Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Catalyst Composition on MEA Performance
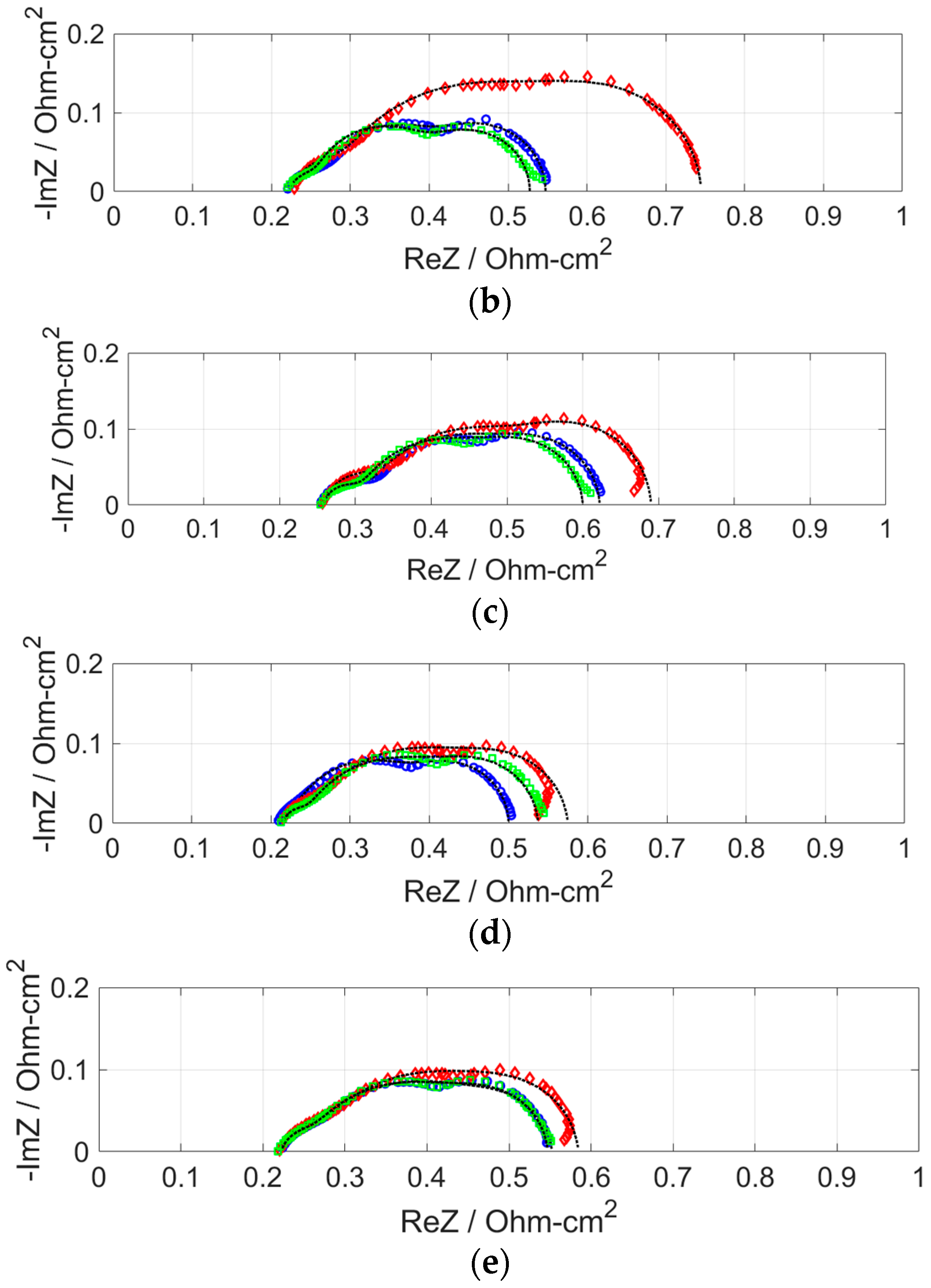
2.2. Effect of H2S on Cell Performance at Various Anode Pt Loadings
2.3. Effect of H2S on Cell Performance under Various Operating Current Density Levels
2.4. Durability Test
3. Experimental
3.1. MEA Preparation
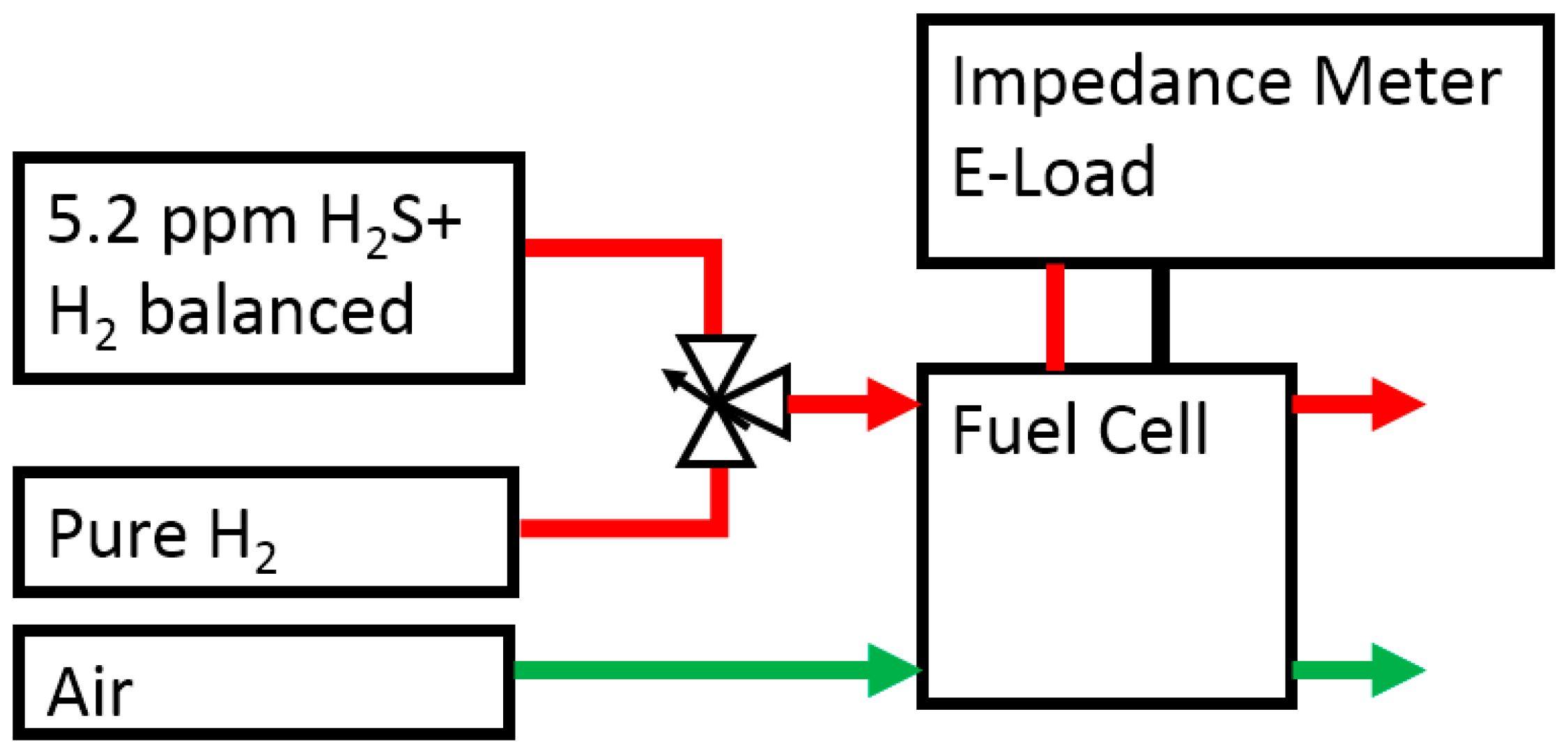
3.2. Experimental Procedure
4. Conclusions
- When PVDF was employed as the binder, excessive PVDF covered the electrochemical surface area of the catalyst and reduced the continuous path for electron transfer. The optimal PVDF loading in the catalyst layer was 10 wt % in this study.
- MEA performance did not increase significantly when the anode Pt loading was higher than 0.4 mg cm−2 under pure H2 operation.
- After the anode was supplied with H2S, the resistance increase was the least for the MEA with anode Pt loading of 0.7 mg cm−2. Because the performances of MEAs with anode Pt loadings higher than 0.4 mg cm−2 were almost the same, the excess electrochemical surface area can be said to have worked as a buffer that adsorbed H2S. The larger increase in area resistance at 0.2 A cm−2 was caused by slow gas flow rate.
- In the durability test, the voltage decrease rate was approximately 300 μV h−1 in the presence of H2S, and it recovered to 200 μV h−1 after H2 was supplied to the anode.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and off-design performance of a 1kWe HT-PEMFC (high temperature-proton exchange membrane fuel cell)-based residential micro-CHP (combined-heat-and-power) system for Danish single-family households. Energy 2011, 36, 993–1002. [Google Scholar] [CrossRef]
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and parametric study of a 1kWe HT-PEMFC-based residential micro-CHP system. Int. J. Hydrog. Energy 2011, 36, 5010–5020. [Google Scholar] [CrossRef]
- Giacoppo, G.; Barbera, O.; Carbone, A.; Gatto, I.; Saccà, A.; Pedicini, R.; Passalacqua, E. 1.5 kWe HT-PEFC stack with composite MEA for CHP application. Int. J. Hydrog. Energy 2013, 38, 11619–11627. [Google Scholar] [CrossRef]
- Najafi, B.; Haghighat Mamaghani, A.; Rinaldi, F.; Casalegno, A. Long-term performance analysis of an HT-PEM fuel cell based micro-CHP system: Operational strategies. Appl. Energy 2015, 147, 582–592. [Google Scholar] [CrossRef]
- Lei, J.; Yue, H.; Tang, H.; Liang, B. Heat integration and optimization of hydrogen production for a 1 kW low-temperature proton exchange membrane fuel cell. Chem. Eng. Sci. 2015, 123, 81–91. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-based polymer membranes for high temperature fuel cells–preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Ruiz-López, C.; Linares, J.J. Influence of the Teflon loading in the gas diffusion layer of PBI-based PEM fuel cells. J. Appl. Electrochem. 2008, 38, 793–802. [Google Scholar] [CrossRef]
- Mamlouk, M.; Scott, K. Phosphoric acid-doped electrodes for a PBI polymer membrane fuel cell. Int. J. Energy Res. 2011, 35, 507–519. [Google Scholar] [CrossRef]
- Jung, G.-B.; Tseng, C.-C.; Yeh, C.-C.; Lin, C.-Y. Membrane electrode assemblies doped with H3PO4 for high temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2012, 37, 13645–13651. [Google Scholar] [CrossRef]
- Su, H.; Pasupathi, S.; Bladergroen, B.; Linkov, V.; Pollet, B.G. Optimization of gas diffusion electrode for polybenzimidazole-based high temperature proton exchange membrane fuel cell: Evaluation of polymer binders in catalyst layer. Int. J. Hydrog. Energy 2013, 38, 11370–11378. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Pasupathi, S.; Bladergroen, B.J.; Linkov, V.; Pollet, B.G. Enhanced performance of polybenzimidazole-based high temperature proton exchange membrane fuel cell with gas diffusion electrodes prepared by automatic catalyst spraying under irradiation technique. J. Power Sources 2013, 242, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, membrane, free electrolyte challenges, and pathways to resolutions in high temperature polymer electrolyte membrane fuel cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Linares, J.J.; Pinar, F.J. Study of the influence of the amount of PBI–H3PO4 in the catalytic layer of a high temperature PEMFC. Int. J. Hydrog. Energy 2010, 35, 1347–1355. [Google Scholar] [CrossRef]
- Lin, H.-L.; Wu, T.-J.; Lin, Y.-T.; Wu, H.-C. Effect of polyvinylidene difluoride in the catalyst layer on high-temperature PEMFCs. Int. J. Hydrog. Energy 2015, 40, 9400–9409. [Google Scholar] [CrossRef]
- Lwin, L.; Daud, W.R.W.D.; Mohamad, A.B.; Yaakob, Z. Hydrogen production from steam methanol reforming: Thermodynamic analysis. Int. J. Hydrog. Energy 2000, 25, 47–53. [Google Scholar] [CrossRef]
- Joensen, F.; Rostrup-Nielsen, J.R. Conversion of hydrocarbons and alcohols for fuel cells. J. Power Sources 2002, 105, 195–201. [Google Scholar] [CrossRef]
- Hafez, H.; Nakhla, G.; El Naggar, H. Biological Hydrogen production from corn-syrup waste using a novel system. Energies 2009, 2, 445–455. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Rau, M.; Cremers, C.; Tübke, J. Development of anodic materials for HT-PEMFCs with high tolerance to H2S. Int. J. Hydrog. Energy 2015, 40, 5439–5443. [Google Scholar] [CrossRef]
- Qi, Z. Effect of CO in the anode fuel on the performance of PEM fuel cell cathode. J. Power Sources 2002, 111, 239–247. [Google Scholar] [CrossRef]
- Brett, D.; Aguiar, P.; Brandon, N.; Kucernak, A. Measurement and modelling of carbon monoxide poisoning distribution within a polymer electrolyte fuel cell. Int. J. Hydrog. Energy 2007, 32, 863–871. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Bethune, K.; Rocheleau, R. Spatial proton exchange membrane fuel cell performance under carbon monoxide poisoning at a low concentration using a segmented cell system. J. Power Sources 2012, 218, 412–423. [Google Scholar] [CrossRef]
- Decoopman, B.; Vincent, R.; Rosini, S.; Paganelli, G.; Thivel, P.X. Proton exchange membrane fuel cell reversible performance loss induced by carbon monoxide produced during operation. J. Power Sources 2016, 324, 492–498. [Google Scholar] [CrossRef]
- Krishnan, P.; Park, J.-S.; Kim, C.-S. Performance of a poly(2,5-benzimidazole) membrane based high temperature PEM fuel cell in the presence of carbon monoxide. J. Power Sources 2006, 159, 817–823. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Gao, J.-A.; Jensen, J.O.; Bjerrum, N.J. The CO poisoning effect in PEMFCs operational at temperatures up to 200 °C. J. Electrochem. Soc. 2003, 150, A1599–A1605. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; He, R.; Li, Q.; Jensen, J.O.; Bjerrum, N.J.; Hjulmand, H.A.; Jensen, A.B. Integration of high temperature PEM fuel cells with a methanol reformer. J. Power Sources 2005, 145, 392–398. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Durability and reliability in high-temperature reformed hydrogen PEFCs. ECS Trans. 2006, 3, 861–869. [Google Scholar]
- Rau, M.; Niedergesäß, A.; Cremers, C.; Alfaro, S.; Steenberg, T.; Hjuler, H.A. Characterization of membrane electrode assemblies for high-temperature PEM fuel cells. Fuel Cells 2016, 16, 577–583. [Google Scholar] [CrossRef]
- Su, H.; Jao, T.-C.; Pasupathi, S.; Bladergroen, B.J.; Linkov, V.; Pollet, B.G. A novel dual catalyst layer structured gas diffusion electrode for enhanced performance of high temperature proton exchange membrane fuel cell. ECS Trans. 2014, 246, 63–67. [Google Scholar] [CrossRef]
- Andreasen, S.J.; Vang, J.R.; Kær, S.K. High temperature PEM fuel cell performance characterisation with CO and CO2 using electrochemical impedance spectroscopy. Int. J. Hydrog. Energy 2011, 36, 9815–9830. [Google Scholar] [CrossRef]






| Pt Loading (mg cm−2) | Anode Gas | RΩ (Ω cm2) | Ran (Ω cm2) | Rca (Ω cm2) | Rm (Ω cm2) |
|---|---|---|---|---|---|
| 0.3 | H2 | 0.2575 | 0.1250 | 0.2250 | 0.1500 |
| H2S | 0.2750 | 0.1675 | 0.2500 | 0.2200 | |
| Recover | 0.2575 | 0.1125 | 0.1500 | 0.1500 | |
| 0.4 | H2 | 0.2225 | 0.0500 | 0.1375 | 0.1375 |
| H2S | 0.2250 | 0.0650 | 0.2750 | 0.1800 | |
| Recover | 0.2225 | 0.0375 | 0.1425 | 0.1250 | |
| 0.5 | H2 | 0.2575 | 0.0625 | 0.1850 | 0.1175 |
| H2S | 0.2575 | 0.0750 | 0.2000 | 0.1575 | |
| Recover | 0.2575 | 0.0500 | 0.1750 | 0.1175 | |
| 0.6 | H2 | 0.2100 | 0.0250 | 0.1500 | 0.1150 |
| H2S | 0.2175 | 0.0375 | 0.2125 | 0.1075 | |
| Recover | 0.2150 | 0.0300 | 0.1875 | 0.1050 | |
| 0.7 | H2 | 0.2225 | 0.0500 | 0.2000 | 0.0800 |
| H2S | 0.2225 | 0.0500 | 0.2125 | 0.1000 | |
| Recover | 0.2225 | 0.0375 | 0.1925 | 0.0950 |
| Current Density (A cm−2) | Anode Gas | RΩ (Ω cm2) | Ran (Ω cm2) | Rca (Ω cm2) | Rm (Ω cm2) |
|---|---|---|---|---|---|
| 0.2 | H2 | 0.2175 | 0.1375 | 0.2375 | 0.2875 |
| H2S | 0.2175 | 0.1625 | 0.2525 | 0.3025 | |
| Recover | 0.2175 | 0.1375 | 0.2375 | 0.2875 | |
| 0.3 | H2 | 0.2000 | 0.0750 | 0.2500 | 0.2875 |
| H2S | 0.2150 | 0.1075 | 0.2500 | 0.2625 | |
| Recover | 0.2150 | 0.0975 | 0.2225 | 0.2975 | |
| 0.4 | H2 | 0.1875 | 0.0625 | 0.3000 | 0.3125 |
| H2S | 0.1925 | 0.0875 | 0.2625 | 0.3500 | |
| Recover | 0.1950 | 0.0750 | 0.3175 | 0.2975 | |
| 0.5 | H2 | 0.1875 | 0.0625 | 0.3750 | 0.4250 |
| H2S | 0.1850 | 0.0875 | 0.4000 | 0.4125 | |
| Recover | 0.1875 | 0.0625 | 0.3850 | 0.4250 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, R.-J.; Chen, Y.-S. Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy. Catalysts 2018, 8, 441. https://doi.org/10.3390/catal8100441
Kang R-J, Chen Y-S. Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy. Catalysts. 2018; 8(10):441. https://doi.org/10.3390/catal8100441
Chicago/Turabian StyleKang, Ren-Jun, and Yong-Song Chen. 2018. "Experimental Study on the Effect of Hydrogen Sulfide on High-Temperature Proton Exchange Membrane Fuel Cells by Using Electrochemical Impedance Spectroscopy" Catalysts 8, no. 10: 441. https://doi.org/10.3390/catal8100441





