Surface Modification of TiO2 for Obtaining High Resistance against Poisoning during Photocatalytic Decomposition of Toluene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Samples
2.2. Photocatalytic Decomposition of Toluene under Dry Conditions
2.3. Photocatalytic Decomposition of Toluene under Humid Conditions
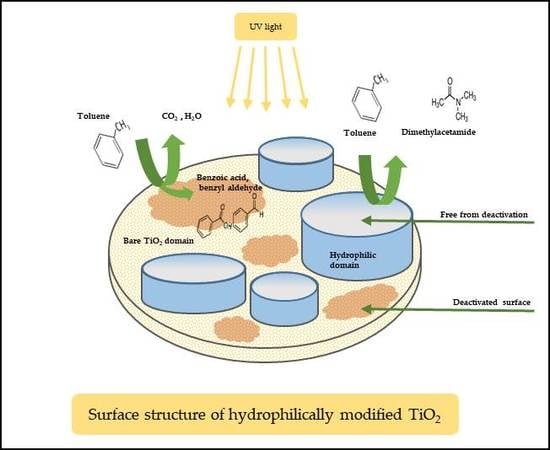
2.4. Proposed Surface Structure of h-TiO2
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
3.3. Photocatalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, S.; Khare, M.; Goyal, R. Sick building syndrome—A case study in a multistory centrally air-conditioned building in the Delhi city. Build. Environ. 2007, 42, 2797–2809. [Google Scholar] [CrossRef]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Odum, J.R.; Jungkamp, T.P.W.; Griffin, R.J.; Flagan, R.C.; Seinfeld, J.H. The atmospheric aerosol-forming potential of whole gasoline vapor. Science 1997, 276, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Ma, Q.; Lu, G.Q.M. VOC removal: Comparison of MCM-41 with hydrophobic zeolites and activated carbon. Energy Fuels 1998, 12, 1051–1054. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D.H. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Martin, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Dettmer, K.; Engewald, W. Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Anal. Bioanal. Chem. 2002, 373, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, B.; Orfao, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Jeong, M.G.; Park, E.J.; Jeong, B.; Kim, D.H.; Kim, Y.D. Toluene combustion over NiO nanoparticles on mesoporous SiO2 prepared by atomic layer deposition. Chem. Eng. J. 2014, 237, 62–69. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2008, 84, 303–312. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.G.; Park, E.J.; Seo, H.O.; Kim, K.D.; Kim, Y.D.; Lim, D.C. Humidity effect on photocatalytic activity of TiO2 and regeneration of deactivated photocatalysts. Appl. Surf. Sci. 2013, 271, 164–170. [Google Scholar] [CrossRef]
- Nakajima, A.; Obata, H.; Kameshima, Y.; Okada, K. Photocatalytic destruction of gaseous toluene by sulfated TiO2 powder. Catal. Commun. 2005, 6, 716–720. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 nanotubes with open channels as deactivation-resistant photocatalyst for the degradation of volatile organic compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Kim, J.; Choi, W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound. Appl. Catal. B Environ. 2018, 220, 1–8. [Google Scholar] [CrossRef]
- Franch, M.I.; Peral, J.; Domenech, X.; Ayllon, J.A. Aluminium(III) adsorption: A soft and simple method to prevent TiO2 deactivation during salicylic acid photodegradation. Chem. Commun. 2005, 2015, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Kim, K.D.; Seo, H.O.; Kim, I.H.; Jeon, C.S.; An, J.E.; Kim, J.H.; Uhm, S.; Kim, Y.D. Oil-water separation using superhydrophobic PET membranes fabricated via simple dip-coating of PDMS-SiO2 nanoparticles. Macromol. Mater. Eng. 2017, 302, 1700218. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, E.J.; Kim, D.H.; Jeong, M.G.; Kim, Y.D. Superhydrophobic surfaces with photocatalytic activity under UV and visible light irradiation. Catal. Today 2016, 260, 32–38. [Google Scholar] [CrossRef]
- Park, E.J.; Cho, Y.K.; Kim, D.H.; Jeong, M.G.; Kim, Y.H.; Kim, Y.D. Hydrophobic polydimethylsiloxane (PDMS) coating of mesoporous silica and its use as a preconcentrating agent of gas analytes. Langmuir 2014, 30, 10256–10262. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, K.D.; Yoon, H.S.; Jeong, M.G.; Kim, D.H.; Lim, D.C.; Kim, Y.H.; Kim, Y.D. Fabrication of conductive, transparent and superhydrophobic thin films consisting of multi-walled carbon nanotubes. RSC Adv. 2014, 4, 30368–30374. [Google Scholar] [CrossRef]
- Park, E.J.; Yoon, H.S.; Kim, D.H.; Kim, Y.H.; Kim, Y.D. Preparation of self-cleaning surfaces with a dual functionality of superhydrophobicity and photocatalytic activity. Appl. Surf. Sci. 2014, 319, 367–371. [Google Scholar] [CrossRef]
- Jeong, M.G.; Seo, H.O.; Kim, K.D.; Kim, Y.D.; Lim, D.C. Enhanced photocatalytic activity of TiO2 by polydimethylsiloxane deposition and subsequent thermal treatment at 800 °C. Thin Solid Films 2012, 520, 4929–4933. [Google Scholar] [CrossRef]
- Cha, B.J.; Woo, T.G.; Park, E.J.; Kim, I.H.; An, J.E.; Seo, H.O.; Kim, Y.D. Photo-catalytic activity of hydrophilic-modified TiO2 for the decomposition of methylene blue and phenol. Curr. Appl. Phys. 2017, 17, 1557–1563. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Park, E.J.; Jeong, B.; Jeong, M.G.; Kim, Y.D. Synergetic effects of hydrophilic surface modification and N-doping for visible light response on photocatalytic activity of TiO2. Curr. Appl. Phys. 2014, 14, 300–305. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Band Gap Implications on Nano-TiO2 Surface Modification with Ascorbic Acid for Visible Light-Active Polypropylene Coated Photocatalyst. Nanomater. Basel 2018, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.G.; Seo, H.O.; Kim, I.H.; Han, S.W.; Cha, B.J.; Kim, Y.D. Unveiling the complexity of the degradation mechanism of semiconducting organic polymers: Visible-light-induced oxidation of P3HT films on ZnO/ITO under atmospheric conditions. J. Phys. Chem. C 2017, 121, 18692–18701. [Google Scholar] [CrossRef]
- Seo, H.O.; Park, E.J.; Kim, I.H.; Han, S.W.; Cha, B.J.; Woo, T.G.; Kim, Y.D. Influence of humidity on the photo-catalytic degradation of acetaldehyde over TiO2 surface under UV light irradiation. Catal. Today 2017, 295, 102–109. [Google Scholar] [CrossRef]
- Jeong, M.G.; Seo, H.O.; Kim, K.D.; Kim, D.H.; Kim, Y.D.; Lim, D.C. Quenching of photocatalytic activity and enhancement of photostability of ZnO particles by polydimethysiloxane coating. J. Mater. Sci. 2012, 47, 5190–5196. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, I.H.; Kim, J.H.; Seo, H.O.; Kim, Y.D. Polydimethylsiloxane thin-film coating on silica nanoparticles and its influence on the properties of SiO2-polyethylene composite materials. Polymer 2018, 138, 24–32. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.C. Opinion on N,N-Dimethylacetamide (DMAC); ECHA: Helsinki, Finland, 2014.
- Lide, D.R.; Kehiaian, H.V. CRC Handbook of Thermophysical and Thermochemical Data; CRC Press: Boca Raton, FL, USA, 1994; p. 518. [Google Scholar]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, B.J.; Woo, T.G.; Han, S.W.; Saqlain, S.; Seo, H.O.; Cho, H.K.; Kim, J.Y.; Kim, Y.D. Surface Modification of TiO2 for Obtaining High Resistance against Poisoning during Photocatalytic Decomposition of Toluene. Catalysts 2018, 8, 500. https://doi.org/10.3390/catal8110500
Cha BJ, Woo TG, Han SW, Saqlain S, Seo HO, Cho HK, Kim JY, Kim YD. Surface Modification of TiO2 for Obtaining High Resistance against Poisoning during Photocatalytic Decomposition of Toluene. Catalysts. 2018; 8(11):500. https://doi.org/10.3390/catal8110500
Chicago/Turabian StyleCha, Byeong Jun, Tae Gyun Woo, Sang Wook Han, Shahid Saqlain, Hyun Ook Seo, Hong Kwan Cho, Jee Yong Kim, and Young Dok Kim. 2018. "Surface Modification of TiO2 for Obtaining High Resistance against Poisoning during Photocatalytic Decomposition of Toluene" Catalysts 8, no. 11: 500. https://doi.org/10.3390/catal8110500