Preparation of Magnetic Cross-Linked Amyloglucosidase Aggregates: Solving Some Activity Problems
Abstract
:1. Introduction
2. Results and Discussion
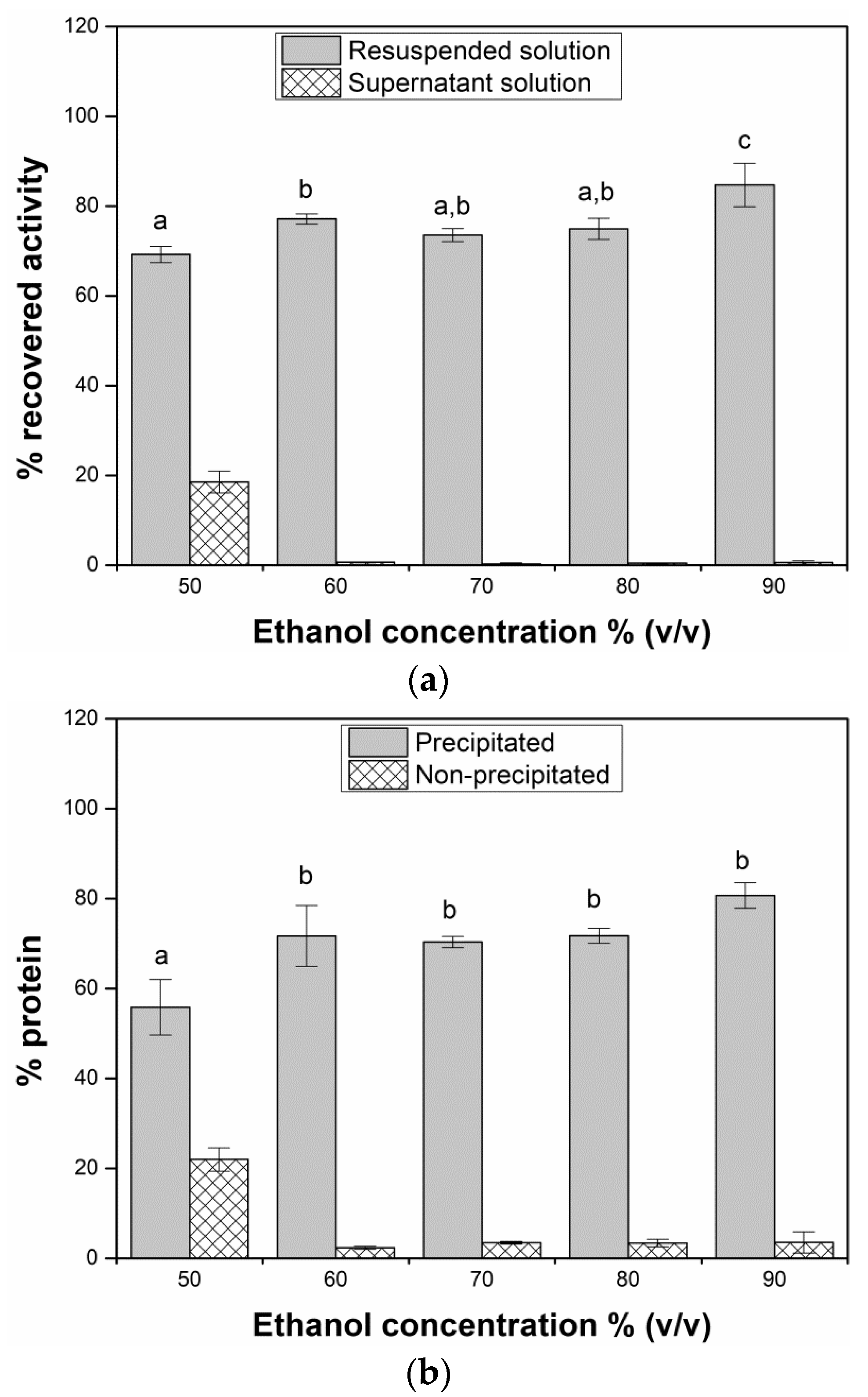
2.1. Precipitant Selection
2.2. Preparation of CLEAs
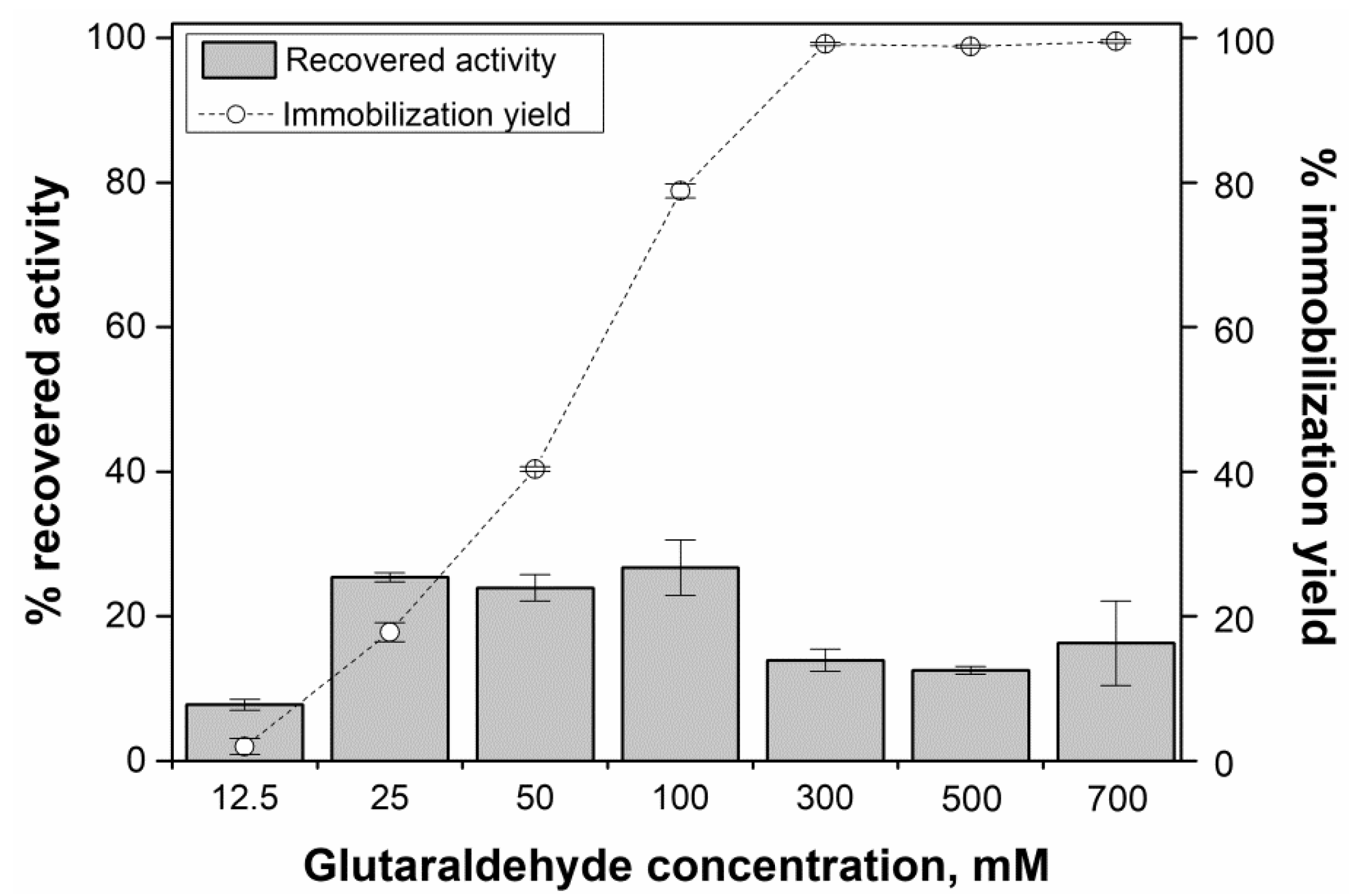
2.3. Preparation of Magnetic AMG CLEAs
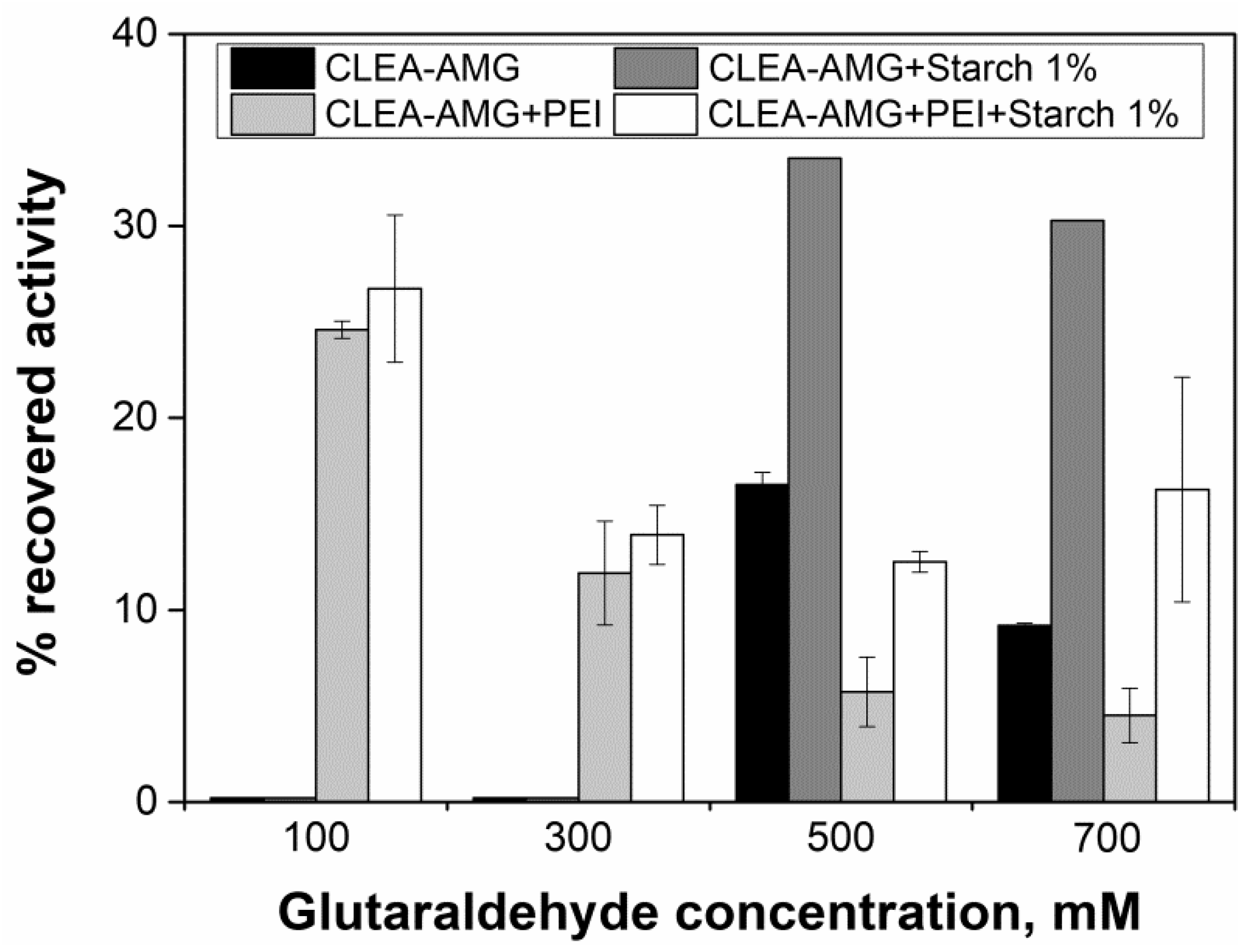
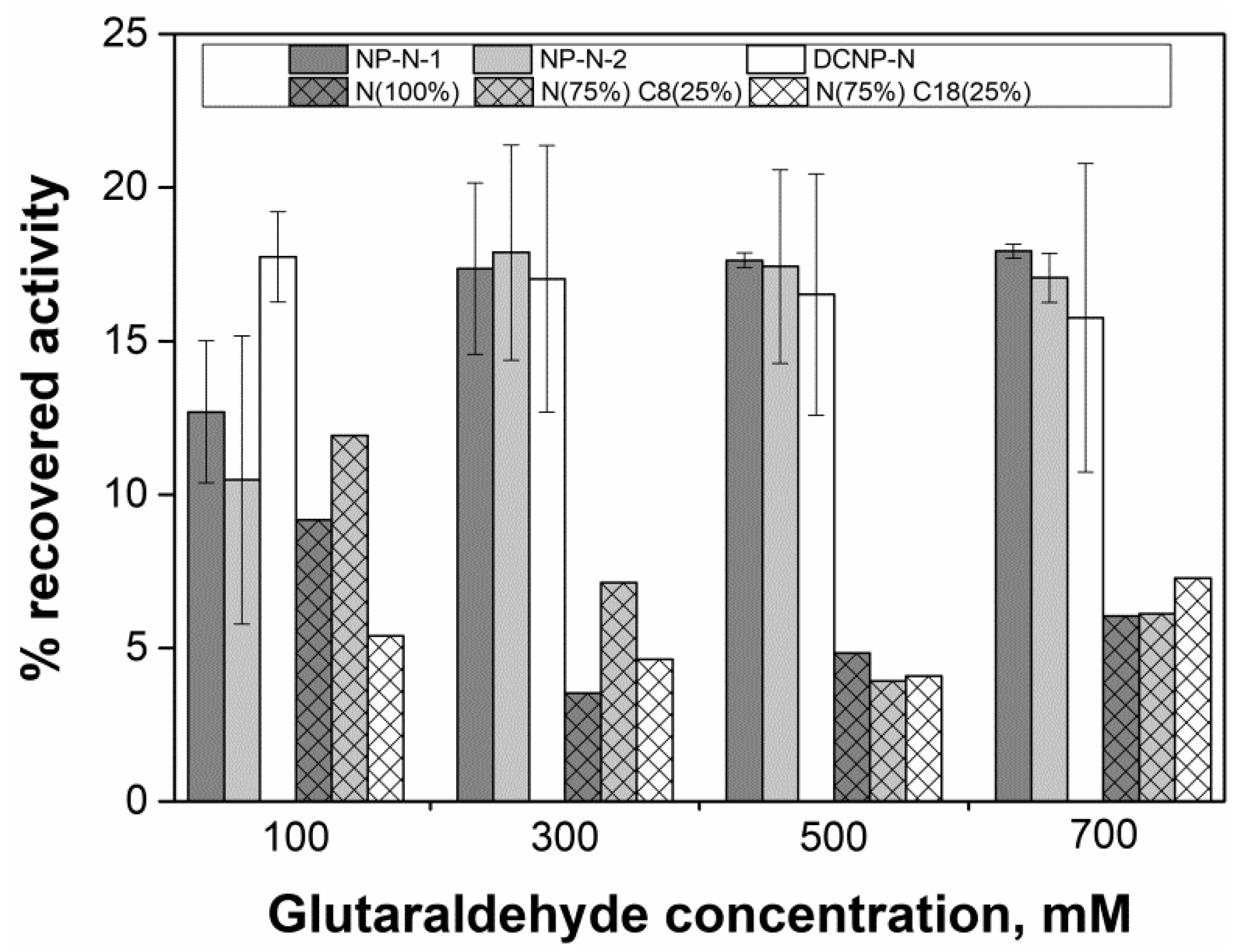
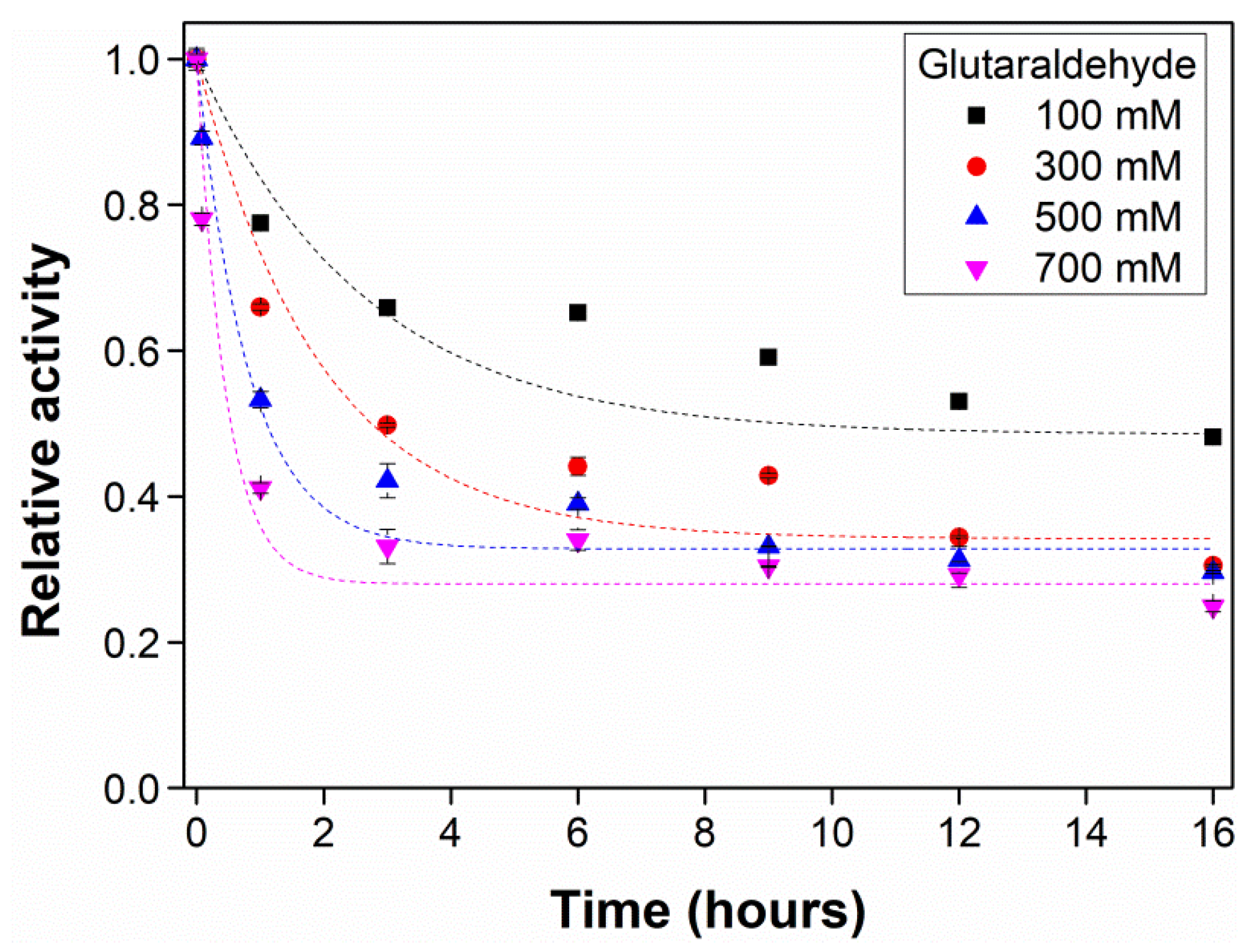
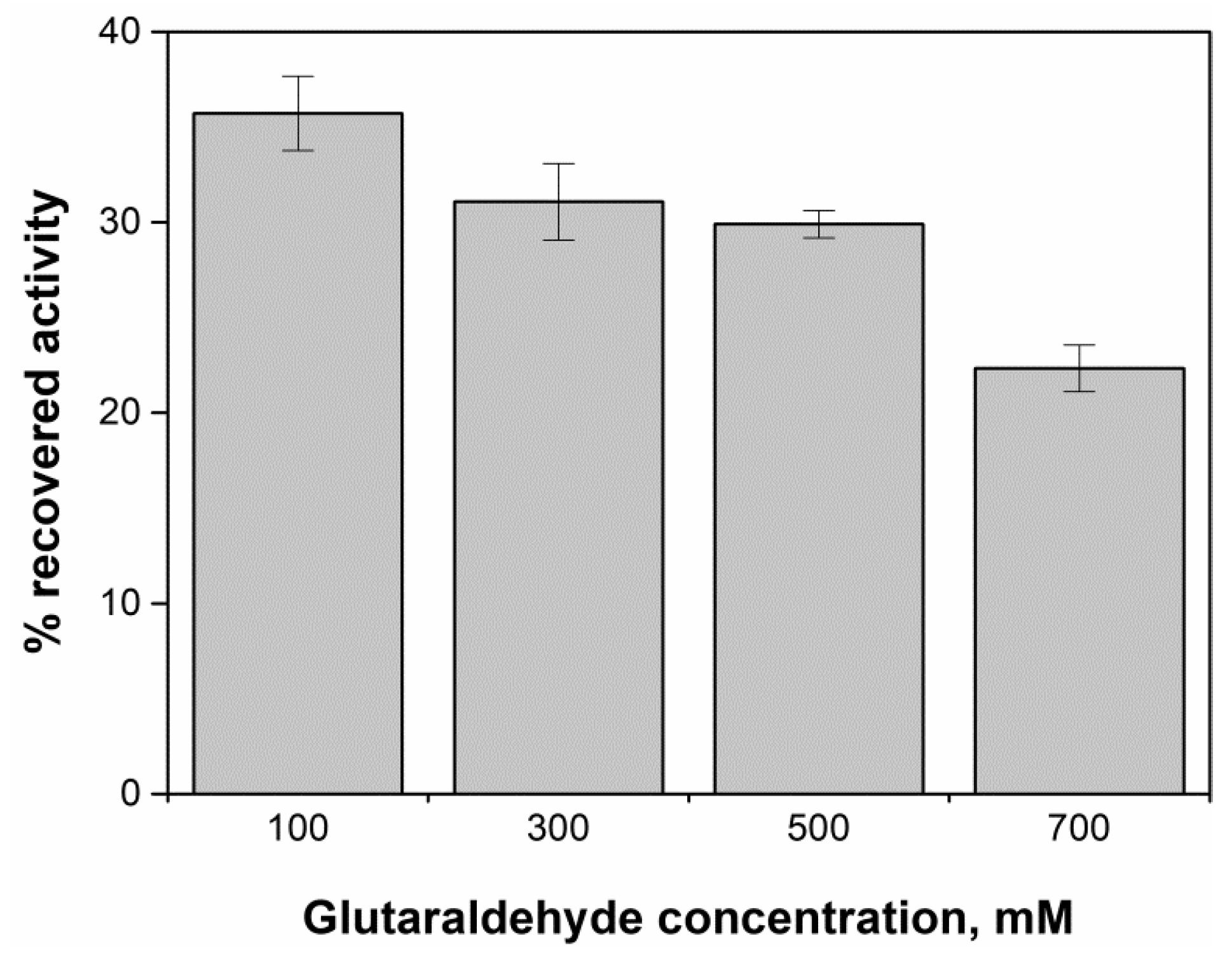
2.4. Evaluation of Glutaraldehyde Effect on Enzyme Activity
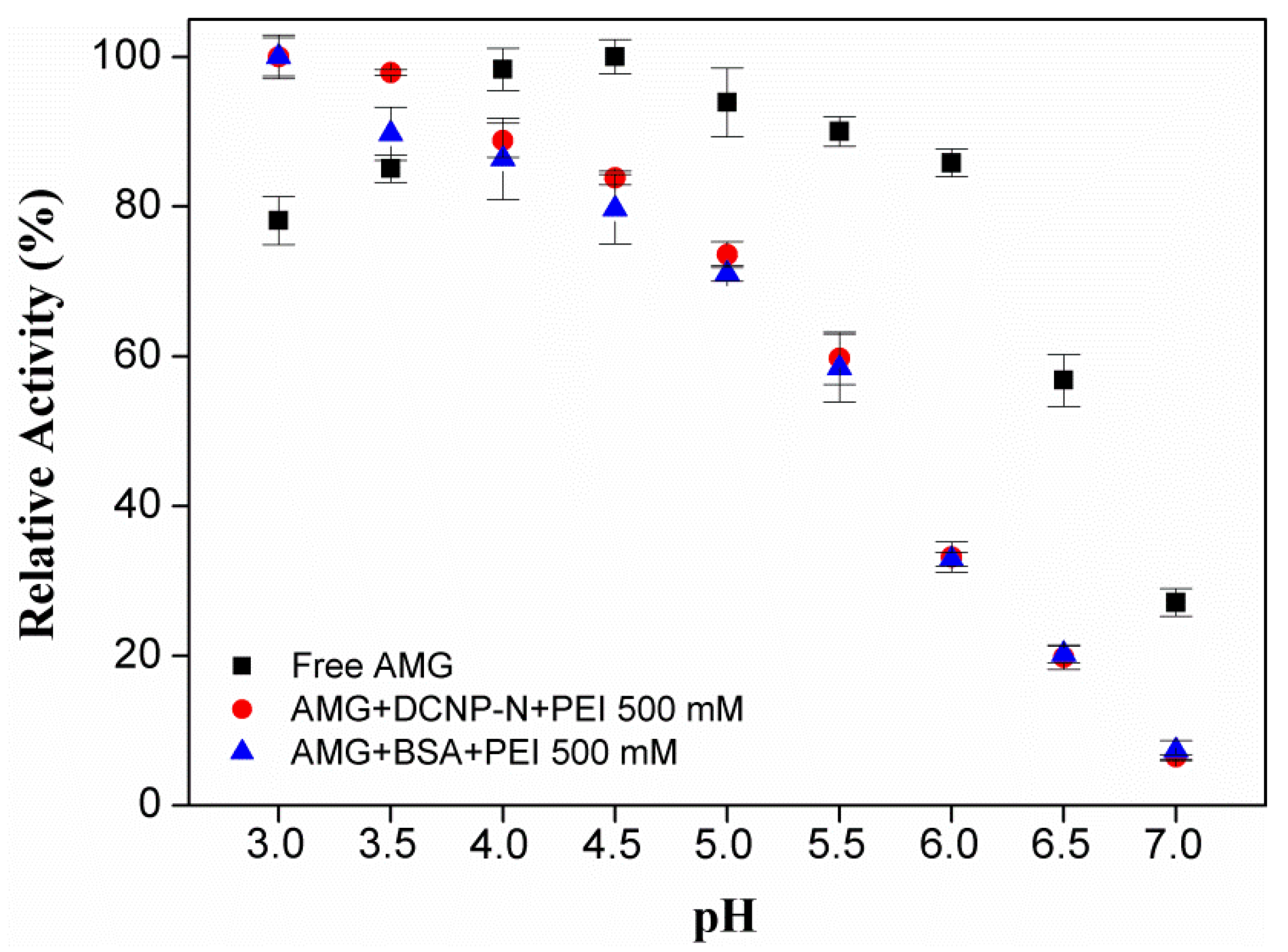
2.5. Study of the Co-Aggregation of AMG and Polyethyleneimine (PEI)
2.6. Influence of Agitation, Glutaraldehyde Treatment Time, Cross-Linker Concentration, and Co-Feeders in the Activity of AMG-CLEAs
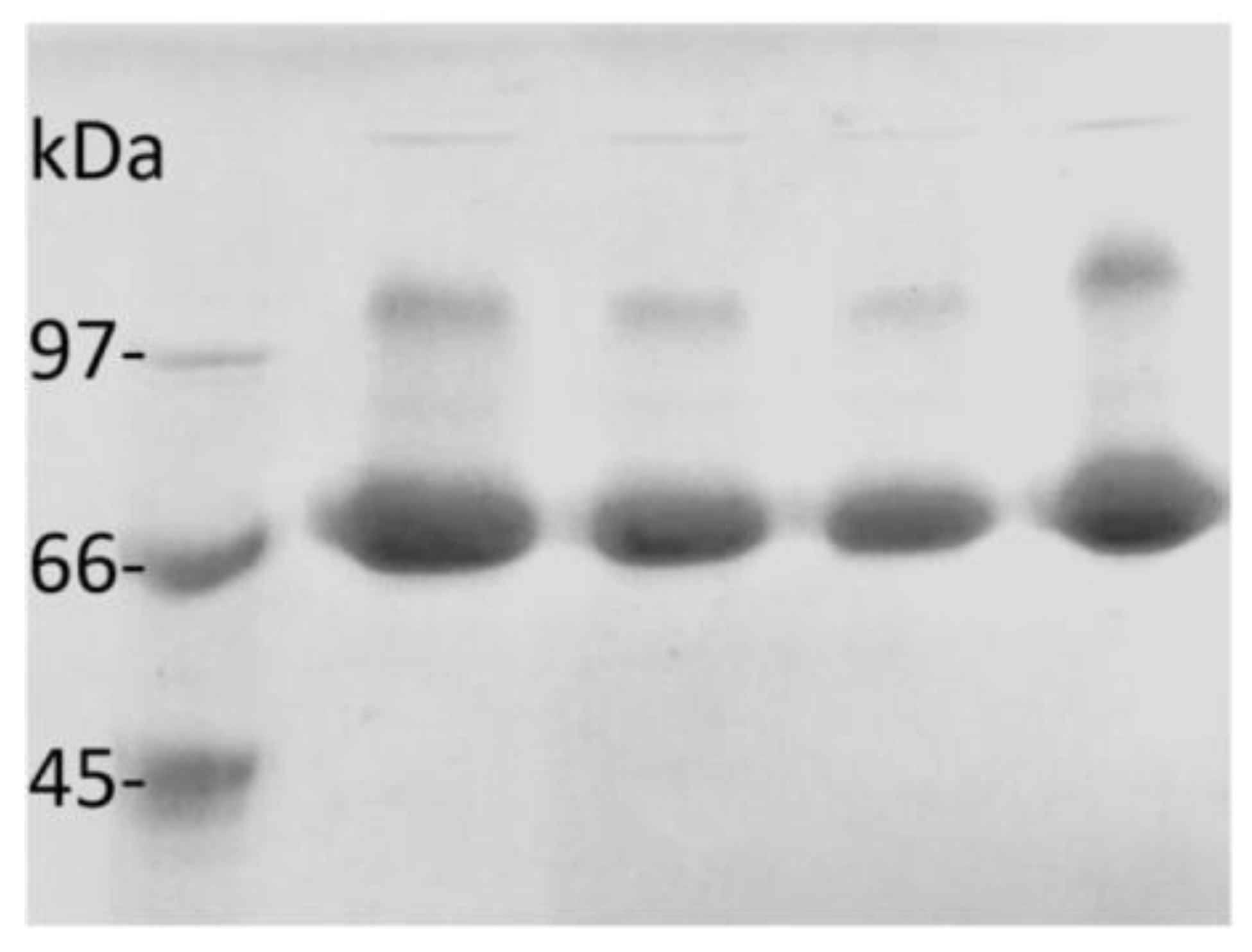
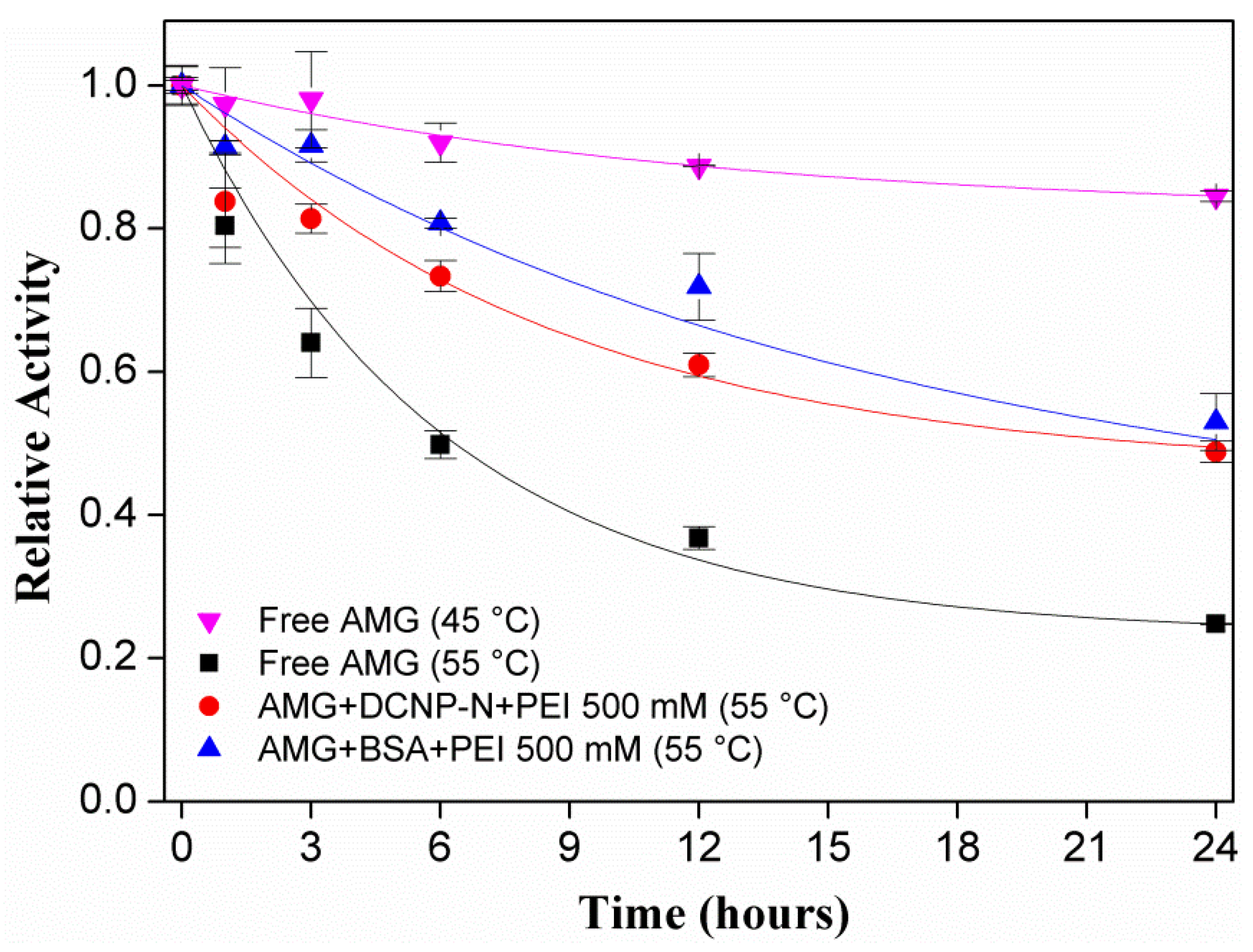
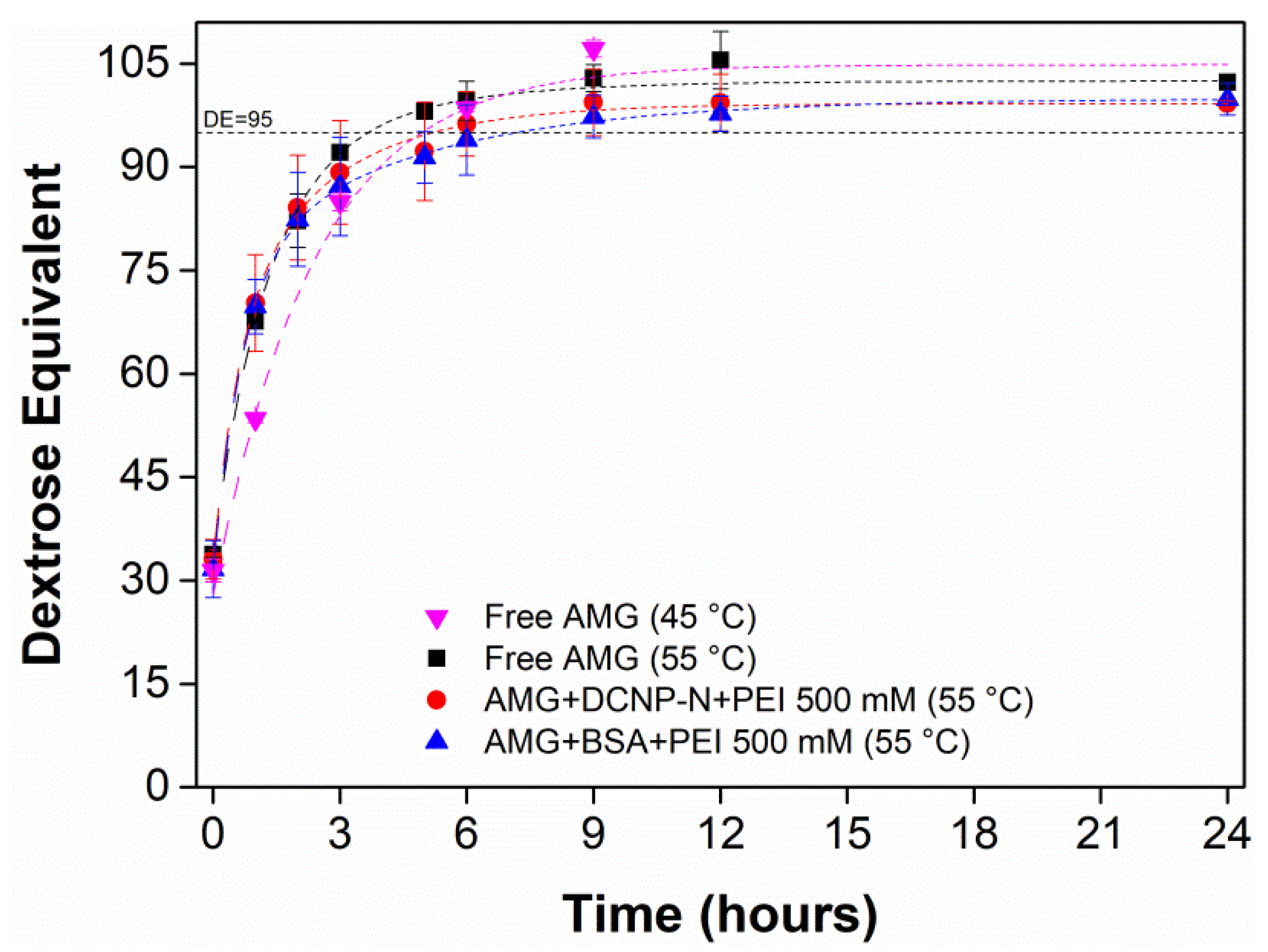
2.7. Characterization of the CLEAs of AMG
2.8. Reuse Assays
3. Materials and Methods
3.1. Materials
3.2. Precipitant Screening
3.3. General Crosslinking Procedure Using Glutaraldehyde
3.4. Characterization of the Biocatalysts
3.5. Hydrolysis of Starch
3.6. Reuse Assays
3.7. Enzymatic Activity Assay
3.8. Determination of Glucose and Protein Concentration
3.9. SDS-PAGE Electrophoresis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parkin, K.L. Enzymes. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 331–435. ISBN 978-0849392726. [Google Scholar]
- Crabb, W.D.; Mitchinson, C. Enzymes involved in the processing of starch to sugars. Trends Biotechnol. 1997, 15, 349–352. [Google Scholar] [CrossRef]
- Svensson, B.; Larsen, K.; Svendsen, I.; Boel, E. The complete amino acid sequence of the glycoprotein, glucoamylase G1, from Aspergillus niger. Carlsberg Res. Commun. 1983, 48, 529–544. [Google Scholar] [CrossRef]
- Lee, J.; Paetzel, M. Structure of the catalytic domain of glucoamylase from Aspergillus niger. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Sorimachi, K.; Gal-Coëffet, M.-F.L.; Williamson, G.; Archer, D.B.; Williamson, M.P. Solution structure of the granular starch binding domain of Aspergillus niger glucoamylase bound to β-cyclodextrin. Structure 1997, 5, 647–661. [Google Scholar] [CrossRef]
- Tomasik, P.; Horton, D. Enzymatic conversions of starch. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Oxford, UK, 2012; Volume 68, pp. 59–436. ISBN 9780123965233. [Google Scholar]
- Pazur, J.H.; Ando, T. The action of an amyloglucosidase of Aspergillus niger on starch and malto-oligosaccharides. J. Biol. Chem. 1959, 234, 1966–1970. [Google Scholar] [PubMed]
- Parker, K.; Salas, M.; Nwosu, V.C. High fructose corn syrup: Production, uses and public health concerns. Biotechnol. Mol. Biol. Rev. 2010, 5, 71–78. [Google Scholar]
- Kennedy, J.F.; Cabral, J.M. Enzyme imobilization. In Biotechnology; Rehm, H.J., Reed, G., Eds.; VCH: Weinheim, Germany, 1987; pp. 347–404. [Google Scholar]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Silva, R.N.; Asquieri, E.R.; Fernandes, K.F. Immobilization of Aspergillus niger glucoamylase onto a polyaniline polymer. Process Biochem. 2005, 40, 1155–1159. [Google Scholar] [CrossRef]
- Bryjak, J. Glucoamylase, α-amylase and β-amylase immobilisation on acrylic carriers. Biochem. Eng. J. 2003, 16, 347–355. [Google Scholar] [CrossRef]
- Shah, C.; Sellappan, S.; Madamwar, D. Entrapment of enzyme in water-restricted microenvironment—Amyloglucosidase in reverse micelles. Process Biochem. 2000, 35, 971–975. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Vieira, M.F.; Vieira, A.M.S.; Zanin, G.M.; Betancor, L.; Mateo, C.; Fernández-Lorente, G.; Guisán, J.M. Immobilization-stabilization of glucoamylase: Chemical modification of the enzyme surface followed by covalent attachment on highly activated glyoxyl-agarose supports. Process Biochem. 2011, 46, 409–412. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Li, Y.; Liu, X.; Hou, P. Reversible immobilization of glucoamylase onto magnetic chitosan nanocarriers. Appl. Microbiol. Biotechnol. 2013, 97, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Mafra, A.C.O.; Beltrame, M.B.; Ulrich, L.G.; Giordano, R.D.L.C.; Ribeiro, M.P.D.A.; Tardioli, P.W. Combined CLEAs of invertase and soy protein for economically feasible conversion of sucrose in a fed-batch reactor. Food Bioprod. Process. 2018, 110, 145–157. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Kate, A.; Samant, N.; Kumar, C.; Gadagkar, S. Preparation and Characterization of Cross-linked Enzyme Aggregates of Saccharomyces cerevisiae Invertase. Aust. J. Basic Appl. Sci. 2010, 4, 4760–4765. [Google Scholar]
- Ramos, M.D.; Miranda, L.P.; Giordano, R.L.C.; Fernandez-Lafuente, R.; Kopp, W.; Tardioli, P.W. 1,3-Regiospecific ethanolysis of soybean oil catalyzed by crosslinked porcine pancreas lipase aggregates. Biotechnol. Prog. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Betancor, L.; Fernández-Lorente, G.; Fuentes, M.; Hidalgo, A.; Guisán, J.M.; Pessela, B.C.C.; Fernández-Lafuente, R. Cross-Linked Aggregates of Multimeric Enzymes: A Simple and Efficient Methodology to Stabilize Their Quaternary Structure. Biomacromolecules 2004, 5, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; de Lima Camargo Giordano, R.; de Arruda Ribeiro, M.P.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; Giordano, R.D.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic macromolecular cross linked enzyme aggregates (CLEAs) of glucoamylase. Enzyme Microb. Technol. 2016, 83, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jana, A.K.; Kumar, S.; Maiti, M. Immobilization of amyloglucosidase from SSF of Aspergillus niger by crosslinked enzyme aggregate onto magnetic nanoparticles using minimum amount of carrier and characterizations. J. Mol. Catal. B Enzym. 2013, 98, 30–36. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Fernández-Lorente, G.; Fernández-Lafuente, R.; Illanes, A.; Guisán, J.M.; Palomo, J.M. CLEAs of lipases and poly-ionic polymers: A simple way of preparing stable biocatalysts with improved properties. Enzyme Microb. Technol. 2006, 39, 750–755. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Ruiz, M.; Cruz, J.; Ortiz, C.; Torres, R.; Fernandez-Lafuente, R. Chemical amination of lipase B from Candida antarctica is an efficient solution for the preparation of crosslinked enzyme aggregates. Process Biochem. 2012, 47, 2373–2378. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Hidalgo, A.; Alonso, N.; Fernández-Lafuente, R.; Guisán, J.M. Co-aggregation of enzymes and polyethyleneimine: A simple method to prepare stable and immobilized derivatives of glutaryl acylase. Biomacromolecules 2005, 6, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, Y.; Yang, L.; Li, M.; Zhang, J. Preparation of Cross-Linked Enzyme Aggregates of Trehalose Synthase via Co-aggregation with Polyethyleneimine. Appl. Biochem. Biotechnol. 2014, 174, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 7461–7490. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Vázquez-Duhalt, R.; Mateos-Díaz, J.C.; Guisán, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W.; Silva, F.A.; Lima, L.N.; Masunaga, S.H.; Tardioli, P.W.; Giordano, R.C.; Araújo-Moreira, F.M.; Giordano, R.L.C. Synthesis and characterization of robust magnetic carriers for bioprocess applications. Mater. Sci. Eng. B 2015, 193, 217–228. [Google Scholar] [CrossRef]
- Panek, A.; Pietrow, O.; Synowiecki, J. Characterization of glucoamylase immobilized on magnetic nanoparticles. Starch/Staerke 2012, 64, 1003–1008. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support-polyethylenimine composites. Biotechnol. Bioeng. 2000, 68, 98–105. [Google Scholar] [CrossRef]
- Wang, M.; Jia, C.; Qi, W.; Yu, Q.; Peng, X.; Su, R.; He, Z. Porous-CLEAs of papain: Application to enzymatic hydrolysis of macromolecules. Bioresour. Technol. 2011, 102, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Shah, V.; Patil, S.; Nimbalkar, M. Porous cross linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase. Catal. Sci. Technol. 2012, 2, 1575–1579. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.L.; Sobocinski, P.Z. An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal. Biochem. 1975, 64, 284–288. [Google Scholar] [CrossRef]
- Talekar, S.; Nadar, S.; Joshi, A.; Joshi, G. Pectin cross-linked enzyme aggregates (pectin-CLEAs) of glucoamylase. RSC Adv. 2014, 4, 59444–59453. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Hobbs, L. Sweeteners from Starch: Production, Properties and Uses. In Starch; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, UK, 2009; pp. 797–832. ISBN 9780127462752. [Google Scholar]
- Synowiecki, J. The Use of Starch Processing Enzymes in the Food Industry. In Industrial Enzymes; Springer: Dordrecht, The Netherlands, 2007; pp. 19–34. [Google Scholar]
- Guzmán-Maldonado, H.; Paredes-López, O.; Biliaderis, C.G. Amylolytic enzymes and products derived from starch: A review. Crit. Rev. Food Sci. Nutr. 1995, 35, 373–403. [Google Scholar] [CrossRef] [PubMed]
- Sadana, A.; Henley, J.P. Single-step unimolecular non-first-order enzyme deactivation kinetics. Biotechnol. Bioeng. 1987, 30, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kearsley, M.W.; Dziedzic, S.Z. Physical and chemical properties of glucose syrups. In Handbook of Starch Hydrolysis Products and Their Derivatives; Springer: Boston, MA, USA, 1995; pp. 129–154. [Google Scholar]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]






| Magnetic Nanoparticle | ‒NH2 Content (μmol/g) | ‒C8 or ‒C18 (μmol/g) | |
|---|---|---|---|
| NP-N-1 | 1013.1 1 | Not applicable | |
| NP-N-2 | 265.5 1 | ||
| DCNP-N | 522.1 1 | ||
| N (100%) | 275.7 1 | 348 ± 15 2 | |
| N (75%) C8 (25%) | 136 ± 10 2 | 282 ± 19 2 | |
| N (75%) C18 (25%) | 310 ± 39 2 | 479 ± 21 2 | |
| Glutaraldehyde Concentration | Residual Activity (%) | |||
|---|---|---|---|---|
| Without Starch | Starch 1% | Starch 2.5% | Starch 5% | |
| 100 mM | 34.46 | 48.12 | 43.18 | 36.37 |
| 300 mM | 20.25 | 30.53 | 24.25 | 23.22 |
| 500 mM | 19.48 | 29.63 | 26.80 | 24.40 |
| 700 mM | 17.33 | 24.92 | 25.73 | 20.29 |
| Glutaraldehyde Concentration | Recovered Activity (%) | |||
|---|---|---|---|---|
| Without Starch | Starch 1% | DCNP-N | DCNP-N+Starch 1% | |
| 100 mM | - | - | 17.74 ± 1.47 | 18.72 ± 0.29 |
| 300 mM | - | - | 17.03 ± 4.34 | 18.81 ± 0.31 |
| 500 mM | 16.53 ± 0.64 | 33.53 | 16.52 ± 3.93 | 39.40 ± 1.44 |
| 700 mM | 9.20 ± 0.11 | 30.28 | 15.76 ± 5.03 | 29.75 ± 1.15 |
| Biocatalyst | t1/2 (h) | SF | kd | α | Adj. R2 |
|---|---|---|---|---|---|
| Free AMG | 6.34 | 1.00 | 0.167 ± 0.014 | 0.234 ± 0.005 | 0.99 |
| AMG+DCNP-N+PEI 500 mM | 22.67 | 3.58 | 0.117 ± 0.016 | 0.462 ± 0.027 | 0.98 |
| AMG+BSA+PEI 500 mM | 24.60 | 3.88 | 0.062 ± 0.019 | 0.361 ± 0.119 | 0.95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral-Fonseca, M.; Kopp, W.; Giordano, R.d.L.C.; Fernández-Lafuente, R.; Tardioli, P.W. Preparation of Magnetic Cross-Linked Amyloglucosidase Aggregates: Solving Some Activity Problems. Catalysts 2018, 8, 496. https://doi.org/10.3390/catal8110496
Amaral-Fonseca M, Kopp W, Giordano RdLC, Fernández-Lafuente R, Tardioli PW. Preparation of Magnetic Cross-Linked Amyloglucosidase Aggregates: Solving Some Activity Problems. Catalysts. 2018; 8(11):496. https://doi.org/10.3390/catal8110496
Chicago/Turabian StyleAmaral-Fonseca, Murilo, Willian Kopp, Raquel de Lima Camargo Giordano, Roberto Fernández-Lafuente, and Paulo Waldir Tardioli. 2018. "Preparation of Magnetic Cross-Linked Amyloglucosidase Aggregates: Solving Some Activity Problems" Catalysts 8, no. 11: 496. https://doi.org/10.3390/catal8110496







