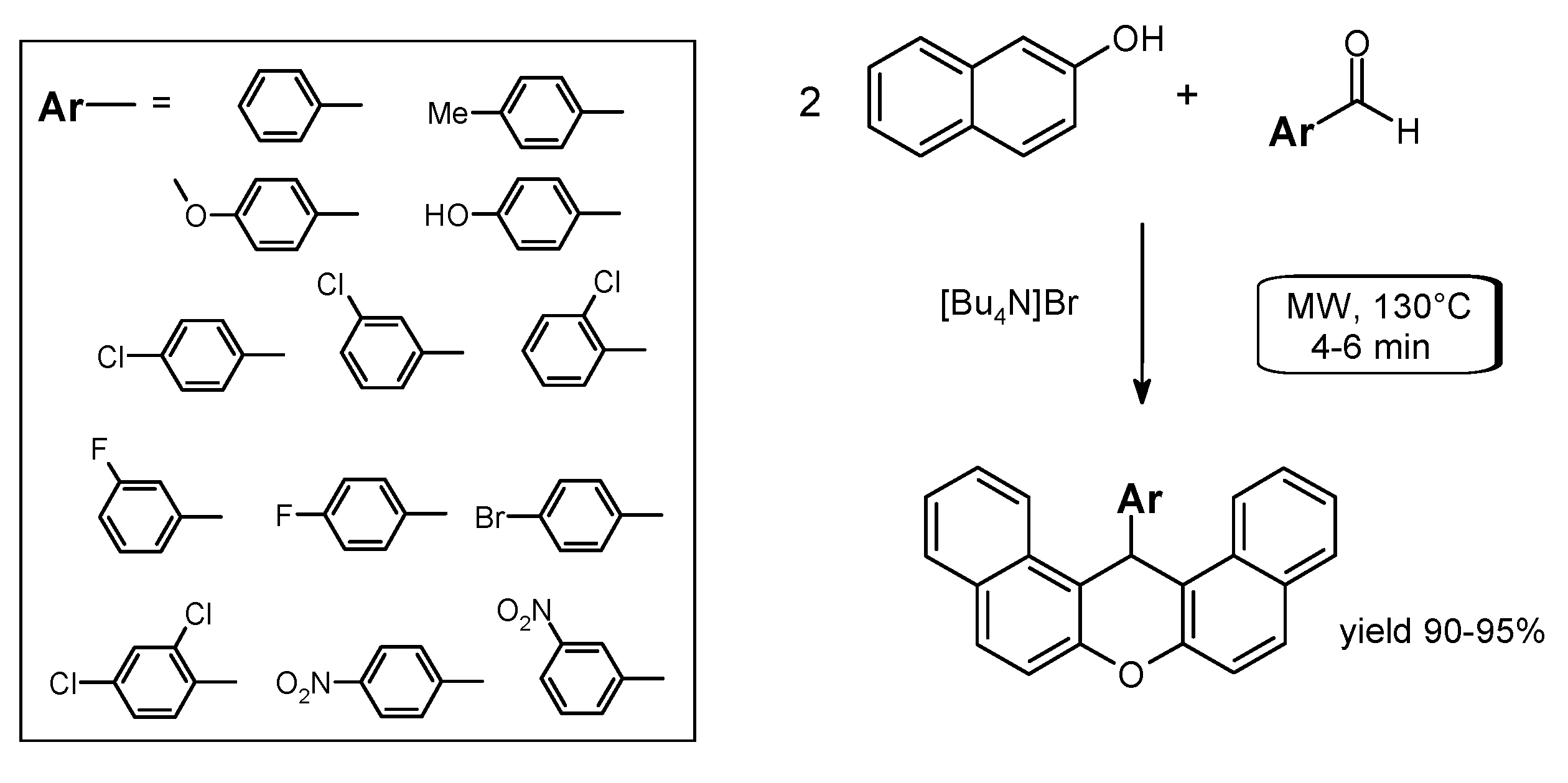
- Kantevari, S.; Chary, M.V.; Rudra Das, A.P.; Vuppalapati, S.V.N.; Lingaiah, N. Catalysis by an ionic liquid: Highly efficient solvent-free synthesis of aryl-14H-dibenzo[a.j]xanthenes by molten tetrabutylammonium bromide under conventional and microwave heating. Catal. Commun. 2008, 9, 1575–1578. [Google Scholar]
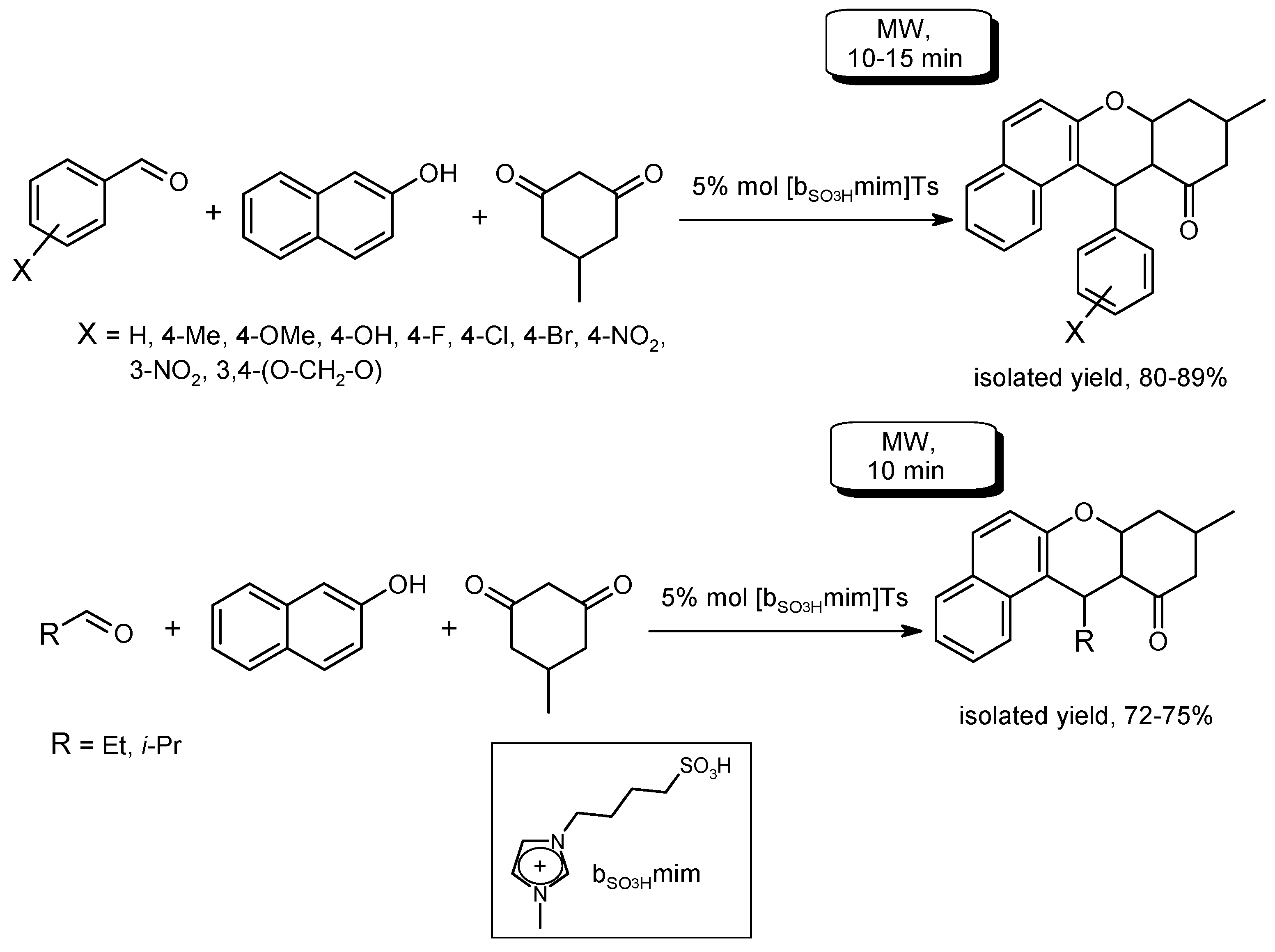
- Kundu, D.; Majee, A.; Hajra, A. Task-specific ionic liquid-catalyzed efficient microwave-assisted synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones under solvent-free conditions. Green Chem. Lett. Rev. 2011, 4, 205–209. [Google Scholar] [CrossRef]
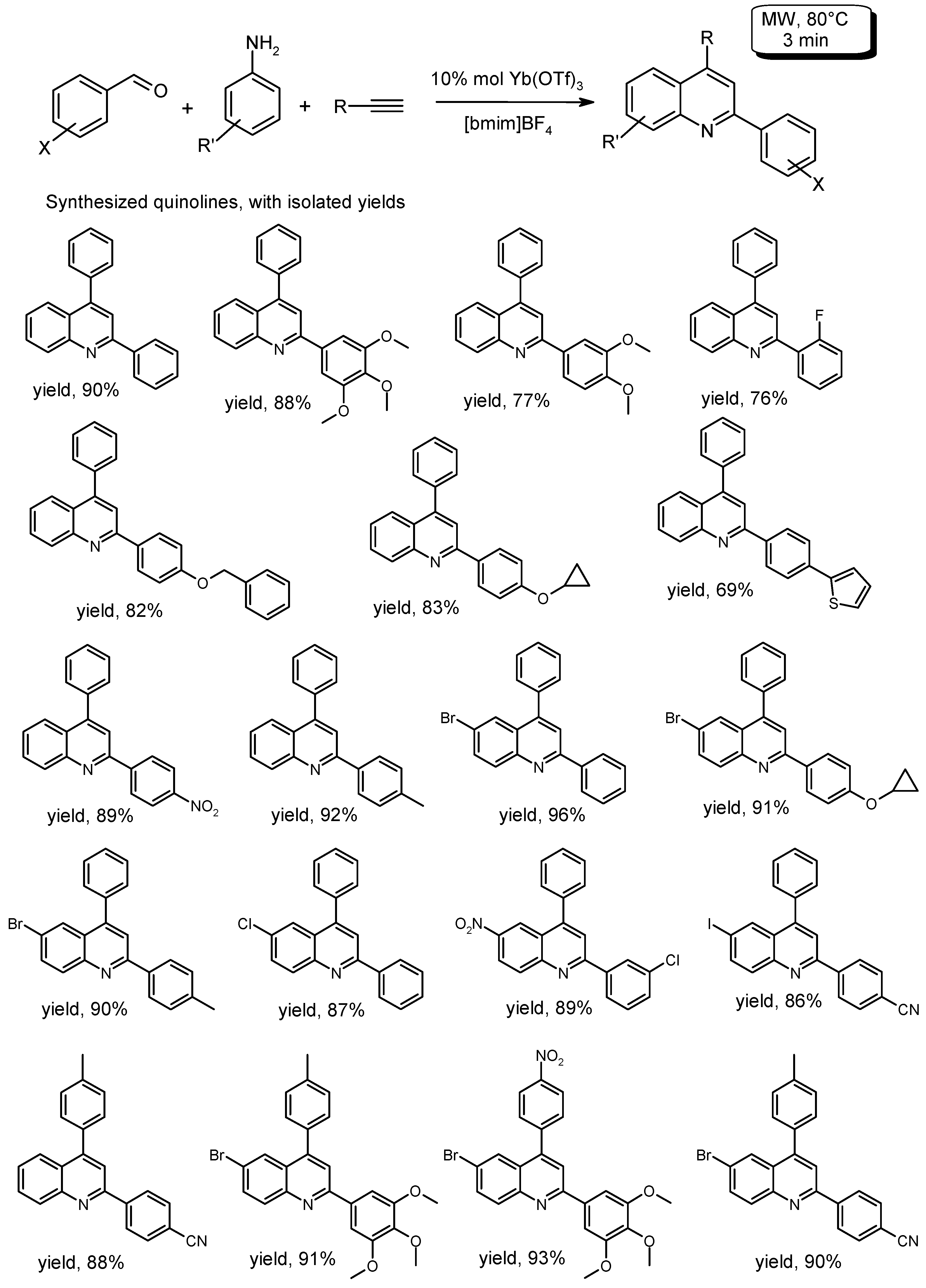
- Kumar, A.; Rao, V.K. Microwave-assisted and Yb(OTf)3-promoted one-pot multicomponent synthesis of substituted quinolines in ionic liquid. Synlett 2011, 2011, 2157–2162. [Google Scholar] [CrossRef]
- Mert-Balci, F.; Conrad, J.; Beifuss, U. Microwave-assisted three-component reaction in conventional solvents and ionic liquids for the synthesis of amino-substituted imidazo[1,2-a]pyridines. Arch. Org. Chem. 2012, 2012, 243–256. [Google Scholar]
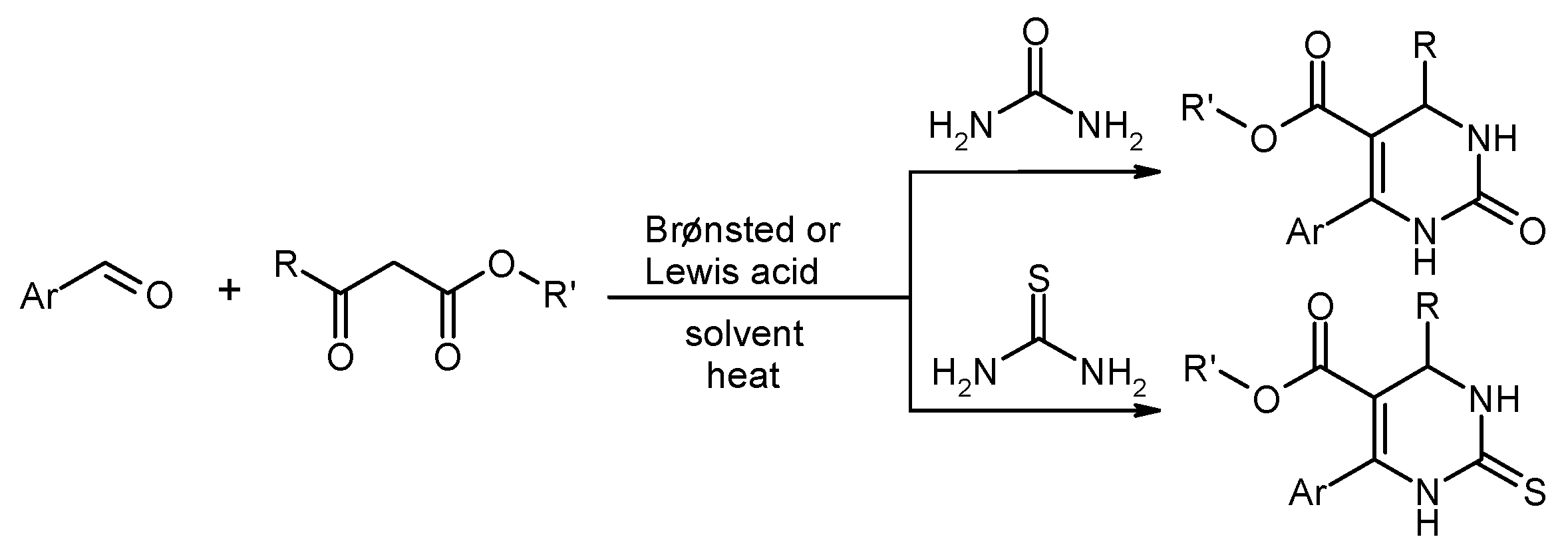
- Chebanov, V.A.; Gorobets, N.Y.; Sedash, Y.V. Reactions involving a carbonyl compound as electrophilic component. Third component 1,3-dicarbonyl compound (with ureas: Biginelli reaction). In Science of Synthesis, Multicomponent Reactions; Mueller, T.J.J., Ed.; Thieme: Stuttgart, Germany, 2014; Volume 1, pp. 29–66. [Google Scholar]
- Suresh; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. Arch. Org. Chem. 2011, 2012, 66–133. [Google Scholar]
- Kolosov, M.A.; Orlov, V.D.; Beloborodov, D.A.; Dotsenko, V.V. A chemical placebo: NaCl as an effective, cheapest, non-acidic and greener catalyst for Biginelli-type 3,4-dihydropyrimidin-2(1H)-ones (-thiones) synthesis. Mol. Divers. 2009, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Deng, Y. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001, 42, 5917–5919. [Google Scholar] [CrossRef]
- Arfan, A.; Paquin, L.; Bazureau, J.P. Acidic task-specific ionic liquid as catalyst of microwave-assisted solvent-free Biginelli reaction. Russ. J. Org. Chem. 2007, 43, 1058–1064. [Google Scholar] [CrossRef]
- Rong, L.; Han, H.; Wang, H.; Jiang, H.; Tu, S.; Shi, D. An efficient and facile synthesis of pyrimidine and quinazoline derivatives via one-pot three-component reaction under solvent-free conditions. J. Heterocycl. Chem. 2009, 46, 152–157. [Google Scholar] [CrossRef]
- Zare, A.; Hasaninejad, A.; Salimi Beni, A.; Moosavi-Zare, A.R.; Merajoddin, M.; Kamali, E.; Akbari-Seddigh, M.; Parsaee, Z. Ionic liquid 1-butyl-3-methylimidazolium bromide ([Bmim]Br) as a green and neutral reaction media for the catalyst-free synthesis of 1-amidoalkyl-2-naphthols. Sci. Iran. 2011, 18, 433–438. [Google Scholar] [CrossRef]
- Srivastava, V. An improved protocol for Biginelli reaction. Green Sustain. Chem. 2013, 3, 38–40. [Google Scholar] [CrossRef]
- Dadhania, A.N.; Patel, V.K.; Raval, D.K. A facile approach for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones using a microwave promoted Biginelli protocol in ionic liquid. J. Chem. Sci. 2012, 124, 921–926. [Google Scholar] [CrossRef]
- Mirzai, M.; Valizadeh, H. Microwave-promoted synthesis of 3,4-dihydropyrimidin-2(1H)-(thio)ones using IL-ONO as recyclable base catalyst under solvent-free conditions. Synth. Commun. 2012, 42, 1268–1277. [Google Scholar] [CrossRef]
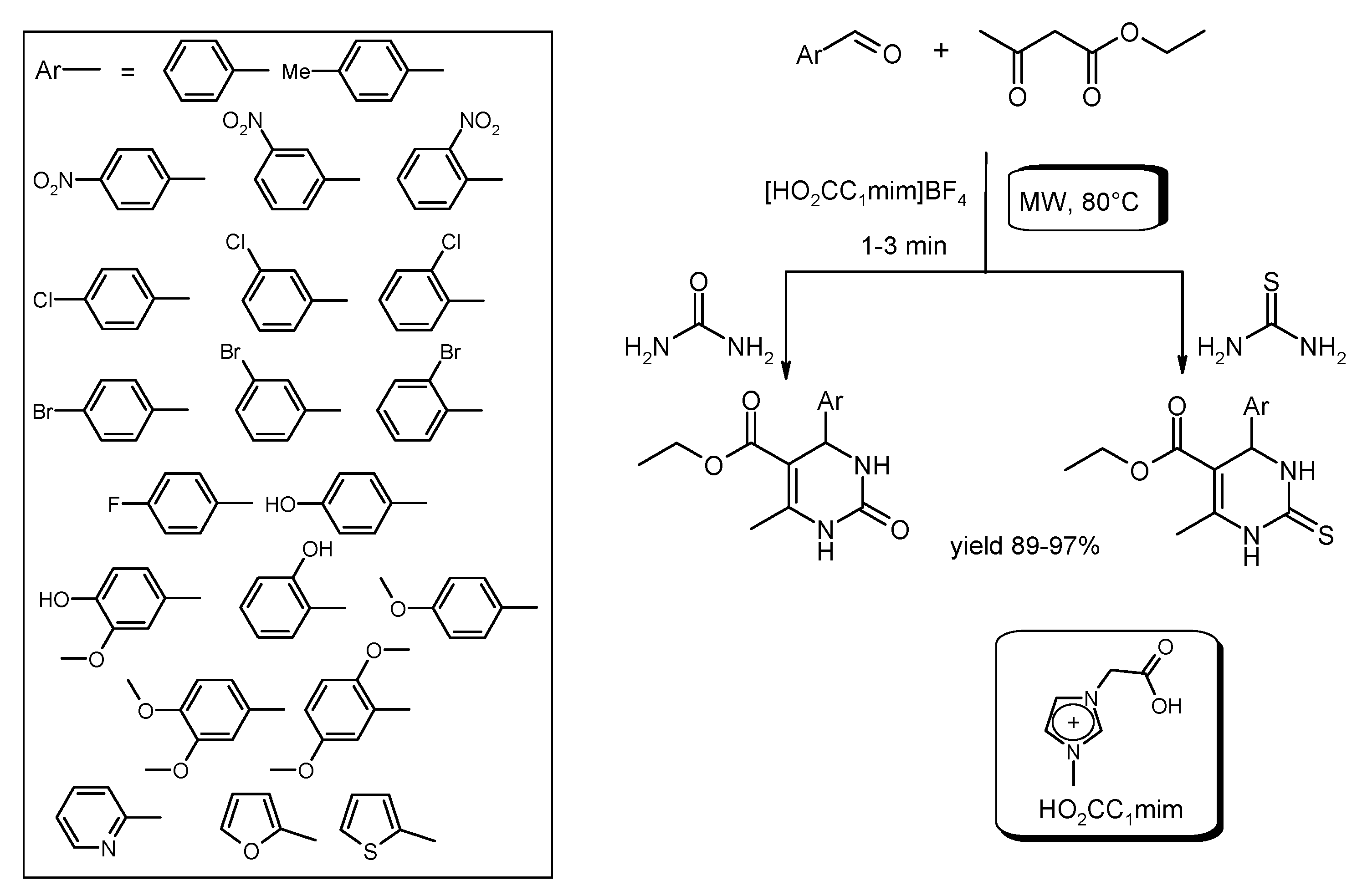
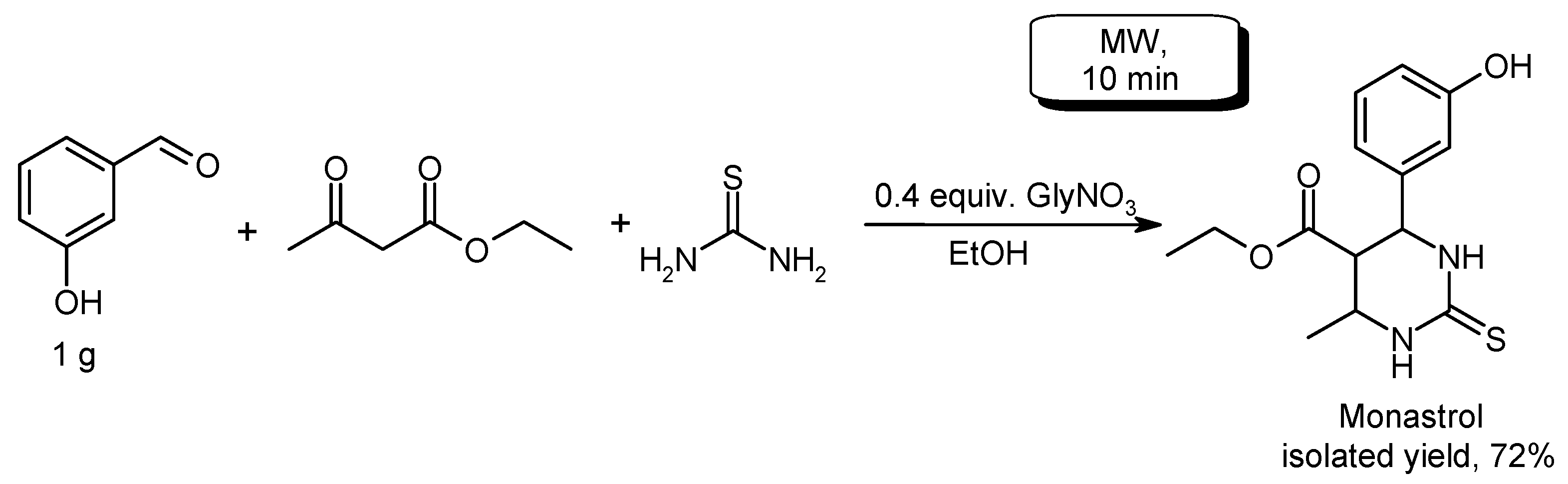
- Sharma, N.; Sharma, U.K.; Kumar, R.; Richa; Sinha, A.K. Green and recyclable glycine nitrate (GlyNO3) ionic liquid triggered multicomponent Biginelli reaction for the efficient synthesis of dihydropyrimidinones. RSC Adv. 2012, 2, 10648–10651. [Google Scholar] [CrossRef]
- Fu, R.; Yang, Y.; Lai, W.; Ma, Y.; Chen, Z.; Zhou, J.; Chai, W.; Wang, Q.; Yuan, R. Efficient and green microwave-assisted multicomponent Biginelli reaction for the synthesis of dihydropyrimidinones catalyzed by heteropolyanion-based ionic liquids under solvent-free conditions. Synth. Commun. 2015, 45, 467–477. [Google Scholar] [CrossRef]
- Zakeri, M.; Nasef, M.M.; Abouzari-Lotf, E. Eco-safe and expeditious approaches for synthesis of quinazoline and pyrimidine-2-amine derivatives using ionic liquids aided with ultrasound or microwave irradiation. J. Mol. Liquids 2014, 199, 267–274. [Google Scholar] [CrossRef]
- Bhatt, J.D.; Chudasama, C.J.; Patel, K.D. Microwave assisted synthesis of pyrimidines in ionic liquid and their potency as non-classical malarial antifolates. Arch. Pharm. Chem. Life Sci. 2016, 349, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Zarnegar, Z.; Safari, J. Magnetic nanoparticles supported imidazolium-based ionic liquids as nanocatalyst in microwave-mediated solvent-free Biginelli reaction. J. Nanoparticle Res. 2014, 16, 2509. [Google Scholar] [CrossRef]
- Martens, J.A.; Bogaerts, A.; De Kimpe, N.; Jacobs, P.A.; Marin, G.B.; Rabaey, K.; Saeys, M.; Verhelst, S. The chemical route to a carbon dioxide nutral world. ChemSusChem 2017, 10, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Kreye, O.; Over, L.C.; Nitsche, T.; Lange, R.Z.; Meier, M.A.R. Organic carbonates: Sustainable and environmentally-friendly ethylation, allylation, and benzylation reagents. Tetrahedron 2015, 71, 293–300. [Google Scholar] [CrossRef]
- Kim, Y.J.; Varma, R.S. Tetrahaloindate(III)-based ionic liquids in the coupling reaction of carbon dioxide and epoxides to generate cyclic carbonates: H-bonding and mechanistic studies. J. Org. Chem. 2005, 70, 7882–7891. [Google Scholar] [CrossRef] [PubMed]
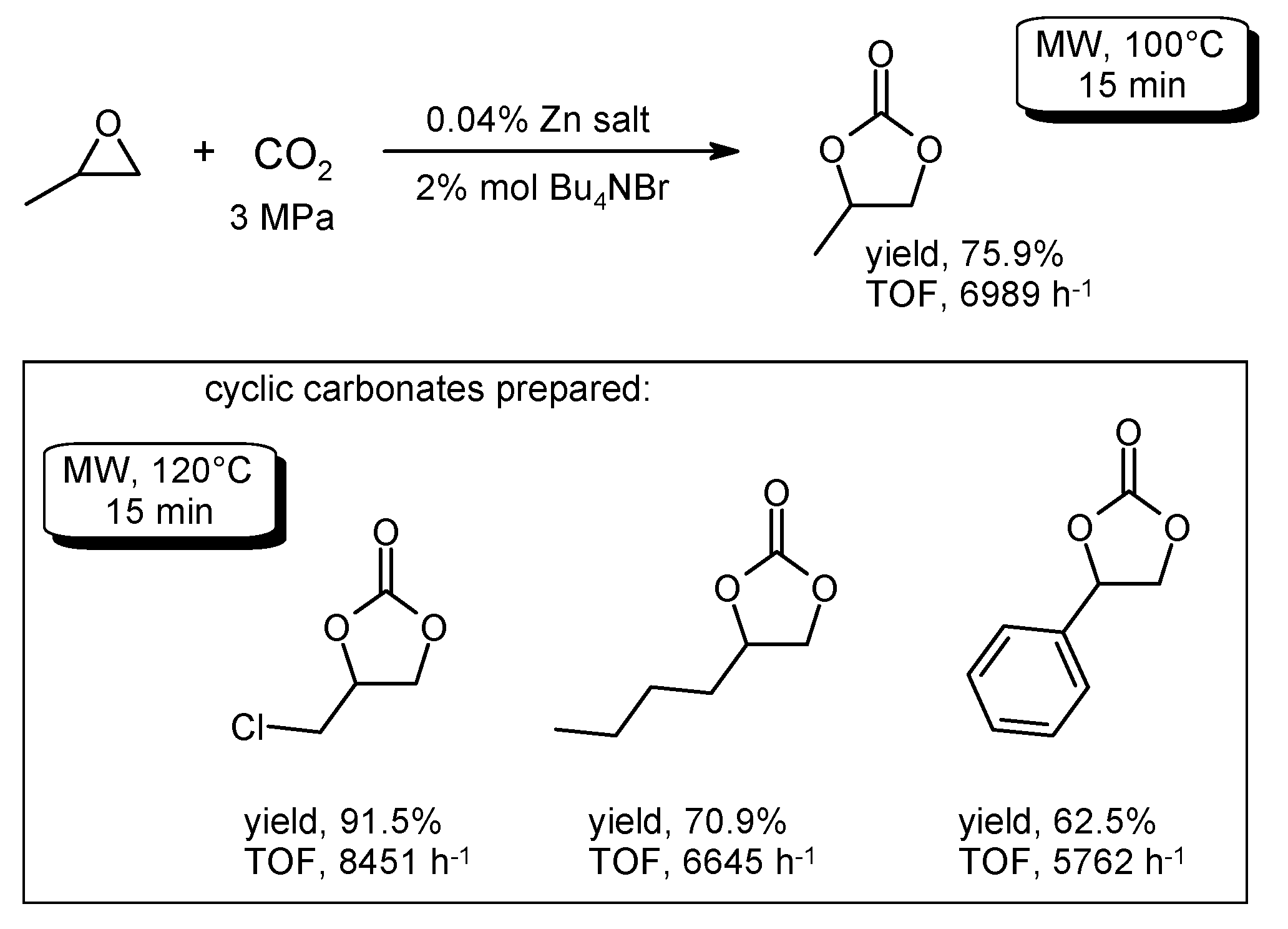
- Ono, F.; Qiao, K.; Tomida, D.; Yokoyama, C. Rapid synthesis of cyclic carbonates from CO2 and epoxides under microwave irradiation with controlled temperature and pressure. J. Mol. Catal. A Chem. 2007, 263, 223–226. [Google Scholar] [CrossRef]
- Tharun, J.; Kim, D.W.; Roshan, R.; Hwang, Y.; Park, D.-W. Microwave assisted preparation of quaternized chitosan catalyst for the cycloaddition of CO2 and epoxides. Catal. Commun. 2013, 31, 62–65. [Google Scholar] [CrossRef]
- Dharman, M.M.; Yu, J.-I.; Ahn, J.-Y.; Park, D.-W. Selective production of cyclic carbonate over polycarbonate using a double metal cyanide-quaternary ammonium salt catalyst system. Green Chem. 2009, 11, 1754–1757. [Google Scholar] [CrossRef]
- Tharun, J.; Mathai, G.; Kathalikkattil, A.C.; Roshan, R.; Kwak, J.-Y.; Park, D.-W. Microwave-assisted synthesis of cyclic carbonates by formic acid/KI catalytic system. Green Chem. 2013, 15, 1673–1677. [Google Scholar] [CrossRef]
- Ju, H.-Y.; Dharman, M.M.; Kim, K.-H.; Park, S.-W.; Park, D.-W. Catalytic performance of quaternary ammonium salts in the reaction of butyl glycidyl ether and carbon dioxide. J. Ind. Eng. Chem. 2008, 14, 157–160. [Google Scholar] [CrossRef]
- Udayakumar, S.; Lee, M.-K.; Shim, H.-L.; Park, S.-W.; Park, D.-W. Imidazolium derivatives functionalized MCM-41 for catalytic conversion of carbon dioxide to cyclic carbonate. Catal. Commun. 2009, 10, 659–664. [Google Scholar] [CrossRef]
- Udayakumar, S.; Lee, M.-K.; Shim, H.-L.; Park, D.-W. Functionalization of organic ions on hybrid MCM-41 for cycloaddition reaction: The effective conversion of carbon dioxide. Appl. Catal. A Gen. 2009, 365, 88–95. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Tharun, J.; Roshan, R.; Soek, H.-G.; Park, D.-W. Efficient route for oxazolidinone synthesis using heterogeneous biopolymer catalysts from unactivated alkyl aziridine and CO2 under mild conditions. Appl. Catal. A Gen. 2012, 447–448, 107–114. [Google Scholar] [CrossRef]
- Roshan, K.R.; Mathai, G.; Kim, J.; Tharun, J.; Park, G.-A.; Park, D.-W. A biopolymer mediated efficient synthesis of cyclic carbonates from epoxides and carbon dioxide. Green Chem. 2012, 14, 2933–2940. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, D.-W. Functionalized IRMOF-3: An efficient heterogeneous catalyst for the cycloaddition of allyl glycidyl ether and CO2. J. Nanosci. Nanotechnol. 2013, 13, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Dharman, M.M.; Choi, H.-J.; Park, S.-W.; Park, D.-W. Microwave assisted synthesis of cyclic carbonate using homogeneous and heterogeneous ionic liquid catalysts. Top. Catal. 2010, 53, 462–469. [Google Scholar] [CrossRef]
- Dharman, M.M.; Choi, H.-J.; Kim, D.-W.; Park, D.-W. Synthesis of cyclic carbonate through microwave irradiation using silica-supported ionic liquids: Effect of variation in the silica support. Catal. Today 2011, 164, 544–547. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, D.-K.; Kim, M.-I.; Park, D.-W. Microwave assisted synthesis of allyl glycidyl carbonate by using ionic liquid immobilized onto montmorillonite clay. Catal. Today 2012, 185, 217–223. [Google Scholar] [CrossRef]
- Tharun, J.; Kathalikkattil, A.C.; Roshan, R.; Kang, D.-H.; Woo, H.-C.; Park, D.-W. Microwave-assisted, rapid cycloaddition of allyl glycidyl ether and CO2 by employing pyridinium-based ionic liquid catalysts. Catal. Commun. 2014, 54, 31–34. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Kim, D.-W.; Tharun, J.; Soek, H.-G.; Roshan, R.; Park, D.-W. Aqueous-microwave synthesized carboxyl functional molecular ribbon coordination framework catalyst for the synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2014, 16, 1607–1616. [Google Scholar] [CrossRef]
- Du, J.; Shi, J.; Li, Z.; Liu, Z.; Fan, X.; Tao, C. Ionic liquid mediated CO2 activation for DMC synthesis. J. Nat. Gas Chem. 2012, 21, 476–479. [Google Scholar] [CrossRef]
- Du, H.; Liu, X.; Yu, Y.; Xu, Y.; Wang, Y.; Liang, Z. Microwave-induced poly(ionic liquid)/poly(vinyl alcohol) shape memory composites. Macromol. Chem. Phys. 2016, 217, 2626–2634. [Google Scholar] [CrossRef]
- Long, D.; Liu, T.; Tan, L.; Shi, H.; Liang, P.; Tang, S.; Wu, Q.; Yu, J.; Dou, J.; Meng, X. Multisynergistic platform for tumor therapy by mild microwave irradiation-activated chemotherapy and enhanced ablation. ACS Nano 2016, 10, 9516–9528. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Du, Q.; Liu, T.; Tan, L.; Niu, M.; Gao, L.; Huang, Z.; Fu, C.; Ma, T.; Meng, X.; et al. In vivo magnetic resonance imaging and microwave thermotherapy of cancer using novel chitosan microcapsules. Nanoscale Res. Lett. 2016, 11, 334. [Google Scholar] [CrossRef] [PubMed]
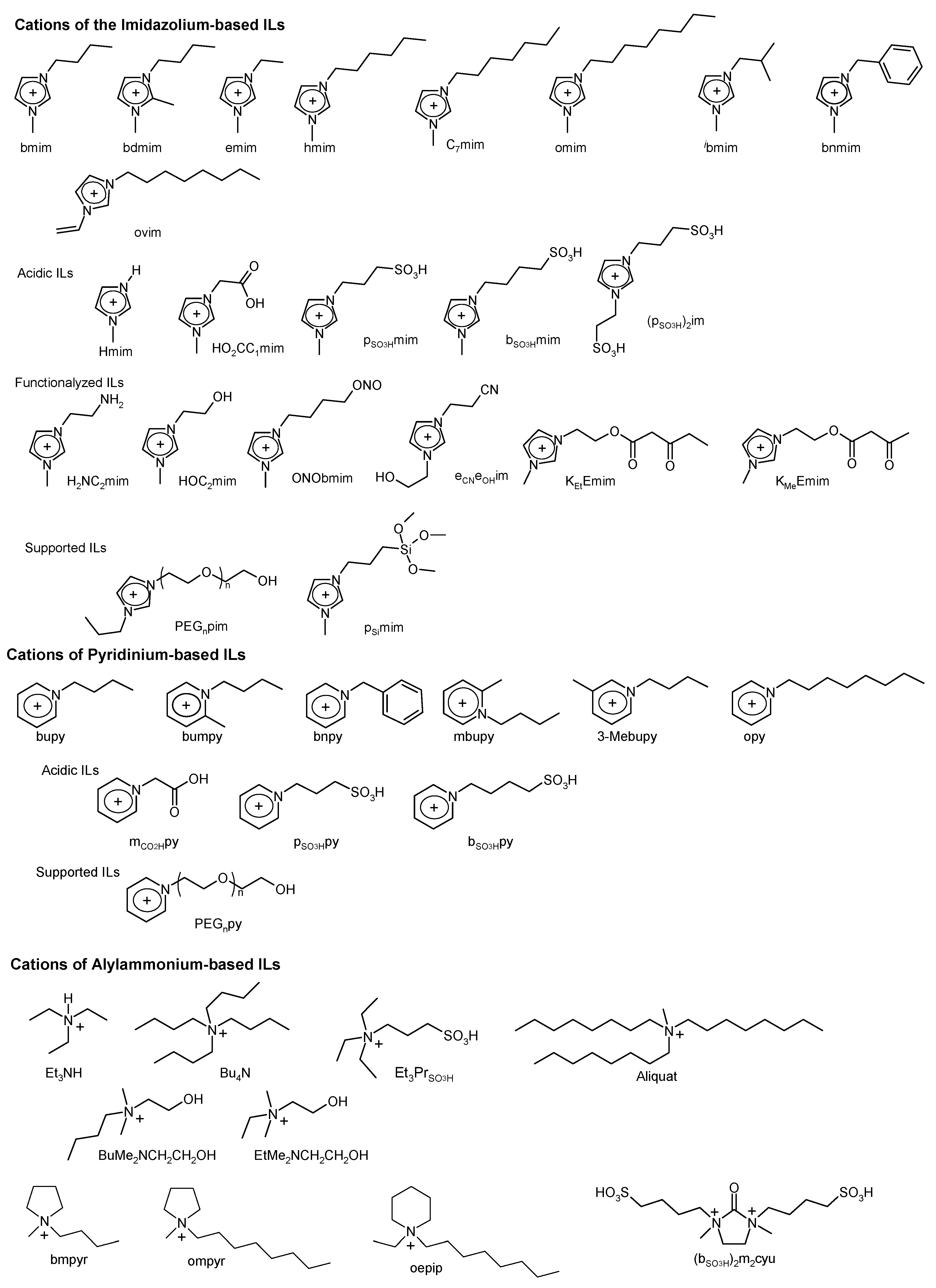



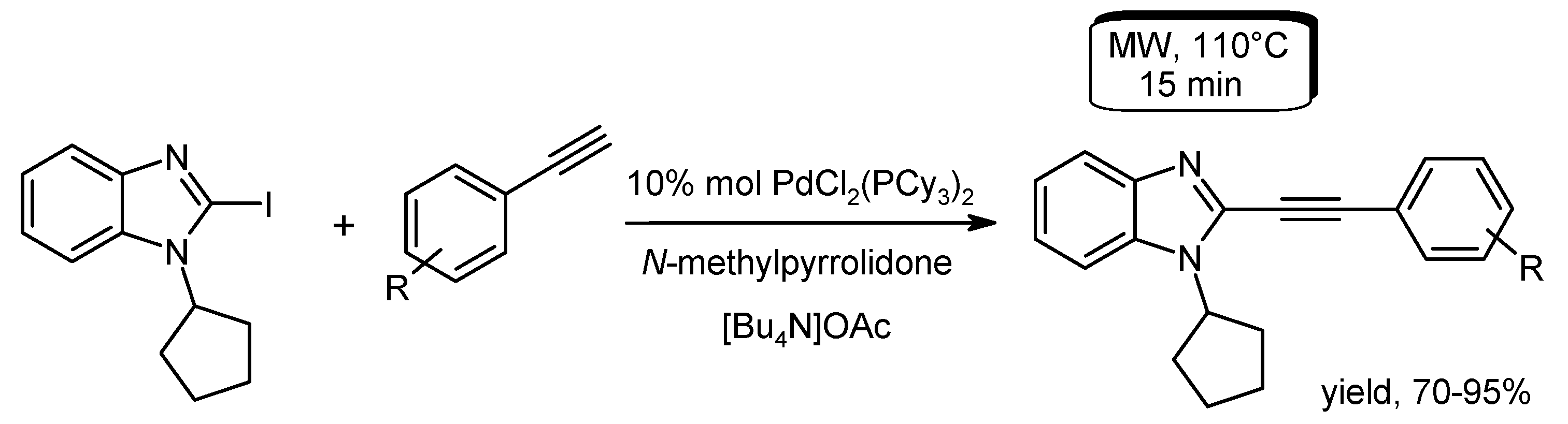
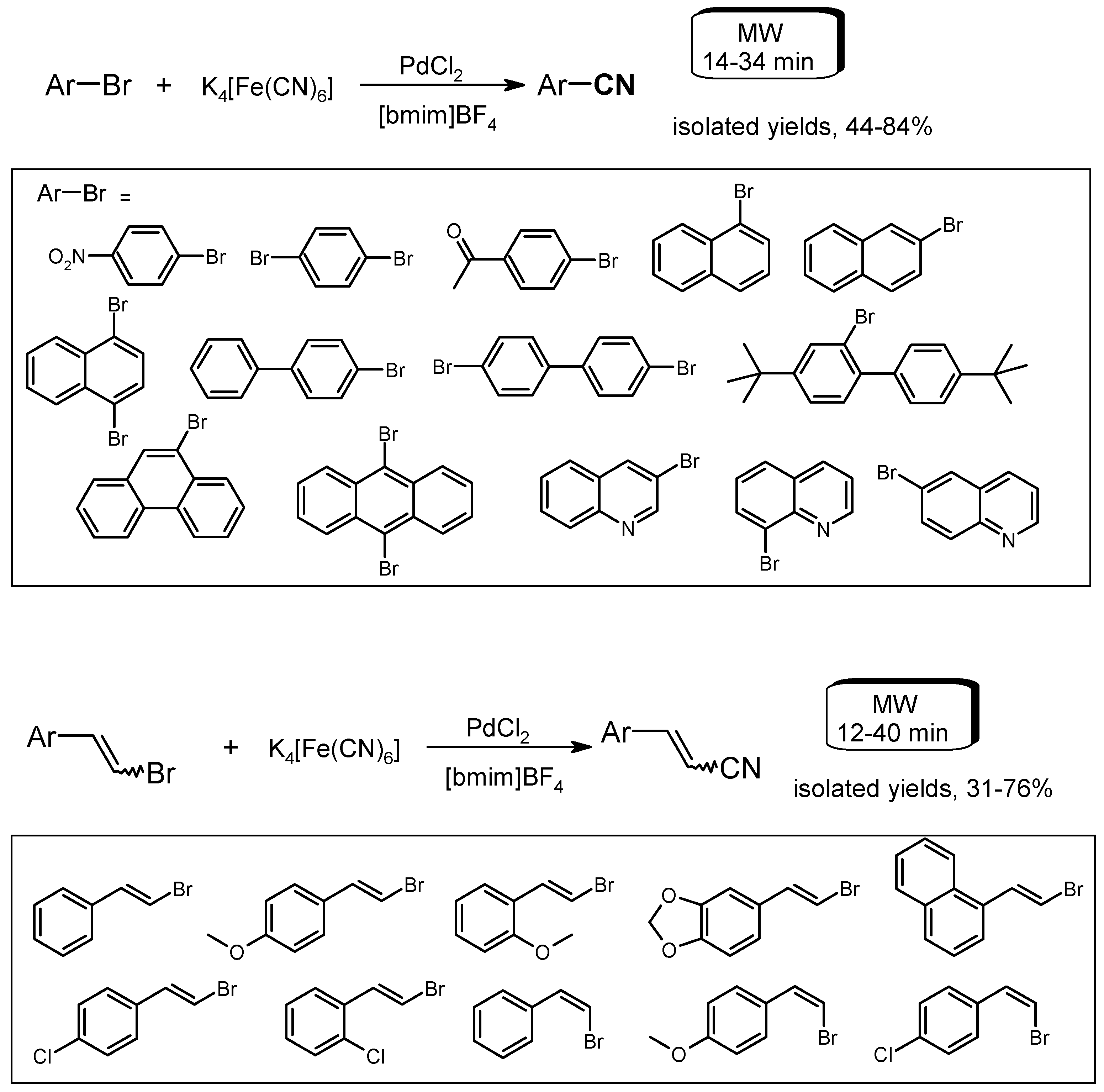








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floris, B.; Sabuzi, F.; Galloni, P.; Conte, V. The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions. Catalysts 2017, 7, 261. https://doi.org/10.3390/catal7090261
Floris B, Sabuzi F, Galloni P, Conte V. The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions. Catalysts. 2017; 7(9):261. https://doi.org/10.3390/catal7090261
Chicago/Turabian StyleFloris, Barbara, Federica Sabuzi, Pierluca Galloni, and Valeria Conte. 2017. "The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions" Catalysts 7, no. 9: 261. https://doi.org/10.3390/catal7090261