Mass Transfer Performance of a String Film Reactor: A Bioreactor Design for Aerobic Methane Bioconversion
Abstract
:1. Introduction
2. Results
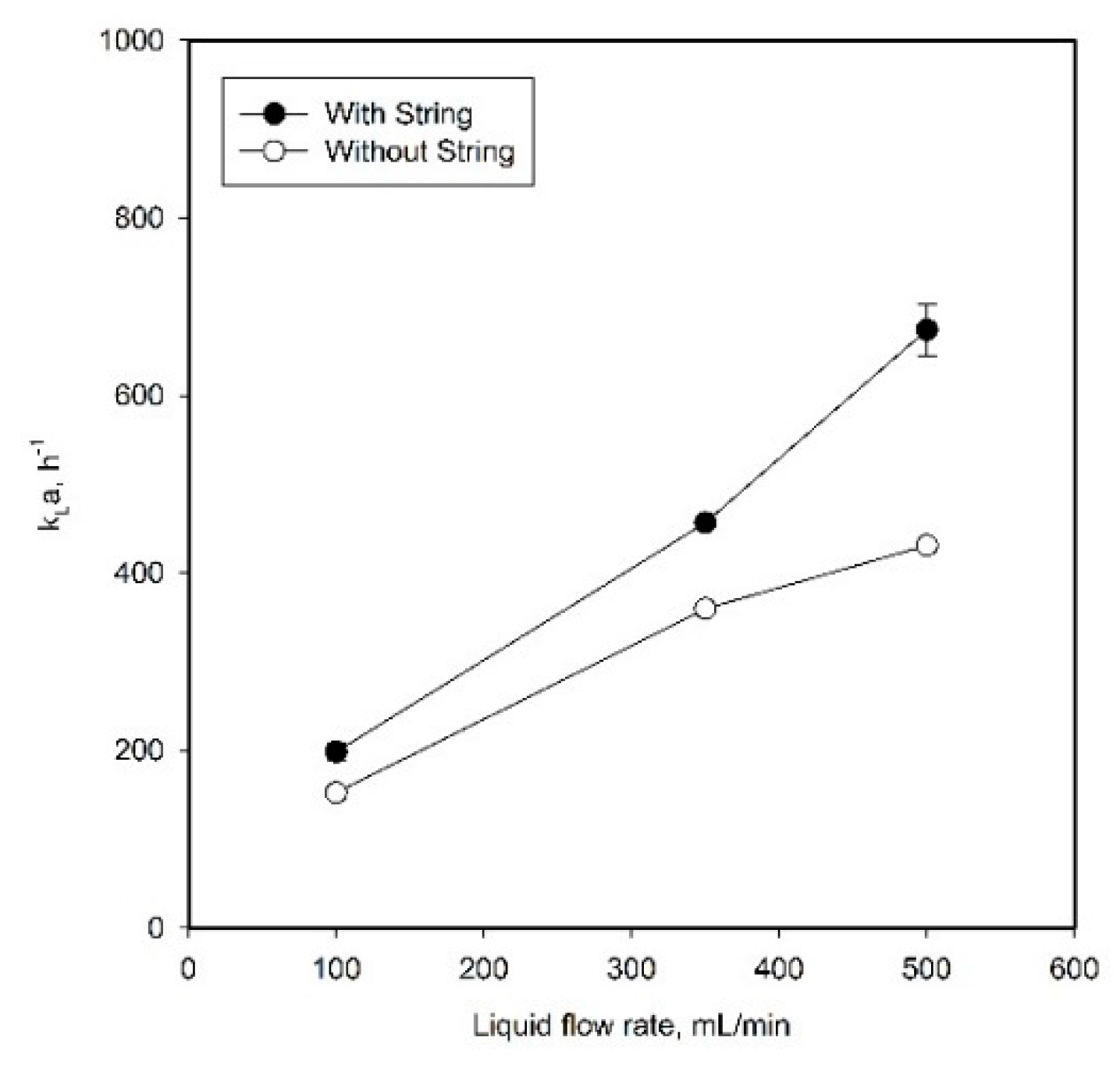
2.1. Effect of Liquid Flow Guidance by Strings on Mass Transfer Performance
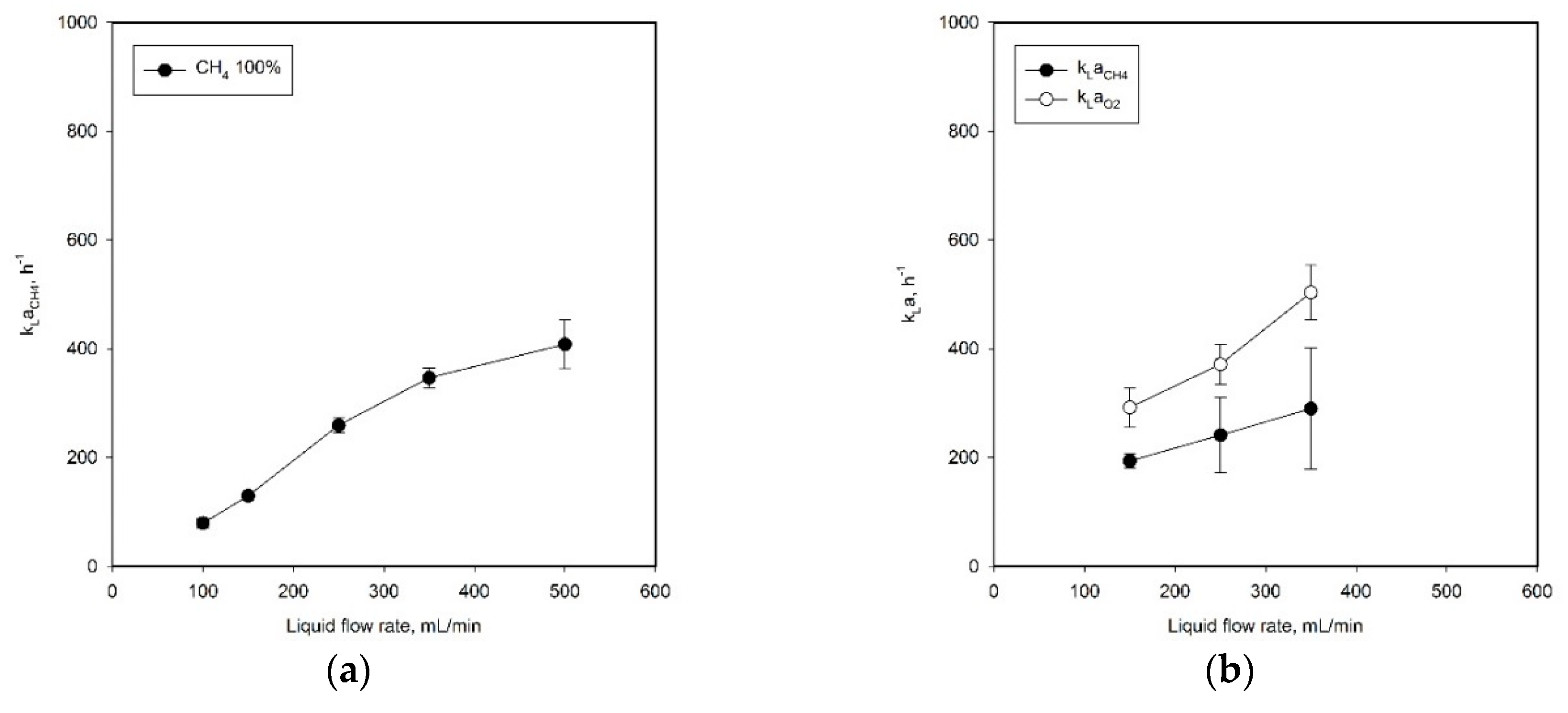
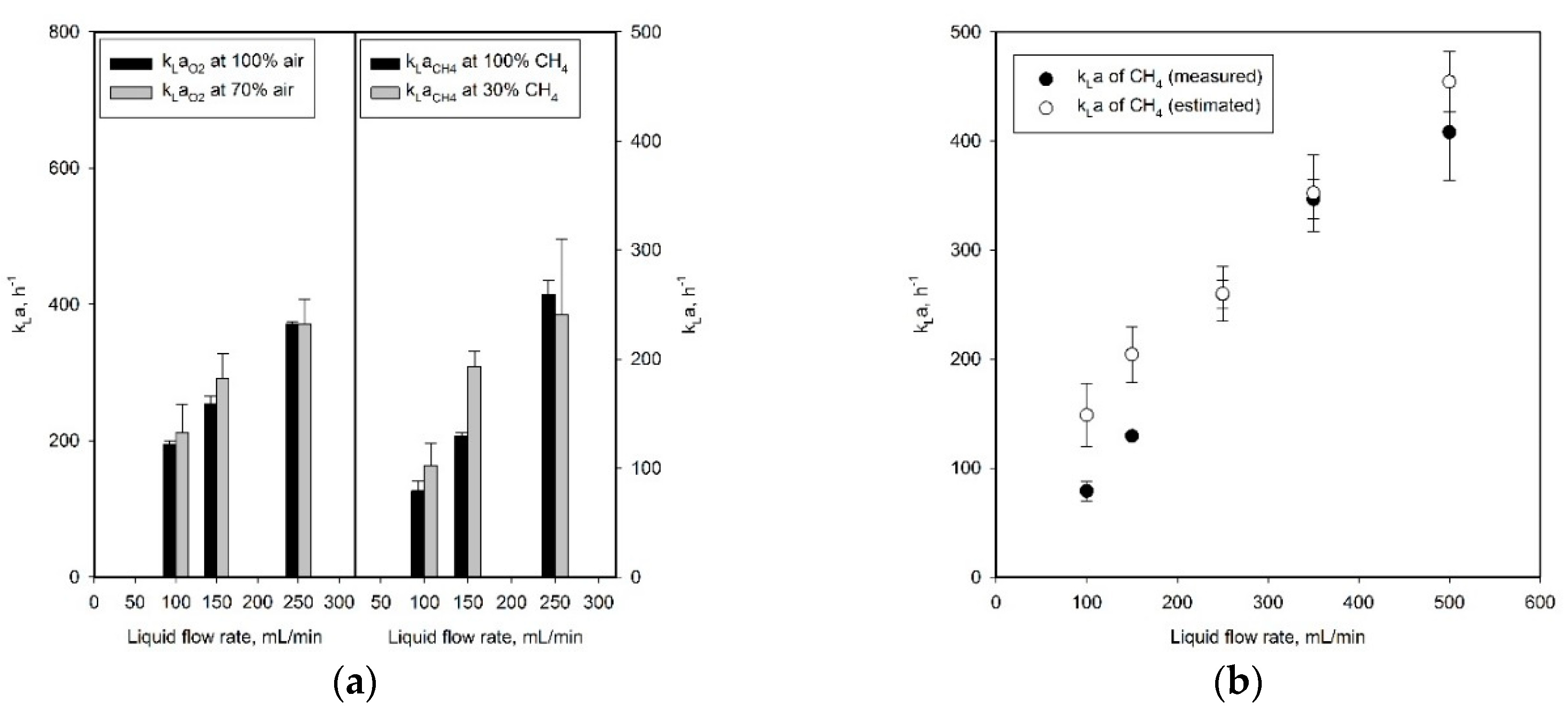
2.2. Investigation of Mass Transfer Characteristics in SFR Using Methane–Air Mixed Gas
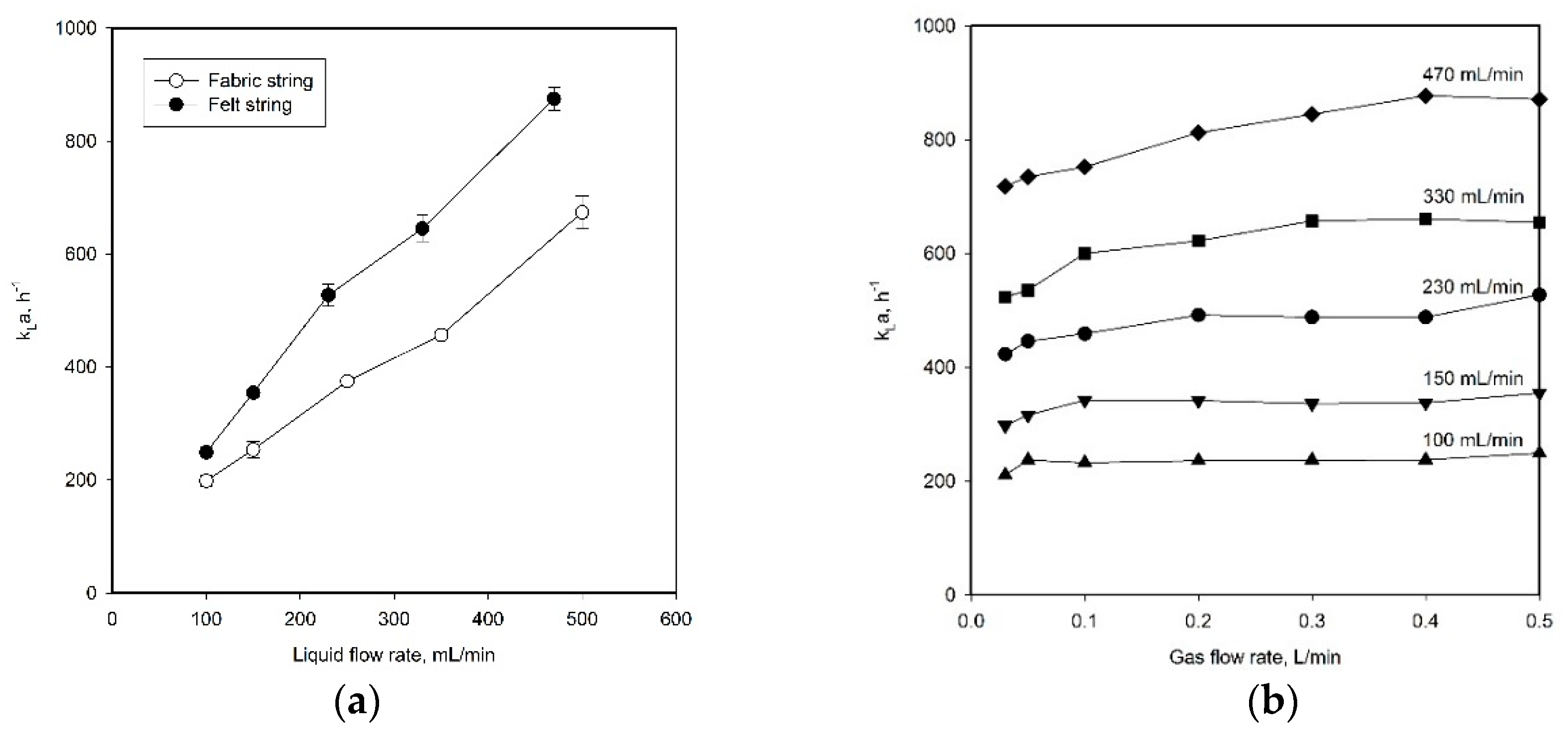
2.3. Improvement of Mass Transfer Performance by Using Hydrophilic Porous Strings
3. Discussion
4. Materials and Methods
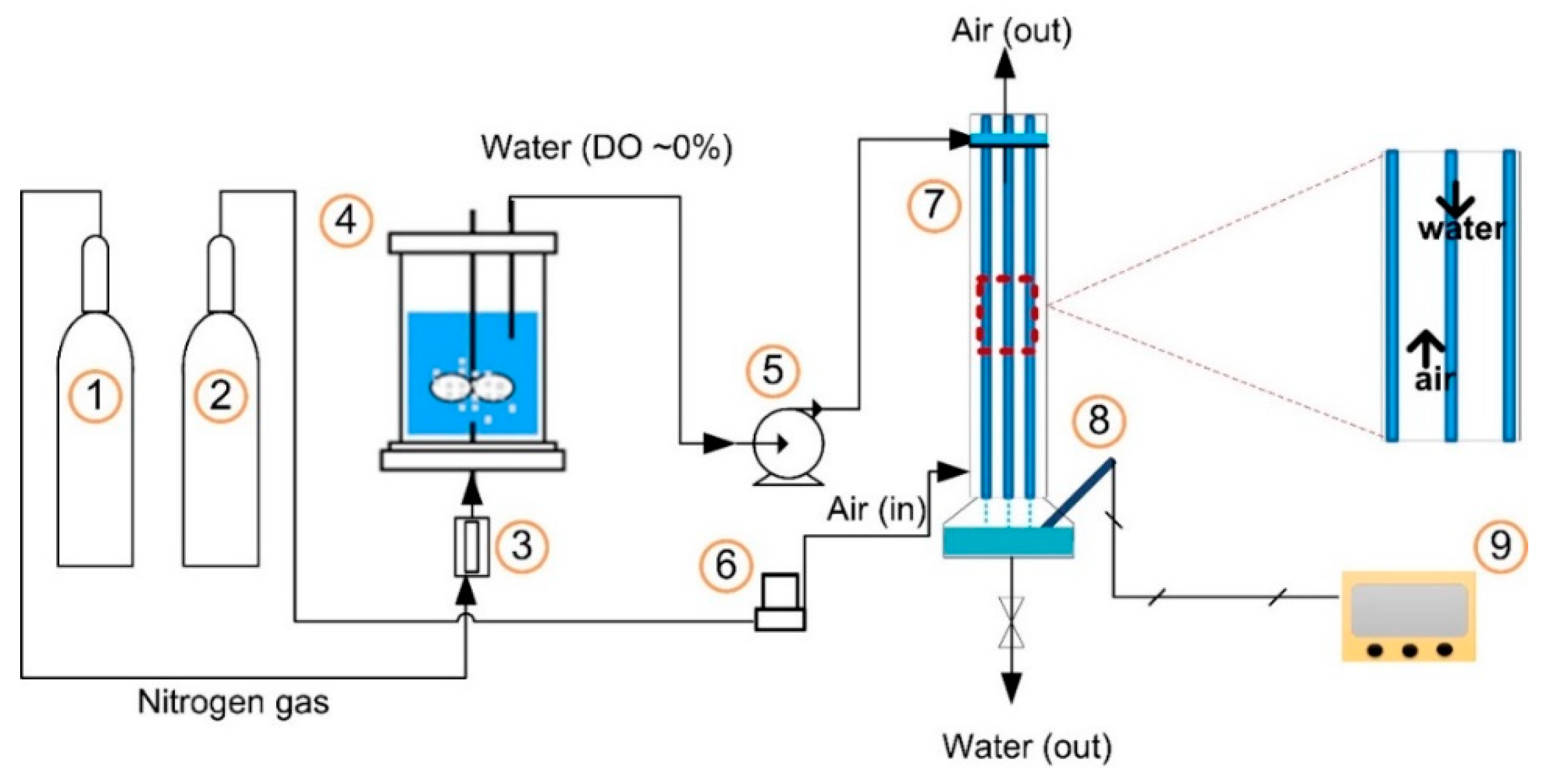
4.1. Schematic of the String Film Reactor (SFR)
4.2. Experimental Procedure for Gas–Liquid Contact Using the SFR System
4.3. Determination of Volumetric Mass Transfer Performance
5. Conclusions
- The liquid flow rate, which had a significant effect on the mass transfer performance;
- The gas flow rate, where the mass transfer performance increased with gas flow rate values below the critical gas flow rate, while it was almost constant above the critical rate. The value of the critical gas flow rate depends on the liquid flow rate;
- The hydrophilicity of the string material, where a more hydrophilic material could significantly improve the mass transfer performance.
Author Contributions
Funding
Conflicts of Interest
References
- Fei, Q.; Guarnieri, M.T.; Tao, L.; Laurens, L.M.L.; Dowe, N.; Pienkos, P.T. Bioconversion of natural gas to liquid fuel: Opportunities and challenges. Biotechnol. Adv. 2014, 32, 596–614. [Google Scholar] [CrossRef] [PubMed]
- U.S. Energy Information Administration, Annual Energy Outlook 2012 with Projections to 2035 (EIA Publication 0383, 2012). Available online: https://www.eia.gov/outlooks/archive/aeo12/ (accessed on 23 October 2018).
- Kidanu, W.G.; Trang, P.T.; Yoon, H.H. Hydrogen and volatile fatty acids production from marine macroalgae by anaerobic fermentation. Biotechnol. Bioproc. Eng. 2017, 22, 612–619. [Google Scholar] [CrossRef]
- Conrado, R.J.; Gonzalez, R. Envisioning the bioconversion of methane to liquid fuels. Science 2014, 343, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Hur, D.H.; Na, J.-G.; Lee, E.Y. Highly efficient bioconversion of methane to methanol using a novel type I Methylomonas sp. DH-1 newly isolated from brewery waste sludge. J. Chem. Technol. Biotechnol. 2017, 92, 311–318. [Google Scholar] [CrossRef]
- Canul-Chan, M.; Chable-Naal, J.; Rojas-Herrera, R.; Zepeda, A. Hydrocarbon degradation capacity and population dynamics of a microbial consortium obtained using a sequencing batch reactor in the presence of molasses. Biotechnol. Bioproc. Eng. 2017, 22, 170–177. [Google Scholar] [CrossRef]
- David, Y.; Baylon, M.G.; Pamidimarri, S.D.V.N.; Baritugo, K.-A.; Chae, C.G.; Kim, Y.J.; Kim, T.W.; Kim, M.S.; Na, J.-G.; Park, S.J. Screening of microorganisms able to degrade low-rank coal in aerobic conditions: Potential coal biosolubilization mediators from coal to biochemical. Biotechnol. Bioproc. Eng. 2017, 22, 178–185. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, H.U.; Choi, S.; Yi, J.; Lee, S.Y. Microbial production of building block chemicals and polymers. Curr. Opin. Biotechnol. 2011, 22, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Hwang, I.Y.; Lee, O.K.; Kim, D.; Kalyuzhnaya, M.G.; Mariyana, R.; Hadiyati, S.; Kim, M.S.; Lee, E.Y. Systematic metabolic engineering of Methylomicrobium alcaliphilum 20Z for 2,3-butanediol production from methane. Metab. Eng. 2018, 47, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Fitriana, H.N.; Kim, T.W.; Kang, S.G.; Jeon, S.G.; Chung, S.H.; Park, G.W.; Na, J.-G. Enhancement of the hydrogen productivity in microbial water gas shift reaction by Thermococcus onnurineus NA1 using a pressurized bioreactor. Int. J. Hydrogen Energy. 2017, 42, 27593–27599. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Golbabaei, F.; Mehrnia, M.R.; Neghab, M.; Mohammad, K. Oxygen mass transfer in a stirred tank bioreactor using different impeller configurations for environmental purposes. Iranian J. Environ. Health Sci. Eng. 2013, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredwell, M.D.; Worden, R.M. Mass-transfer properties of microbubbles. 1. Experimental Studies. Biotechnol. Prog. 1998, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, P.C.; Khanal, S.K. Syngas fermentation to biofuel: evaluation of carbon monoxide mass transfer and analytical modeling using a composite hollow fiber (chf) membrane bioreactor. Bioresour. Technol. 2012, 122, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-H.; Ni, S.-Q.; Chang, S.-Y.; Sung, S.; Kim, S.-H. Enhancement of carbon monoxide mass transfer using an innovative external hollow fiber membrane (hfm) diffuser for syngas fermentation: experimental studies and model development. Chem. Eng. J. 2012, 184, 268–277. [Google Scholar] [CrossRef]
- Lau, R.; Lee, P.H.V.; Chen, T. Mass transfer studies in shallow bubble column reactors. Chem. Eng. Process. 2012, 62, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Krishna, R.; Ellenberger, J. Improving gas–liquid contacting in bubble columns by vibration excitement. Int. J. Multiph. Flow 2002, 28, 1223–1234. [Google Scholar] [CrossRef]
- Kim, Z.H.; Park, Y.S.; Ryu, Y.J.; Lee, C.G. Enhancing biomass and fatty acid productivity of Tetraselmis sp. in bubble column photobioreactors by modifying light quality using light filters. Biotechnol. Bioproc. Eng. 2017, 22, 397–404. [Google Scholar] [CrossRef]
- Jajuee, B.; Margaritis, A.; Karamanev, D.; Bergougnou, M.A. Mass transfer characteristics of a novel three-phase airlift contactor with a semipermeable membrane. Chem. Eng. J. 2006, 125, 119–126. [Google Scholar] [CrossRef]
- Bekassy-Molnar, E.; Majeed, J.G.; Vatai, G. Overall volumetric oxygen transfer coefficient and optimal geometry of airlift tube reactor. Chem. Eng. J. 1997, 68, 29–33. [Google Scholar] [CrossRef]
- Bredwell, M.D.; Srivastava, P.; Worden, R.M. Reactor design issues for synthesis-gas fermentations. Biotechnol. Prog. 1999, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, C.N.; Pelossof, A.A.; Sherwood, T.K. Mass transfer limitations in a trickle-bed reactor. AIChE J. 1969, 15, 226–234. [Google Scholar] [CrossRef]
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: Employing potential of enhanced reaction rates. Microb. Cell. Fact. 2005, 4. [Google Scholar] [CrossRef] [PubMed]
- Arkles, B. Hydrophobicity, Hydrophilicity and Silanes. Paint & Coat. Ind. 2006. Available online: https://www.pcimag.com/ext/resources/PCI/Home/Files/PDFs/Virtual_Supplier_Brochures/Gelest_Additives.pdf (accessed on 23 October 2018).
- Vasiljevic, J.; Tomsic, B.; Jerman, I.; Simoncic, B. Organofunctional Trialkoxysilane Sol-Gel Precursors for Chemical Modification of Textile Fibres. Tekstilec 2017, 60, 198–213. [Google Scholar] [CrossRef]
- Critical Surface Tension and Contact Angle with Water for Various Polymers. Available online: https://www.accudynetest.com/polytable_03.html?sortby_contact_angle (accessed on 13 October 2018).
- Charpentier, J.C. Mass transfer in gas-liquid absorbers and reactors. Adv. Chem. Eng. 1981, 11, 1–123. [Google Scholar] [CrossRef]
- Onda, K.; Takeuchi, H.; Okumoto, Y. Gas absorption with chemical reaction in packed column. J. Chem. Eng. Jpn. 1968, 1, 62–66. [Google Scholar] [CrossRef]
- Han, M.W.; Lee, W.K.; Choi, D.K. Effect of shape and wettability of packing materials on the efficiency of packed column. Korean J. Chem. Eng. 1985, 2, 25–31. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Mass Diffusivity. Available online: https://en.wikipedia.org/wiki/Mass_diffusivity (accessed on 24 September 2018).
- Budzynski, P.; Gwiazda, A.; Dziubinski, M. Intensification of mass transfer in a pulsed bubble column. Chem. Eng. Process. 2017, 112, 18–30. [Google Scholar] [CrossRef]


| Liquid Flow Rate (mL/min) | Critical Gas Flow Rate (L/min) |
|---|---|
| 100 | 0.05 |
| 150 | 0.10 |
| 250 | 0.20 |
| 350 | 0.30 |
| 500 | 0.40 |
| Reactor Type | Operating Condition | Highest kLa (h−1) Obtained for Oxygen | Reference | ||||
|---|---|---|---|---|---|---|---|
| Gas Flow Rate (mL/min) | Liquid Flow Rate (L/min) | Superficial Gas Velocity (cm/s) | Reactor Volume (L) | Liquid Volume (L) | |||
| String film (SFR) | 500 | 0.4 | 0.36 | 0.486 | 0.06 | 874.67 L | This study |
| Stirred tank (900 rpm) | 400 | - | - | 3.5 | 2.5 | 114 R | [11] |
| Stirred tank (1000 rpm) | 5000 | - | - | 2.44 | 1.77 | 216 R | [12] |
| Bubble column | - | - | 1.2 | 8.64 | 6.90 | 180 R | [17] |
| Bubble column | - | - | 10.8 | 22 | 4.0 | 360 R | [16] |
| Bubble column | 10,000 | - | 0.93 | 32 | 11 | 126 R | [32] |
| Air-lift | - | - | 1.77–7.07 | 1.75 | 0.85 | 360 R | [20] |
| Packed bed–trickle flow | 131 | 0.05 | - | 1.2 | 0.0081 | 421 L | [11] |
| Hollow fiber membrane | 1000 | 0.4 | - | - | 0.018 | 1062 L | [11] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariyana, R.; Kim, M.-S.; Lim, C.I.; Kim, T.W.; Park, S.J.; Oh, B.-K.; Lee, J.; Na, J.-G. Mass Transfer Performance of a String Film Reactor: A Bioreactor Design for Aerobic Methane Bioconversion. Catalysts 2018, 8, 490. https://doi.org/10.3390/catal8110490
Mariyana R, Kim M-S, Lim CI, Kim TW, Park SJ, Oh B-K, Lee J, Na J-G. Mass Transfer Performance of a String Film Reactor: A Bioreactor Design for Aerobic Methane Bioconversion. Catalysts. 2018; 8(11):490. https://doi.org/10.3390/catal8110490
Chicago/Turabian StyleMariyana, Rina, Min-Sik Kim, Chae Il Lim, Tae Wan Kim, Si Jae Park, Byung-Keun Oh, Jinwon Lee, and Jeong-Geol Na. 2018. "Mass Transfer Performance of a String Film Reactor: A Bioreactor Design for Aerobic Methane Bioconversion" Catalysts 8, no. 11: 490. https://doi.org/10.3390/catal8110490





